Implementing the Surviving Sepsis Campaign in an Ambulatory Clinic for Patients With Hematologic Malignancies
Background: Infectious complications can occur in patients receiving cancer treatment and are the most common cause of death not directly related to malignancy. Established international best practices for recognition and management of early sepsis with bundled interventions reduce sepsis-related morbidity and mortality in many patient populations. Integration of these practices is common in emergency departments but has not been documented in ambulatory oncology clinics, where many patients with cancer present for evaluation of infectious symptoms.
Objectives: The current quality improvement project embedded sepsis best practices into routine care for ambulatory clinic patients receiving chemotherapy or undergoing hematopoietic stem cell transplantation for hematologic disease or malignancies.
Methods: An interprofessional protocol was implemented that included guideline-based universal screening, nurse-activated standing orders for recommended interventions, and clinician-supported decision making for the first six hours.
Findings: Evaluation of implementation of the protocol showed improved timeliness and adherence to sepsis practice guidelines. Postintervention adherence to threshold times for obtaining blood cultures and blood lactate and start of antibiotics showed improvement. All recommended interventions were completed within the target time frame for the majority of patients.
Jump to a section
As many as 10%–75% of patients receiving cancer treatment experience infectious complications, and those complications are the most common cause of death not directly related to malignancy (National Comprehensive Cancer Network [NCCN], 2015). Sepsis is the leading cause of death among hospitalized patients and the most expensive disease to treat (Joint Commission Center for Transforming Healthcare, 2016). Sepsis is particularly relevant in oncology, where leukopenia and sepsis occur in 21% of patients (NCCN, 2015) and are associated with a 15% increase in mortality (Lyman et al., 2010). The Surviving Sepsis Campaign has shown that early detection and appropriate treatment can decrease mortality by 7%–17%, even in resource-challenged settings (Dellinger et al., 2013; Levy et al., 2010, 2012; Surviving Sepsis Campaign, 2016; Wang, Xiong, Schorr, & Dellinger, 2013). Evidence-based sepsis strategies include elements to be performed within the first six hours, called early goal-directed therapy. Additional elements should be completed within the first 24 hours. Implementation of early goal-directed therapies is cost effective and saves one life for every seven to nine patients treated (Cannon et al., 2012; Puskarich, Marchick, Kline, Steuerwald, & Jones, 2009; Sebat et al., 2005) (see Table 1). Although research has challenged the value of central venous pressure monitoring and central venous oxygen saturation goals, other interventions are still strongly recommended (Yealy et al., 2014). 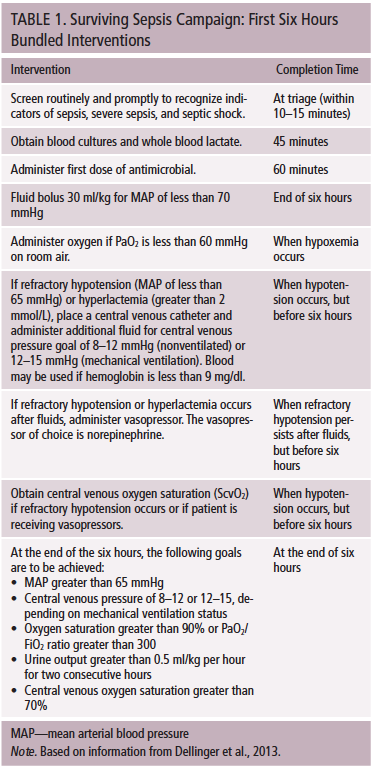
One challenge to implementing timely sepsis care in patients with cancer is that, instead of going to an emergency department (ED) with infectious symptoms, patients present to oncology clinics. Compared to the ED, the staff in clinics is less likely to implement the sepsis bundle. The aim of this project was to implement sepsis screening and initial bundled six-hour interventions in an ambulatory clinic, where specialized care is provided to patients undergoing intensive chemotherapy or hematopoietic stem cell transplantation (HSCT) for hematologic disease or malignancy.
