Optimizing Care and Compliance for the Treatment of Mycosis Fungoides Cutaneous T-Cell Lymphoma With Mechlorethamine Gel
Background: Mycosis fungoides is the most common form of cutaneous T-cell lymphoma (MF-CTCL). Quality nursing care is necessary for effective diagnosis and treatment of patients with MF-CTCL. Early-stage MF-CTCL (stages Ia and Ib) is most often managed in both dermatology and multidisciplinary settings. These stages can be effectively controlled by skin-directed therapies such as mechlorethamine gel (Valchlor®). Topical mechlorethamine has been used since the 1940s as an alkylating agent; however, compounded formulas have disadvantages that limit patient compliance. In contrast, newly approved mechlorethamine gel has demonstrated an efficacious and well-tolerated profile that has longer stability and is quicker to dry than its compounded counterpart.
Objectives: This article aims to provide recommendations for optimal nursing care of patients who have been diagnosed with stage Ia or Ib MF-CTCL.
Methods: Four real-world patient cases are examined, along with practical considerations for the use of mechlorethamine gel to treat patients with MF-CTCL.
Findings: Nurses can promote patient adherence through specific interventions and strategies, such as education about mechlorethamine gel, its mechanism of action, and safety and efficacy, as well as connecting patients with patient assistance programs or other supportive services.
Jump to a section
Cutaneous T-cell lymphomas (CTCLs), primarily skin-affecting non-Hodgkin lymphomas, represent about 70%–80% of all cutaneous lymphomas reported in the United States (Bradford, Devesa, Anderson, & Toro, 2009; Imam, Shenoy, Flowers, Phillips, & Lechowicz, 2013). Mycosis fungoides (MF-CTCL) is the most common type of CTCL (50%–70% of CTCL cases) and can present as cutaneous patches, plaques, or tumors, as well as Sézary syndrome (a more aggressive leukemic variant) (Bradford et al., 2009; Criscione & Weinstock, 2007; Imam et al., 2013). An estimated 16,000–20,000 cases of MF-CTCL are reported annually in the United States (Cutaneous Lymphoma Foundation, 2011; Lymphoma Research Foundation, 2012), with about 1,000–1,500 new MF-CTCL cases diagnosed per year (Bradford et al., 2009; Stanford School of Medicine Multidisciplinary Cutaneous Lymphoma Group, 2015). The five-year age-adjusted incidence of MF-CTCL for all stages has been reported as 0.55–0.56 per 100,000 person-years (Imam et al., 2013; Korgavkar, Xiong, & Weinstock, 2013).