Literature Review
MEDLINE®, Embase, and Cochrane Libraries were searched for evidence published from January 2000 to March 2013 to support best practices in sepsis management. The international standards from the Surviving Sepsis Campaign have been validated (Dellinger et al., 2004, 2008, 2013) and endorsed by more than 15 countries and 8 international organizations. Review of the articles revealed that patients with cancer, with their unique clinical risks and symptomatology, were rarely included. No contraindications were noted to implementing Surviving Sepsis Campaign best practices with patients with cancer; however, no studies describing use of these strategies in patients with cancer were found.
Searching the same databases for febrile neutropenia and sepsis in cancer care yielded 59 articles describing risks and poor prognostic variables associated with infections in patients with cancer. Risk models that accurately predict poor outcomes in patients with febrile neutropenia were identified (Baskaran, Gan, & Adeeba, 2008; Klastersky et al., 2000; Lyman et al., 2011). Based on the literature review, cancer-specific risks for sepsis and poor outcomes, such as presence of mucositis or concomitant immunosuppressive agents, were added to the screening tool (Lyman et al., 2011; NCCN, 2015).
International guidelines for sepsis management were established in 2004 and revised in 2012 (Dellinger et al., 2004, 2013). Moderate to high levels of evidence exist for initiating key “bundled interventions” for patients with possible sepsis. The four cornerstones of sepsis bundled strategies are (a) early screening and completion of diagnostic studies, (b) source evaluation, (c) timely administration of appropriate antibiotics, and (d) aggressive management of perfusion (Dellinger et al., 2013). Several trials have shown reduced mortality even with suboptimal compliance with all bundle elements (Cannon et al., 2012; Chamberlain, Willis, & Bersten, 2011; Funk, Sebat, & Kumar, 2009; McKinley et al., 2011), which suggests that some elements may be more important than others or that host factors influence the impact of individual interventions (Levy et al., 2012; Machado & Mazza, 2010).
Because strong evidence supports early intervention after patients present with sepsis, another literature search was conducted using the same databases and dates to identify best methods to implement recommended guidelines. Sixty-nine articles described successful implementation of sepsis bundle elements through standardized processes, including protocols, electronic prompts, and staff reminders (Cannon et al., 2012; Cruz et al., 2011; Focht, Jones, & Lowe, 2009; Levy et al., 2004; Phua et al., 2012). Patient safety studies reinforced use of automation to avoid care omissions (Micek et al., 2006; Shekelle et al., 2013; Wachter, Pronovost, & Shekelle, 2013). Development of teams and redundant systems also affected achievement of desired clinical outcomes (Beale et al., 2009; Campbell, 2008; Capuzzo et al., 2012; Ferrer et al., 2008).
This project was designed to improve the care of neutropenic patients with cancer and patients undergoing HSCT who present to the ambulatory clinic with signs or symptoms of sepsis. Sepsis criteria used for this protocol were modified from the original criteria to include oncology-specific risks and symptoms (see Figure 1). The two aims of this project were to reduce the time from initial patient presentation to first intervention for sepsis to less than 20 minutes and to ensure that 80% of patients receive all of the three- and six-hour sepsis bundle elements within the recommended time frame. Based on the evidence, a nurse-managed protocol, with complementary electronic orders to guide patient evaluation and emergent treatment, was adopted to accomplish the aims. In addition, healthcare providers were to respond immediately to a sepsis alert, search for potential infectious sources, prescribe appropriate management (including antibiotics), and choose other emergent interventions. 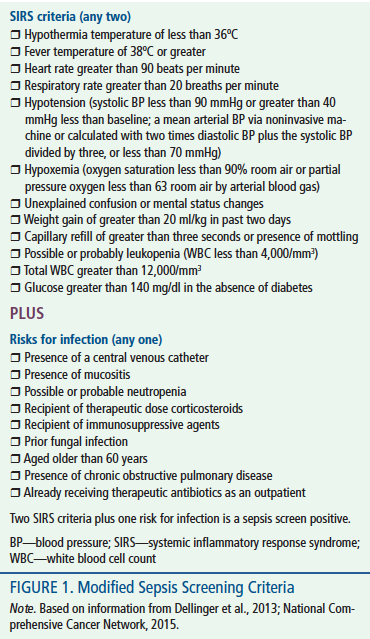
Methods
This project was reviewed by the Johns Hopkins Institutional Review Board and determined to be exempt as a quality improvement study. The project was completed in a clinic where patients receive oncology care (active chemotherapy and transplantation) for hematologic malignancy. The clinic typically provides service for 60 patients undergoing active therapy, with 25–45 patient visits daily. Three to eight patients are admitted from the clinic weekly for chemotherapy or treatment of complications, the most common being infection.
Before initiating the project, existing practices were evaluated. Because febrile neutropenia can be used in the presence of presumed infection to define sepsis, patients admitted with these three criteria (fever, neutropenia, and presumed infection) served as the baseline group for the project (NCCN, 2015). Forty of 222 patients admitted from the clinic to the hospital with possible sepsis from July 1, 2012, to March 31, 2013, were randomly selected as a baseline group.
Power was calculated based on a projected improvement of adherence to guideline recommendations one month after protocol implementation from 10%–40%, a beta of 0.8, and a 95% confidence interval. This adherence rate was selected based on data showing that a guideline adherence rate of 37% could induce a 7%–17% reduction in sepsis mortality in a broad population at an academic medical center (Chamberlain et al., 2011; Focht et al., 2009; Rivers et al., 2001). Without other thresholds to assess the optimum dose of intervention, this offered a guide for sample size deemed most likely to enhance patient outcomes.
Innovation
This project implemented an evidence-based protocol for management of early sepsis in a population of ambulatory patients with hematologic malignancies or undergoing HSCT. The innovation included an interprofessional guideline-based protocol, including a nurse-activated set of standing orders within the existing physician order entry system. The staff was provided with 30–45-minute face-to-face educational sessions in small groups from December 2013 to March 2014. They were also given reference materials prior to implementation of the project.
Upon arrival at the clinic, nursing staff screened each patient for the presence of sepsis using sepsis screening criteria. If the patient screened positive for possible sepsis, the nurse activated the standing orders. Blood lactate levels and two sets of blood cultures, drawn peripherally or from a semipermanent venous access device, were obtained immediately, and the provider was notified. Providers completed a clinical evaluation and determined whether additional diagnostic and therapeutic actions were indicated. Applicable orders and detailed instructions supported provider decision making to manage patients presenting with symptoms ranging from the minimal complaints to hypoxemia, hypotension, and septic shock. Effectiveness of this protocol was assessed by comparing the time from screening positive for sepsis to initiation of sepsis bundle interventions recommended for use during the first six hours of care in the intervention and baseline groups.
Data Collection
Data to describe the sample and evaluate the impact of the innovation on specific aims were collected by a trained research assistant. Data included (a) demographic, disease, and treatment-related variables; (b) unique sepsis screen inclusion criteria of patients and time of screen positive status; and (c) recommended bundled interventions performed and time of completion. Data were collected directly from the patient’s electronic health record, entered into Microsoft® Excel®, version 8.0, deidentified after validation, and imported into SPSS®, version 22.0, for analysis.
Data extraction from the electronic health record was piloted three times with two extractors, and conflicting data were resolved with further clarification of extraction rules, then reassessed by a third extractor. Because this new practice posed a risk for missed cases, all clinic visits were secondarily screened daily by the quality project coordinator throughout the phase-in and data collection periods. During the phase-in week, missed cases were discussed with staff daily until there were no missed cases. Nine cases were missed because of a lack of recognition of positive screening by nursing staff during data collection (3% of all clinic visits; 11% of positive sepsis screen cases). Nine positive sepsis screen cases were withdrawn from the protocol by providers who believed that unique clinical circumstances confounded the accuracy of the screening criteria.
Demographic, disease, and treatment data were analyzed using chi-square analysis. Mean time to completion of sepsis interventions was analyzed using independent t test. Chi-square analysis was used to compare the collection of blood lactate and cultures within 45 minutes, start of antibiotics within 60 minutes, achievement of six-hour goals, and completion of all bundled interventions within recommended time frames.