Patients with early-stage disease (National Comprehensive Cancer Network [NCCN], 2014; Olsen et al., 2007) typically have a more favorable prognosis (median survival of 10–35 years) (Scarisbrick et al., 2014) (see Table 1). In several studies, patients with large cell transformation (a biopsy showing cells four times larger or greater than a small lymphocyte in 25% or more of dermal infiltrate) (Salhany et al., 1988) have shown poorer outcomes (Scarisbrick et al., 2014). Decreases in overall survival, overall disease-specific survival, and median survival have been seen with relative risk for disease-related death (21.6 times more likely in patients with stage IV compared with stage Ia) (Agar et al., 2010). 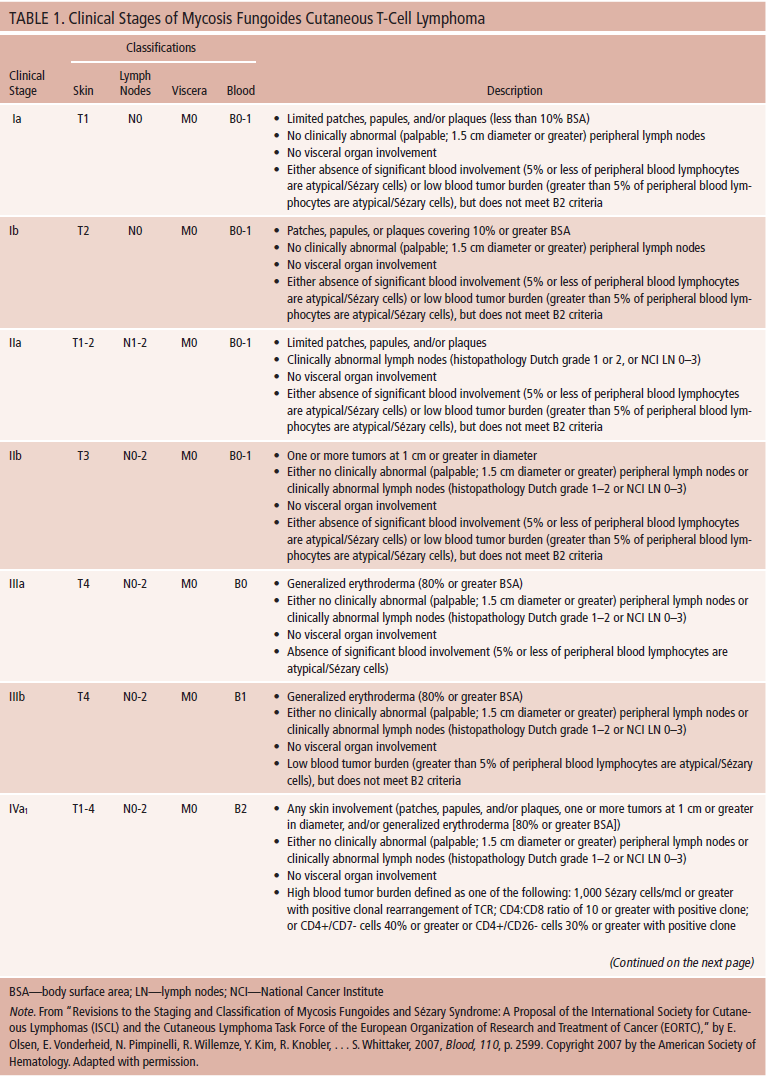
Delayed diagnosis or misdiagnosis leading to under-reporting and incorrect treatment may occur because MF-CTCL can present similarly as other types of skin conditions such as psoriasis, vitiligo, chronic contact dermatitis, eczema, folliculitis, pityriasis lichenoides chronica, pigmented purpuric dermatoses, and pityriasis lichenoides et varioliformis acuta (Girardi, Heald, & Wilson, 2004; Sarantopoulos et al., 2013; Smedby et al., 2008; Zackheim, & McCalmont, 2002). To diagnose MF-CTCL, the NCCN (2014) guidelines recommend biopsy and subsequent dermatopathology, immunohistochemistry, and molecular analysis (T-cell receptor [TCR] gene rearrangement) of suspicious skin sites. Physical examination, laboratory tests (complete blood count with manual differential [CBC diff], lactic acid dehydrogenase [LDH], TCR gene rearrangement, Sézary flow cytometry), and, if more advanced disease is suspected, human T-cell lymphotropic virus serology and bone marrow or lymph node biopsy also are important factors in diagnosis.
Treatment for MF-CTCL is contingent on disease stage and patient response. The goals of treatment are to relieve symptoms and to achieve remission and delay progression while minimizing the emergent side effects related to treatment. Therapeutic options for early-stage MF-CTCL include topical treatments such as corticosteroids, mechlorethamine (Mustargen®), and carmustine (BiCNU®); localized radiation; retinoids; phototherapy, such as psoralen plus ultraviolet A (PUVA) or ultraviolet B; and total skin electron beam. Systemic treatments such as oral retinoids, interferons (INFa, INF-g), histone deacetylase inhibitors, extracorporeal photopheresis, or single- or multi-agent chemotherapy regimens also may be used if blood involvement is positive (Girardi et al., 2004; NCCN, 2014; Reavely & Wilson, 2004). In advanced disease, treatments that reduce tumor burden or delay disease progression are implemented (Jawed, Myskowski, Horwitz, Moskowitz, & Querfeld, 2014). Patients with advanced disease may receive more aggressive single-agent or multimodal strategies such as radiation, systemic first-line therapies (gemcitabine [Gemzar®], liposomal doxorubicin [Adriamycin®]), or second-line therapies (pentostatin [Nipent®], chlorambucil [Leukeran®], temozolomide [Temodar®], cyclophosphamide [Cytoxan®], etoposide [VePesid®], higher doses of methotrexate [Rheumatrex®], or low-dose pralatrexate [Folotyn®]) (Jawed et al., 2014; NCCN, 2014).