Results
Data on 119 patients, 40 in the baseline group and 79 in the intervention group, were collected and analyzed to evaluate the impact of this project. Demographic and clinical characteristics of patients in each group are reported on Table 2. Age and gender distribution was similar in both groups. Mean age in the baseline group was 52.2 years (SD = 14.9), and mean age in the intervention group was 49.7 years (SD = 18.3). Twenty-four men (60%) were in the baseline group, and 47 men (59.5%) were in the intervention group. 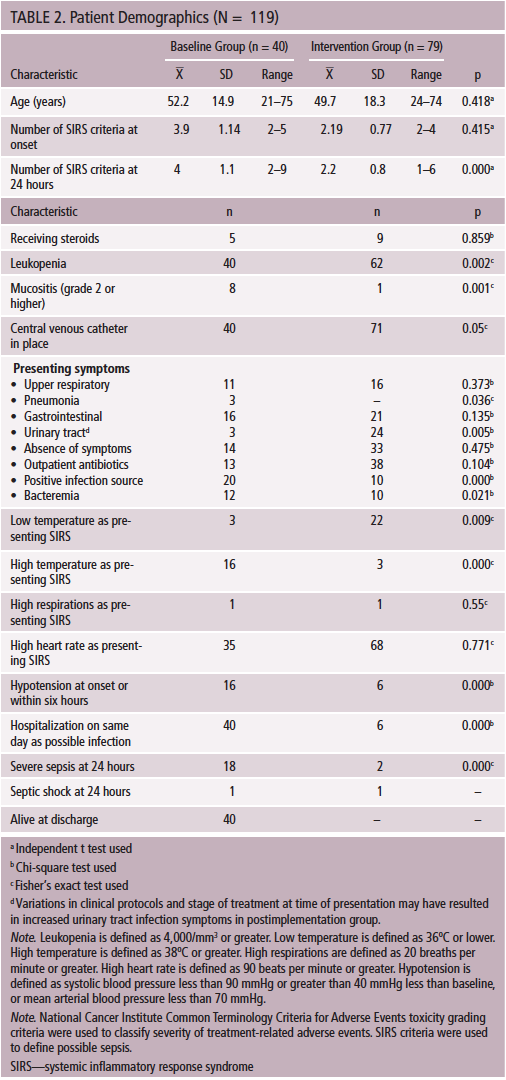
Oncologic diagnoses were distributed equally across both groups, but the intervention group included more patients with hematologic disorders (p = 0.002). The treatments used were different across groups, with less use of myeloablative chemotherapy (p = 0.000) and greater use of nonmyeloablative transplantation in the intervention group (p = 0.002) (see Table 3). These differences represent typical treatment of hematologic malignancy and may have contributed to the lower level of acuity found in the intervention group. The baseline group had a significantly higher incidence of severe mucositis, hyperthermia, hypotension, leukopenia, positive infection source, bacteremia, hospitalizations, number of systemic inflammatory response syndrome criteria, and severe sepsis at 24 hours. Patients in the baseline group appeared to be more acutely ill than patients in the intervention group. Detailed data on clinical characteristics of the two groups are found in Table 4. 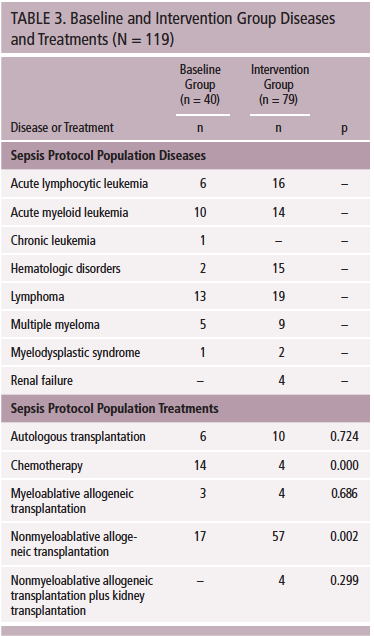
The first aim of this project was to reduce the time from screening positive to the first intervention for sepsis to less than 20 minutes. Initial sepsis interventions included collection of blood tests (serum lactate, blood cultures) and/or administration of an antibiotic when prescribed by providers. This time was selected to ensure adequate time for providers to assess patients and prescribe antibiotics and for nurses to start the antimicrobial by 60 minutes from the positive sepsis screen. Practice in the baseline group varied widely, with mean time to the first intervention of 291 minutes (SD = 535 minutes, range = 0–2,927) and only 29% achieving the 20-minute goal. The mean time to first intervention for the intervention group was 23 minutes (SD = 22, range = 0–131), with 51% receiving the first intervention within 20 minutes (p = 0.029). The time from positive sepsis screen to the first sepsis intervention decreased significantly after the innovation but did not reach the goal of 20 minutes.