Topical mechlorethamine has been used to treat leukemic diseases since the 1940s (Goodman et al., 1946) and has been recommended as a primary treatment option for MF-CTCL (NCCN, 2014; Trautinger et al., 2006). Mechlorethamine is an alkylating agent that inhibits quickly proliferating cells and, possibly via immune mechanisms, affects keratinocyte-Langerhans cell–T-cell interactions (Kim et al., 2005). Initially prescribed as compounded aqueous- or petrolatum-based ointment, these mechlorethamine formulations had disadvantages (e.g., greasy, less stable, more difficult to apply) that limited patient adherence (Kim, Martinez, Varghese, & Hoppe, 2003; Ramsay, Parnes, & Dubin, 1984). The U.S. Food and Drug Administration approved a topical mechlorethamine gel (Valchlor®) formulation in 2013 for patients diagnosed with stage Ia and Ib MF-CTCL who have received prior skin-directed therapy. Overall clinical experience (Kim, Duvic, Guitart, & Lessin, 2014; Lessin et al., 2013) has shown that mechlorethamine gel is generally well tolerated and similar in effectiveness to compounded mechlorethamine ointment. The most common adverse effects seen in the clinical trials were mild to moderate in severity and manageable and included pruritus, dermatitis, and hyperpigmentation. Following topical mechlorethamine use, nonmelanoma skin cancers have been reported in patients who received treatments known to cause nonmelanoma skin cancer, such as long-term phototherapy (particularly PUVA), radiation, and immunosuppressive chemotherapy; however, topical mechlorethamine did not show an increased risk of secondary cancers or comorbidities (Lindahl, Fenger-Grøn, & Iversen, 2014). Patients should be monitored for redness, inflammation, swelling, itchiness, blisters, secondary skin infections, ulceration, and nonmelanoma skin cancers during and following treatment with mechlorethamine gel. Contrary to mechlorethamine compounded in ointment, mechlorethamine gel has longer stability and is a quick-drying, greaseless gel (Actelion Pharmaceuticals Inc., 2013).
Although guidelines are available to help stage MF-CTCL, institutions may follow their own protocol for MF-CTCL diagnosis, treatment, patient follow-up, and maintenance plans. Real-world patient cases along with practical management tips are presented in this article to help healthcare providers recognize the signs and symptoms of the disease and educate nurses about topical mechlorethamine applications for use in the MF-CTCL treatment plan.
Case Reports
Case Study 1
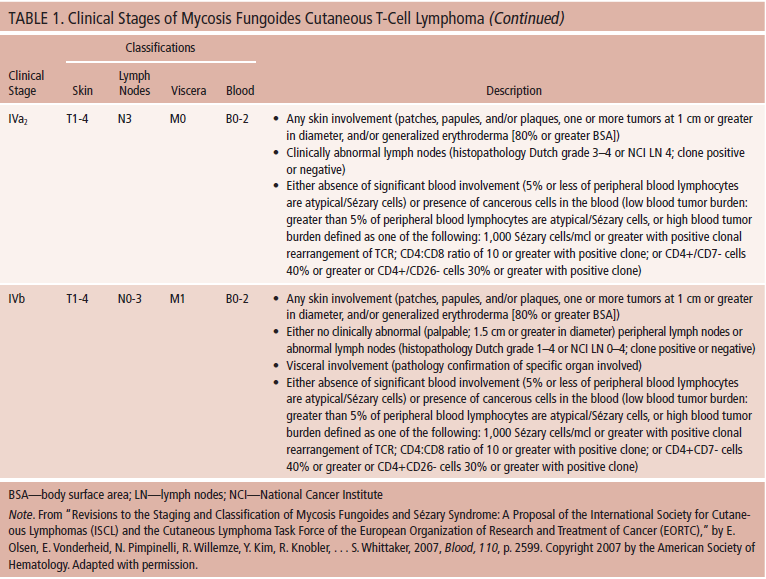
A 60-year-old Caucasian man, Fitzpatrick skin type 2 (a numerical classification schema for human skin color), body mass index (BMI) 27 kg/m2, presented with a persistent, non-pruritic rash (see Figure 1). Past medical history was remarkable for nine years of atopic dermatitis with generalized eczematous eruption treated by over-the-counter moisturizers. Physical examination in August 2013 revealed 1–3 cm atrophic erythematous patches with a body surface area (BSA) of 5%–8%. The lesions were thin, scaly, and annular/arciform, located at upper inner thighs and buttocks. The patient described the eruption as having a waxing and waning clinical course, improving after sun exposure and worsening after shower. The patient was bothered by the physical appearance of the rash and had irritation and discomfort when wearing clothing. 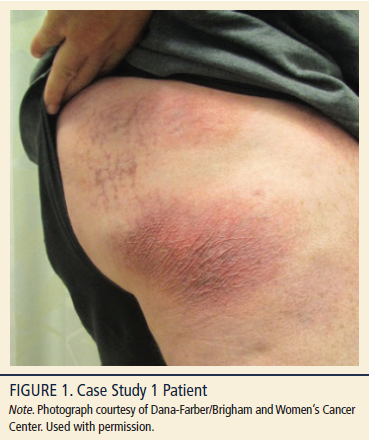
In August 2013, the patient was diagnosed with MF-CTCL stage Ia (T1N0M0B0). Skin biopsy of a representative patch revealed infiltrate of small-sized lymphocytes with irregular nuclear contours, condensed chromatin, scant cytoplasm, indistinct nucleoli present at the dermal-epidermal junction (tagging), focally extending into the epidermis and adnexal structures. A superficial dermal and perivascular lymphoid infiltrate also was present. Immunoperoxidase studies demonstrated that the lymphoid infiltrate was composed primarily of CD3-positive T-cells with intact expression of CD2 and CD5, and CD7 loss. The majority of the T-cells, including those at the dermal-epidermal junction and extending into the epidermis, expressed CD4 (CD4:CD8 ratio of about 8:1).