[[{"type":"media","view_mode":"media_original","fid":"23916","attributes":{"alt":"","class":"media-image","height":"623","typeof":"foaf:Image","width":"373"}}]]
The second aim of the project was to increase the proportion of patients who screen positive for sepsis and receive all of the three- and six-hour sepsis bundle elements within those time frames to 40%. This included collection of blood cultures and lactate within 45 minutes, and start of first antibiotic dose within 60 minutes. Interventions for hypoxemia, hypotension, and achievement of all pertinent six-hour goals were also assessed. Only four patients in the baseline group had lactate levels drawn. Consequently, mean time to collection of lactate was 907 minutes (SD = 507, range = 547–1,267) at baseline. Blood lactate was obtained in the intervention group after an average of 23 minutes (SD = 22, range = 0–131; p = 0.000). Because of the wide range of times, interpreting these findings and deriving clinical implications is difficult.
The mean time to collection of blood cultures for the baseline group was 303 minutes (SD = 540, range = 0–2,927) and 25 minutes (SD = 31, range = 0–239; p = 0.002) for the intervention group. Mean time to start of antibiotics for the baseline group was 362 minutes (SD = 548, range = 0–2,992) and 56 minutes (SD = 37.5, range = 13–100; p = 0.002) for the intervention group. Patients in the intervention group received all three of the initial interventions (collection of blood lactate and blood cultures and start of antibiotics) earlier than patients in the baseline group.
Blood cultures were drawn within the 45-minute threshold only 40% of the time in the baseline group, but this time threshold was met in 86% of patients in the intervention group. Although the mean time to start of the first antibiotic was significantly different between the baseline and intervention groups (p = 0.002), the proportion of the two groups who met the 60-minute time threshold for starting an antibiotic did not differ significantly (p = 0.131). Only five patients in the intervention group received an antibiotic at all compared to 37 in the baseline group. Between-group differences based on analysis of variance in illness acuity and the low number of patients in the intervention group receiving an antibiotic make it difficult to interpret differences in receipt of antibiotics.
None of the baseline patients received all the recommended interventions within the designated six-hour time frame. However, 65 of 79 of the patients in the intervention group received all appropriate interventions defined in the sepsis protocol, exceeding the project’s goal of 40%. Adherence to guideline recommendations after implementation of the protocol was complete 82% of the time. Implementation of the innovation produced a significant improvement in adherence with the sepsis bundled elements during the first six hours (p = 0.000).
Discussion
This project demonstrated that an interprofessional protocol that included guideline-based universal screening, nurse-initiated actions, electronic standing orders, and clinician-supported decision making in the first six hours after positive screening for sepsis was useful. It resulted in timely completion of early goal-directed sepsis interventions in patients with hematologic diagnoses or malignancies treated in an ambulatory setting. After the protocol was implemented, the mean time from screening positive to first intervention improved dramatically from 291 to 23 minutes but did not meet the desired 20-minute time frame. The protocol improved care practices so that 82% of patients who screened positive for sepsis received all of the appropriate sepsis bundle interventions within the first six hours of care. Few patients developed hypotension, making it difficult to determine whether the use of the protocol would improve time to implementation for all the six-hour recommendations and subsequent attainment of six-hour goals.
During staff education sessions, more than 75% of staff thought they were adequately prepared to care for patients with sepsis but were not familiar with the recommendations from the Surviving Sepsis Campaign (2016). Significant improvements in practice could be partially linked to an increased awareness of best practices in sepsis care derived from the education intervention. Follow-up assessment of staff knowledge and practice would help researchers understand the importance of this observation.
Significant differences in patient diagnoses and acuity were found between the baseline and intervention groups. Patients in the baseline group were generally sicker and had been admitted to the hospital. Attention to the signs and symptoms of sepsis and the ability to implement initial sepsis bundled actions might be greater in an inpatient setting. However, many sepsis elements were omitted in this group.