Relevant laboratory tests, including CBC diff and platelet count, liver function tests (LFT), and LDH were all found to be within normal limits. Flow cytometry (CD4+/CD7- or CD4+/CD26-) revealed no evidence of blood involvement.
Following the diagnosis of MF-CTCL, a three-month trial of topical corticosteroids twice per day achieved only a partial response and a few new sites of emerging disease appeared. Topical corticosteroid treatment was discontinued and the patient was switched to mechlorethamine gel applied nightly. The patient was instructed to apply a thin film of mechlorethamine gel nightly to affected areas of the skin and allow the skin to dry completely for at least four hours before or 30 minutes after a shower or washing. The patient was informed to continue treatment for three months, unless any adverse reactions occurred such as redness, itching, swelling, blisters, ulcers, and/or skin infections, which would require a return to the clinic for follow-up. The patient was informed that a positive response may include pigment changes (darker or lighter), a smoother texture, and/or no new patches emerging. No concomitant therapy was given.
At the time of this writing, the patient has tolerated treatment for five months with no dermatitis. The patches have faded, smoothed, have a mild-brawny hyperpigmentation, and the involved BSA has been reduced from 5%–8% to 2%, indicating stable-to-improved disease. The patient was educated that a mild degree of hyperpigmentation may be a positive response to treatment. The patient’s quality of life has improved because clothing is more comfortable to wear and the patient is less distressed about the appearance of the patches. The treatment plan is to continue the mechlorethamine gel treatment until patches are cleared (complete response).
Case Study 2
A 45-year-old Hispanic man, Fitzpatrick skin type 6, BMI of 29 kg/m2, presented with hyperpigmented patches and moderate pruritus. Past medical history included a diagnosis of dermatitis 15 years prior, having a waxing and waning clinical course, but worsening during the past two years. Physical examination revealed mildly xerotic, darkly hyperpigmented patches on the upper inner arms, abdomen, back, buttocks, and upper thighs with a 25%–30% BSA (see Figure 2). Lesions were distributed over axilla, upper inner arms, lower abdomen, lower back, buttocks, and hips. The patient described his skin as inflamed and tender to the touch during the past six months, resulting in disrupted sleep and difficulty in working (the patient worked many hours as a manual laborer). His wife attends his clinical visits and she also aids in applying topical treatments. 
Topical corticosteroids applied twice per week as pulse therapy (two weeks on treatment/two weeks off treatment) and an oral antihistamine provided relief of pruritus initially, but more recent response was poor and the patient discontinued the corticosteroids. Compounded mechlorethamine ointment (10 mg%) was used three years ago but was discontinued because of dermatitis.
In February 2014, the patient was diagnosed with MF-CTCL stage Ib (T2N0M0B0). A 4 mm skin punch biopsy taken from two patches presenting on his back and thigh revealed infiltrate of small-sized lymphocytes with irregular nuclear contours, indistinct nucleoli, condensed chromatin, and scant cytoplasm present at the dermal-epidermal junction (tagging) and focally extending into the epidermis and adnexal structures. Superficial dermal and perivascular lymphoid infiltrate also was present. Immunoperoxidase studies demonstrated that the lymphoid infiltrate was composed primarily of CD3-positive T-cells with intact expression of CD2, CD5, and CD7. The majority of the T-cells, including those at the dermal-epidermal junction and extending into the epidermis, expressed CD4 (CD4:CD8 ratio of about 8:1). The TCR gene rearrangement studies identified a clonal T-cell receptor gamma chain gene rearrangement. Relevant laboratory tests revealed no elevation of white blood cells and no evidence of blood involvement.