A total of 37% of all clinic visits resulted in a positive sepsis screen in the intervention group. The screening instrument and sepsis management protocol did not miss any cases of sepsis. In the intervention group, the high sensitivity and low specificity of the screening tool led to high numbers of patients screening positive for sepsis, who were later deemed by clinicians not to be infected. In fact, 79 of 87 patients who screened positive for sepsis were later found to be false positive based on absence of positive cultures or evidence of postscreening systemic infection. More accurate criteria to define sepsis in this population is essential for optimal translation and implementation of these guidelines in oncology practice.
Clinic workflow did not readily support attaining the 20-minute time frame for performing the initial sepsis intervention. Only a small number of patients in the intervention group received antibiotics because providers often felt that presenting symptoms did not indicate the presence of infection. Feasibility of timely administration of antibiotics in the ambulatory environment is important to consider and requires evaluation of a larger patient sample. Some sepsis interventions were well represented in this study (e.g., collection of blood cultures and lactate), but others occurred in such low numbers that statistical analysis was not possible (e.g., fluid and vasopressor administration). Several patients with tachycardia received fluid boluses despite no antibiotics being prescribed. This unanticipated consequence of fluid administration based on sepsis screening was not part of planned evaluation but may be valuable to include in future analysis.
Patients in the intervention group experienced a low incidence of sepsis-related complications and were not admitted to the hospital. Based on incidence rates in the literature, an intervention group of 40–80 patients were anticipated to yield at least two patients with severe sepsis or septic shock, but this occurred in only one individual during the project. Evaluation of a larger number of patients is needed to assess the true mean time for interventions, such as antimicrobial administration, fluid administration, or management of hemodynamic instability. 
Conclusion
Standards for early management of sepsis are well established in the emergency setting. This project demonstrated that implementation of initial sepsis interventions using nurse-driven, prewritten, conditional orders within recommended time frames is possible in an ambulatory oncology care setting. Screening for sepsis is within the expertise of oncology nurses and can truly be considered a nurse-sensitive outcome (Aitken et al., 2011; de Papathanassoglou, 2009; Kleinpell, Aitken, & Schorr, 2013). This project demonstrated that protocol-driven, independent nursing actions in high-risk patients can be performed reliably and consistently. Patient engagement in their care using infection prevention strategies, monitoring for symptoms outside the clinic setting, and seeking medical care promptly can complement the effectiveness of a sepsis screening and early intervention protocol (Centers for Disease Control and Prevention, 2011). The intervention group in this project did not include enough patients with severe sepsis, hypotension, or hypoxemia to evaluate the effectiveness of recommended interventions for these complications. Additional patients receiving emergent antibiotics must be evaluated to determine if the threshold of 60 minutes is realistic in this practice environment or if systems can be modified to meet this goal. Additional research is needed to define oncology-specific sepsis screening criteria; pertinent prognostic, population-specific indicators for sepsis; and the efficacy of specific interventions. Given that sepsis management has been identified as a national priority and the recommendations from the Surviving Sepsis Campaign are the basis of best practices, it is essential to pursue research to define the trajectory and management of sepsis for patients with cancer in the ambulatory setting.
[[{"type":"media","view_mode":"media_original","fid":"24056","attributes":{"alt":"","class":"media-image","height":"229","typeof":"foaf:Image","width":"770"}}]]
References
Aitken, L.M., Williams, G., Harvey, M., Blot, S., Kleinpell, R., Labeau, S., . . . Ahrens, T. (2011). Nursing considerations to complement the Surviving Sepsis Campaign guidelines. Critical Care Medicine, 39, 1800–1818.
Baskaran, N.D., Gan, G.G., & Adeeba, K. (2008). Applying the Multinational Association for Supportive Care in Cancer risk scoring in predicting outcome of febrile neutropenia patients in a cohort of patients. Annals of Hematology, 87, 563–569. doi:10.1007/s00277-008-0487-7
Beale, R., Reinhart, K., Brunkhorst, F.M., Dobb, G., Levy, M., Martin, G., . . . Williams, M.D. (2009). Promoting global research excellence in severe sepsis (PROGRESS): Lessons from an international sepsis registry. Infection, 37, 222–232.
Campbell, J. (2008). The effect of nurse champions on compliance with Keystone Intensive Care Unit Sepsis-screening protocol. Critical Care Nursing Quarterly, 31, 251–269.
Cannon, C.M., Holthaus, C.V., Zubrow, M.T., Posa, P., Gunaga, S., Kella, V., . . . Rivers, E.P. (2012). The GENESIS Project (GENeralized Early Sepsis Intervention Strategies): A multicenter quality improvement collaborative. Journal of Intensive Care Medicine, 28, 355–368. doi:10.1177/0885066612453025
Capuzzo, M., Rambaldi, M., Pinelli, G., Campesato, M., Pigna, A., Zanello, M., . . . Toschi, E. (2012). Hospital staff education on severe sepsis/septic shock and hospital mortality: An original hypothesis. BMC Anesthesiology, 12, 28. doi:10.1086/1471-2253-12-28
Centers for Disease Control and Prevention. (2011). Basic infection control and prevention plan for outpatient oncology settings. Retrieved from http://www.cdc.gov/hai/pdfs/guidelines/basic-infection-control-preventi…
Chamberlain, D.J., Willis, E.M., & Bersten, A.B. (2011). The severe sepsis bundles as processes of care: A meta-analysis. Australian Critical Care, 24, 229–243.
Cruz, A.T., Perry, A.M., Williams, E.A., Graf, J.M., Wuestner, E.R., & Patel, B. (2011). Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics, 127, e758–e766. doi:10.1542/peds.2010-2895
Dellinger, R.P., Carlet, J.M., Masur, H., Gerlach, H., Calandra, T., Cohen, J., . . . Levy, M.M. (2004). Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Critical Care Medicine, 32, 858–873.
Dellinger, R.P., Levy, M.M., Carlet, J.M., Bion, J., Parker, M.M., Jaeschke, R., . . . Vincent, J.L. (2008). Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine, 36, 296–327.
Dellinger, R.P., Levy, M.M., Rhodes, A., Annane, D., Gerlach, H., Opal, S.M., . . . Moreno, R. (2013). Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine, 39, 165–228.
de Papathanassoglou, E. (2009). Sepsis bundles: Time for a nursing initiative? Nursing in Critical Care, 14, 162–165.
Ferrer, R., Artigas, A., Levy, M.M., Blanco, J., González-Díaz, G., Garnacho-Montero, J., . . . de la Torre-Pradeos, M.V. (2008). Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA, 299, 2294–2303.
Focht, A., Jones, A.E., & Lowe, T.J. (2009). Early goal-directed therapy: Improving mortality and morbidity in the emergency department. Joint Commission Journal on Quality and Patient Safety, 35, 186–191.
Funk, D., Sebat, F., & Kumar, A. (2009). A systems approach to the early recognition and rapid administration of best practice therapy in sepsis and septic shock. Current Opinion in Critical Care, 15, 301–307.
Joint Commission Center for Transforming Healthcare. (2016). About the center. Retrieved from http://www.centerfortransforminghealthcare.org/about
Klastersky, J., Paesmans, M., Rubenstein, E.B., Boyer, M., Elting, L., Feld, R., . . . Talcott, J. (2000). The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low risk febrile neutropenic cancer patients. Journal of Clinical Oncology, 18, 3038–3051.
Kleinpell, R., Aitken, L, & Schorr, C.A. (2013). Implications of the new international sepsis guidelines for nursing care. American Journal of Critical Care, 22, 212–222.
Levy, M.M., Artigas, A., Phillips, G.S., Rhodes, A., Beale, R., Osborn, T., . . . Dellinger, R.P. (2012). Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet, 12, 919–924.
Levy, M.M., Dellinger, R.P., Townsend, S.R., Linde-Zwirble, W.T., Marshall, J.C., Bion, J., . . . Angus, D.C. (2010). The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine, 38, 367–374
Levy, M.M., Pronovost, P.J., Dellinger, R.P., Townsend, S., Resar, R.K., Clemmer, T.P., & Ramsay, G. (2004). Sepsis change bundles: Converting guidelines into meaningful change in behavior and clinical outcomes. Critical Care Medicine, 32(Suppl.), S595–S597.