Following the diagnosis, phototherapy was considered but was not a viable option because of geographic distance for the patient. Mechlorethamine gel was prescribed. Because of the previous dermatitis reaction following compounded mechlorethamine ointment use, the patient was instructed to patch test the mechlorethamine gel; the patient was instructed to test a small area on one patch by applying mechlorethamine gel nightly for 10 days. The patient reported no adverse reaction. After the successful patch test, the patient was instructed to apply mechlorethamine gel regionally to all affected areas nightly and a lighter amount to skin folds. He did not receive other concomitant therapy. The patient’s wife was instructed to wear gloves when applying to the patient’s difficult-to-reach lesions.
At the three-month follow-up visit, the patient reported a burning sensation at the treatment site, had moderate erythema and increased hyperpigmentation that was diagnosed as severe dermatitis. Mechlorethamine gel was discontinued and class one topical corticosteroids were applied twice per day for two weeks. The dermatitis resolved and the majority of lesions cleared. For the remaining lesions, topical corticosteroids were continued for an additional two weeks (applied once daily in the morning) and concomitant mechlorethamine gel was reinitiated (applied every other night).
The patient has tolerated treatment with mechlorethamine gel for three months with an improved disease response and no return of the dermatitis. The patches are reduced in size and are less inflamed, painful, and itchy with BSA affected by disease reduced from 25%–30% to 5%. The patient’s quality of life has improved; he is sleeping better and is able to work the long hours he would normally work. The plan is to continue mechlorethamine gel treatment until lesions are cleared (complete response). Topical corticosteroids will be used as needed for any early signs of dermatitis.
Case Study 3
An 87-year-old Caucasian man, Fitzpatrick skin type 2, BMI 24.5 kg/m2, with a long-standing, stable 15-year history of MF-CTCL stage Ib presented with chronic scattered patches and plaques (see Figure 3). During this history, treatment with narrowband ultraviolet B phototherapy produced a significant reduction in patches and plaques (partial response). About two years ago, MF-CTCL was remarkable for tumors 1 cm or greater in diameter (erythematous raised nodules). Biopsy revealed large cell transformation (epidermotropism by large atypical lymphoid, CD30+ cells). In light of the patient’s large cell transformation and multidisciplinary care (collaboration with the oncology department), the patient was re-staged to MF-CTCL stage IIb (T3N0M0B0; no lymph node or blood involvement). Localized electron beam treatment was employed to address the tumor stage manifestation on the back and he was treated with an oral retinoid per NCCN (2014) guidelines, producing a strong partial response. The tumors resolved; however, the patient continued to present with persistent plaques in a limited distribution on his forearms. The patient is a retired engineer and is independent in all aspects of daily living. 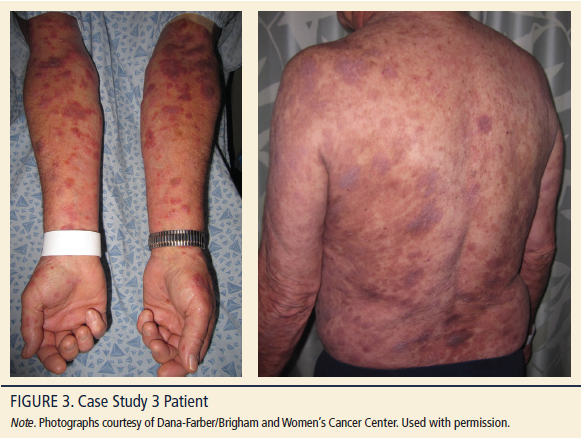
Physical examination in January 2013 revealed erythematous, scaly, annular (2–3 cm) plaques on his forearms at BSA of 3%–5%. No pruritus was noted. Following skin biopsy of multiple lesions, immunostains demonstrated a predominance of CD3+ epidermotropic atypical lymphoid cells. Rare scattered CD20+ B cells were noted. The CD4:CD8 ratio was markedly increased within the epidermis. There was a loss of staining with pan T-cell markers CD5 and CD7. Importantly, clusters and aggregates of large atypical lymphoid cells showed positive staining with antibodies to CD30. The CD30 staining was particularly remarkable.
Relevant laboratory tests revealed no elevation of white blood cells and no evidence of blood involvement. Computerized tomography and positron-emission tomography of the chest, abdomen, and pelvis were negative for lymphadenopathy and visceral involvement.