Lyman, G., Kuderer, N., Crawford, B., Wolff, D.A., Culakova, E., Poniewierski, M.S., & Dale, D.C. (2011). Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer, 117, 1917–1927.
Lyman, G.H., Michels, S.L., Reynolds, M.W., Barron, R., Tomic, K.S., & Yu, J. (2010). Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer, 116, 5555–5563.
Machado, F.R., & Mazza, B.F. (2010). Improving mortality in sepsis: Analysis of clinical trials. Shock, 34(Suppl. 1), 54–58.
McKinley, B.A., Moore, L.J., Sucher, J.F., Todd, S.R., Turner, K.L., Valdivia, A., . . . Moore, F.A. (2011). Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. Journal of Trauma and Acute Care Surgery, 70, 1153–1167.
Micek, S.T., Roubinian, N., Heuring, T., Bode, M., Williams, J., Harrison, C., . . . Kollef, M.H. (2006). Before-after study of a standardized hospital order set for the management of septic shock. Critical Care Medicine, 34, 2702–2713.
National Comprehensive Cancer Network. (2015). Prevention and treatment of cancer-related infection [v.1.2015]. Retrieved from http://www.nccn.org/professionals/physician_gls/PDF/infections.pdf
Phua, J., Ho, B.C., Tee, A., Chan, K.P., Johan, A., Loo, S., . . . Koh, Y. (2012). The impact of clinical protocols in the management of severe sepsis: A prospective cohort study. Anaesthesia and Intensive Care, 40, 663–674.
Puskarich, M.A., Marchick, M., Kline, J.A., Steuerwald, M.T., & Jones, A.E. (2009). One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: A before and after study. Critical Care, 13, R167.
Rivers, E., Nguyen, B., Havstad, S., Ressler, J., Muzzin, A., Knoblich, B., . . . Tomlanovich, M. (2001). Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine, 345, 1368–1377.
Sebat, F., Johnson, D., Musthafa, A.A., Watnik, M., Moore, S., Henry, K., & Saari, M. (2005). A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest, 127, 1729–1743.
Shekelle, P.G., Pronovost, P.J., Wachter, R.M., McDonald, K.M., Schoelles, K., Dy, S.M., . . . Walshe, K. (2013). The top patient safety strategies that can be encouraged for adoption now. Annals of Internal Medicine, 158, 365–368.
Surviving Sepsis Campaign. (2016). Protocols and checklists. Retrieved from http://www.survivingsepsis.org/Resources/Pages/Protocols-and-Checklists…
Yealy, D.M., Kellum, J.A., Huang, D.T., Barnato, A.E., Weissfeld, L.A., Pike, F., . . . Angus, D.C. (2014). A randomized trial of protocol-based care for early septic shock, New England Journal of Medicine, 370, 1683–1693. doi:10.1056/NEJMoa1401602
Wachter, R.M., Pronovost, P.J., & Shekelle, P.G. (2013). Strategies to improve patient safety: The evidence base matures. Annals of Internal Medicine, 158, 350–352.
Wang, Z., Xiong, Y., Schorr, C., & Dellinger, R.P. (2013). Impact of sepsis bundle strategy on outcomes of patients suffering from severe sepsis and septic shock in China. Journal of Emergency Medicine, 44, 735–741. doi:10.1016/j.emermed.2012.07.084
About the Author(s)
Brenda K. Shelton, DNP, RN, APRN-CNS, CCRN, AOCN®, is a clinical nurse specialist in the Sidney Kimmel Comprehensive Cancer Center, Julie Stanik-Hutt, PhD, ACNP, CCNS, FAAN, is an associate professor in the School of Nursing, Joyce Kane, MSN, RN, is a quality and safety manager in the Sidney Kimmel Comprehensive Cancer Center, and Richard J. Jones, MD, is a professor in the School of Nursing, all at Johns Hopkins University in Baltimore, MD. The authors take full responsibility for the content of the article. The authors did not receive honoraria for this work. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors, planners, independent peer reviewers, or editorial staff. Shelton can be reached at sheltbr@jhmi.edu, with copy to editor at CJONEditor@ons.org. (Submitted March 2015. Revision submitted July 2015. Accepted for publication July 15, 2015.)