To address the several lingering plaques, mechlorethamine gel applied nightly was added to supplement the oral retinoid therapy. Six months after mechlorethamine gel was added to the treatment plan, the patient had tolerated treatment with no dermatitis or hyperpigmentation. Lesions were completely resolved. Mechlorethamine gel was discontinued and oral retinoid was continued as maintenance therapy to control the documented transformed (tumor stage) MF-CTCL.
At the time of this writing, the patient continues with long-term oral retinoid maintenance and the plaques have not returned to the affected sites. If the patient presents with new lesions, mechlorethamine gel will be reinitiated.
Case Study 4
A 49-year-old African American woman, Fitzpatrick skin type 5, BMI of 38.1 kg/m2, with a three-year history of MF-CTCL stage Ib presented with scaling patches (see Figure 4) and mild pruritus. Comorbidities include severe osteoarthritis, type 2 diabetes, and hypercholestremia. Physical examination revealed confluent, hyperpigmented, mildly xerotic patches and plaques affecting 40% of the BSA. Lesions were distributed on the patient’s breasts, abdomen, back, and buttocks. Prior treatments included tropical corticosteroids and compounded mechlorethamine ointment. Because of financial constraints and cost of compounded medication, the patient had poor adherence with the compounded mechlorethamine ointment (10 mg%) and had discontinued therapy for several months. The patient’s daughter lives with the patient and helps her by applying the topical treatments to difficult-to-reach areas. 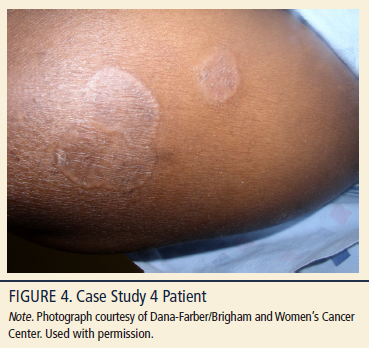
In July 2011, the patient was diagnosed with MF-CTCL stage Ib (T2N0M0B0); a skin punch biopsy from a patch on her buttock revealed that the epidermis had an infiltration of small abnormal lymphoid cells with markedly irregular or convoluted nuclei. The cells lined up at the basal epidermis. Immunostains showed that the atypical lymphocytes were positive for CD2 and CD3, with partial loss of CD4, CD5, and CD7. Relevant laboratory tests revealed no elevation of white blood cells and no evidence of blood involvement.
Following the initial diagnosis in 2011, the patient received a three-month course of topical corticosteroids with no clearing of patches or plaques. Despite the previous intermittent use of compounded mechlorethamine ointment, a strong partial response was seen: involved BSA was reduced to less than 10% BSA. The patient was restarted on therapy and was switched from compounded mechlorethamine ointment to the gel. She was advised to initially apply the gel three times per week and to add an additional day of treatment each subsequent week until applying every day, and in sparing amounts twice weekly to skin fold areas. The patient was instructed to apply regionally because of the generalized and confluent nature of the disease. She did not receive concomitant therapy. The daughter was educated to wear gloves when she applies the mechlorethamine gel to the difficult-to-reach areas.
The patient has tolerated treatment for nine months with no dermatitis or hyperpigmentation. The BSA of the lesions has been reduced from 40% to 10%, with resolution of the pruritus. The patient exhibits gradual improvement; disease regression is noted by skin smoothing, reduction in patch size, and no new areas of disease. The patient prefers the gel to the compounded ointment because it dries quicker, is easier to apply, does not stain her clothing, and the tube can be easily disposed. The plan is to continue the mechlorethamine gel treatment until lesions are cleared (complete response).
Discussion
MF-CTCL is rare and potentially life-threatening. Given the chronicity, long-term nature, and quality-of-life impact of MF-CTCL, correct diagnosis and consistent compliance with treatments are necessary to achieve optimal clinical outcomes. Success in treating patients with stage Ia or Ib MF-CTCL is largely based on patient and caregiver education regarding MF-CTCL and proper treatment.
MF-CTCL is a spectrum disorder; therefore, a multidisciplinary team of dermatology nurses, nurse practitioners, oncology nurses, and physicians may be needed to ensure accurate diagnosis, develop individualized treatment and maintenance plans to prevent disease progression, and to treat the range of symptoms associated with MF-CTCL and its therapies. Early-stage MF-CTCL is most often successfully managed in ambulatory dermatology practices. Later stage or recalcitrant MF-CTCL may be managed in multidisciplinary settings. As part of the multidisciplinary team, nurses educate patients about MF-CTCL diagnosis and staging and provide reassurance about disease progress, symptom management, and response to treatment. Photograph documentation of the disease before and after treatment can serve as a visual learning tool in the electronic health record and help track a patient’s recovery.
Nursing care measures can promote good patient adherence through specific interventions or strategies, such as educating the patient about topical agents (e.g., steroids, mechlorethamine), educating about the mechanism of action and correct application, and assessing the safety and efficacy of treatments. The overall clinical experience with the gel formulation of mechlorethamine has demonstrated a generally well-tolerated profile with most side effects being manageable and mild to moderate in severity. Nurses can connect patients with patient assistance programs offered for these treatments and/or other supportive services for managing MF-CTCL.
Implications for Practice
A patient’s quality of life may be severely affected by his or her MF-CTCL disease, which can include disturbances in functioning and emotional and social well-being (Demierre, Gan, Jones, & Miller, 2006). Other possible coexisting conditions (pruritus, ulceration of tumors, pain) and secondary events (skin infections or other cancers) also may lead to a reduced quality of life (Demierre et al., 2006).
Given the long-term nature of MF-CTCL, the quality-of-life impact, and the potential for misdiagnosis, optimizing the care and treatment compliance of patients with MF-CTCL needs to be addressed. Management of MF-CTCL may be difficult to equate among institutions because individualized patient programs may be implemented in determining disease stage and MF-CTCL diagnosis, applying treatment algorithms, determining duration of maintenance treatments, and responding to adverse outcomes. Figure 5 contains practical tips for healthcare professionals for treating patients with MF-CTCL. 
Conclusion
Nurses’ education of patients regarding topical agents may lead to positive outcomes, such as empowering the patient to be responsible for self-care. Topical agents allow patients to address manifestations of their disease at home, which may reduce travel time to the clinic or phototherapy treatment center and possibly improve patient adherence. Nurses’ assessment of the effectiveness of treatment for patients with MF-CTCL helps guide future decision making and improves patients’ independence and quality of life. 
References
Actelion Pharmaceuticals Inc. (2013). Valchlor® (prescribing information) [Package insert]. San Francisco, CA: Author.
Agar, N.S., Wedgeworth, E., Crichton, S., Mitchell, T.J., Cox, M., Ferreira, S., . . . Whittaker, S.J. (2010). Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. Journal of Clinical Oncology, 28, 4730–4739.
American Academy of Dermatology. (2015). Atopic dermatitis. Retrieved from http://www.aad.org/dermatology-a-to-z/diseases-and-treatments/a---d/ato…
Bradford, P.T., Devesa, S.S., Anderson, W.F., & Toro, J.R. (2009). Cutaneous lymphoma incidence patterns in the United States: A population-based study of 3884 cases. Blood, 113, 5064–5073.
Criscione, V.D., & Weinstock, M.A. (2007). Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Archives of Dermatology, 143, 854–859.
Cutaneous Lymphoma Foundation. (2011). Cutaneous T-cell lymphoma. Retrieved from http://www.clfoundation.org/sites/default/files/content/CTCL_2011.pdf
Demierre, M.F., Gan, S., Jones, J., & Miller, D.R. (2006). Significant impact of cutaneous T-cell lymphoma on patients’ quality of life. Cancer, 107, 2504–2511.
Girardi, M., Heald, P.W., & Wilson, L.D. (2004). The pathogenesis of mycosis fungoides. New England Journal of Medicine. 350, 1978–1988.
Goodman, L.S., Wintrobe, M.M., Dameshek, W., Goodman, M.J., Gilman, A., & McLennan, M.T. (1946). Nitrogen mustard therapy: Use of methyl-bis(beta-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA, 132, 126–132.
Imam, M.H., Shenoy, P.J., Flowers, C.R., Phillips, A., & Lechowicz, M.J. (2013). Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leukemia and Lymphoma, 54, 752–759.
Jawed, S.I., Myskowski, P.L., Horwitz, S., Moskowitz, A., & Querfeld, C. (2014). Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): Part II. Prognosis, management, and future directions. Journal of the American Academy of Dermatology, 70(2), e1–e17.
Kim, E.J., Hess, S., Richardson, S.K., Newton, S., Showe, L.C., Benoit, B.M., . . . Rook, A.H. (2005). Immunopathogenesis and therapy of cutaneous T-cell lymphoma. Journal of Clinical Investigation, 115, 798–812.
Kim, Y.H., Duvic, M., Guitart, J., & Lessin, S.R. (January 23–25, 2014). Tolerability and efficacy of mechlorethamine 0.04% gel in CTCL (mycosis fungoides) after initial treatment with topical mechlorethamine 0.02% gel [Poster TS14_10]. Presented at the T-cell Lymphoma Forum in San Francisco, CA.
Kim, Y.H., Martinez, G., Varghese, A., & Hoppe, R.T. (2003). Topical nitrogen mustard in the management of mycosis fungoides: Update of the Stanford experience. Archives of Dermatology, 139, 165–173.
Korgavkar, K., Xiong, M., & Weinstock, M. (2013). Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatology, 149, 1295–1299.
Lessin, S.R., Duvic, M., Guitart, J., Pandya, A.G., Strober, B.E., Olsen, E.A., . . . Kim, Y.H. (2013). Topical chemotherapy in cutaneous T-cell lymphoma: Positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatology, 149, 25–32.
Lindahl, L.M., Fenger-Grøn, M., & Iversen, L. (2014). Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: A 30-year population-based cohort study. British Journal of Dermatology, 170, 699–704.
Lymphoma Research Foundation. (2012). Cutaneous T-cell lymphoma: Types of cutaneous T-cell lymphoma. Retrieved from http://www.lymphoma.org/site/pp.asp?c=bkLTKaOQLmK8E&b=6300151
National Comprehensive Cancer Network. (2014). NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s lymphomas. Retrieved from http://www.nccn.org/about/nhl.pdf
Olsen, E., Vonderheid, E., Pimpinelli, N., Willemze, R., Kim, Y., Knobler, R., . . . Whittaker, S. (2007). Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood, 110, 1713–1722.
Ramsay, D.L., Parnes, R.E., & Dubin, N. (1984). Response of mycosis fungoides to topical chemotherapy with mechlorethamine. Archives of Dermatology, 120, 1585–1590.
Reavely, M.M., & Wilson, L.D. (2004). Total skin electron beam therapy and cutaneous T-cell lymphoma: A clinical guide for patients and staff. Dermatology Nursing, 16, 36–39.
Salhany, K.E., Cousar, J.B., Greer, J.P., Casey, T.T., Fields, J.P., & Collins, R.D. (1988). Transformation of cutaneous T-cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. American Journal of Pathology, 132, 265–277.
Sarantopoulos, G.P., Palla, B., Said, J., Kinney, M.C., Swerdlow, S.M., Willemze, R., & Binder, S.W. (2013). Mimics of cutaneous lymphoma: Report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. American Journal of Clinical Pathology, 139, 536–551.
Scarisbrick, J.J., Kim, Y.H., Whittaker, S.J., Wood, G.S., Vermeer, M.H., Prince, H.M., & Quaglino, P. (2014). Prognostic factors, prognostic indices and staging in mycosis fungoides and Sézary syndrome: Where are we now? British Journal of Dermatology, 170, 1226–1236.
Smedby, K.E., Vajdic, C.M., Falster, M., Engels, E.A., Martínez-Maza, O., Turner, J., . . . Cozen, W. (2008). Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood, 111, 4029–4038.
Stanford School of Medicine Multidisciplinary Cutaneous Lymphoma Group. (2015). Mycosis fungoides/Sézary syndrome. Retrieved from http://cutaneouslymphoma.stanford.edu/community/mycosis_fungoides.html
Trautinger, F., Knobler, R., Willemze, R., Peris, K., Stadler, R., Laroche, L., . . . Whittaker, S. (2006). EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. European Journal of Cancer, 42, 1014–1030.
Zackheim, H.S., & McCalmont, T.H. (2002). Mycosis fungoides: The great imitator. Journal of the American Academy of Dermatology, 47, 914–918.
About the Author(s)
Allister Benjamin Chase, MSN, FNP-BC, RN, PHN, is an oncology nurse practitioner at the UCLA Medical Center, Santa Monica; Kristen Markel, RN, BSN, is a nurse coordinator for the Multidisciplinary Cutaneous Lymphoma Program in the School of Medicine at Stanford University in California; and Marianne C. Tawa, RN, MSN, ANP, is a nurse practitioner in dermatology and cutaneous oncology at the Dana-Farber Cancer Institute in Boston, MA. The authors take full responsibility for the content of the article. Editorial support was provided by Donna Simcoe, MS, MBA, CMPP, at Simcoe Consultants, Inc., through support from Actelion Pharmaceuticals, Inc. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers or editorial staff. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Clinical Journal of Oncology Nursing or the Oncology Nursing Society. Chase can be reached at achase@mednet.ucla.edu, with copy to editor at CJONEditor@ons.org. (Submitted February 2015. Accepted for publication April 6, 2015.)

