Rationale and Background on Tumor-Treating Fields for Glioblastoma
Background: More than 150 years after the first description of glioma cells, patients with glioblastoma (GBM) continue to have a poor prognosis despite standard-of-care therapy. With the introduction of tumor-treating fields (TTFields) therapy for the treatment of recurrent GBM in 2011 and for newly diagnosed GBM in 2015, the opportunity to increase progression-free survival and overall survival while improving quality of life provides a welcome option.
Objectives: This article describes how TTFields therapy may be used in the treatment of patients with recurrent GBM.
Methods: This article provides oncology nurses with two case studies that examine how TTFields therapy can be integrated into the overall treatment paradigm.
Findings: These two patient case studies demonstrate the autonomy and lack of adverse effects that TTFields therapy offers to patients with GBM.
Jump to a section
Glioblastoma (GBM) is a malignancy of the central nervous system. In the 1860s, Rudolf Virchow was the first to provide a pathologic description of glioma cells (DeAngelis & Mellinghoff, 2011). Johnson (1885) published the first attempted resection by A. Hughes Bennett. Bailey and Cushing (1926) provided the first modern pathologic classification of GBM. Modern medicine has evolved to the current standard of care of maximal safe resection followed by concurrent radiochemotherapy and then adjuvant chemotherapy. However, there remains no cure. Following U.S. Food and Drug Administration ([FDA], 2011) approval, tumor-treating fields (TTFields) entered the oncologist’s arsenal. TTFields use the device Optune® as an innovative therapeutic option approach for treating patients with GBM.
GBM is the most common malignant primary brain tumor. From 12,500–18,000 new cases of GBM are diagnosed annually in the United States (Ostrom et al., 2016; Schwartzbaum, Fisher, Aldape, & Wrensch, 2006). Prognosis remains poor, with about 15-month median survival from diagnosis with the current standard-of-care treatment (Stupp et al., 2005). Prior to 2011, treatments were limited to surgery, radiation, and chemotherapy. Effective chemotherapeutic options are limited, often leaving patients and providers with a sense of desperation in the face of this malignancy. At progression of disease, providers may offer additional chemotherapy from the limited armamentarium of FDA-approved therapies or may suggest clinical trials. At specialized treatment centers, re-resection or re-radiation may be considered.
Case Study: Recurrent Glioblastoma
Jason was a left-handed 28-year-old man approaching his wedding day when he experienced his first seizure while at work. Jason was taken from his office to the hospital by ambulance. Upon arrival to the emergency department, he experienced a second seizure. Jason was slow to recover full consciousness after the second seizure, prompting the healthcare team to intubate Jason for airway protection. During the following few hours, the magnetic resonance imaging (MRI) scan results demonstrated a lesion at the junction of his right temporal and parietal lobes (see Figure 1). Jason was stabilized with high-dose steroids and loaded with levetiracetam (Keppra®). He was taken to the operating room for a craniotomy and resection of the tumor. Jason was discharged from the hospital in time for his wedding day. The following week, Jason and his wife, Teresa, came to the clinic to discuss the pathology results. The tumor was a GBM; Jason and Teresa were crestfallen. 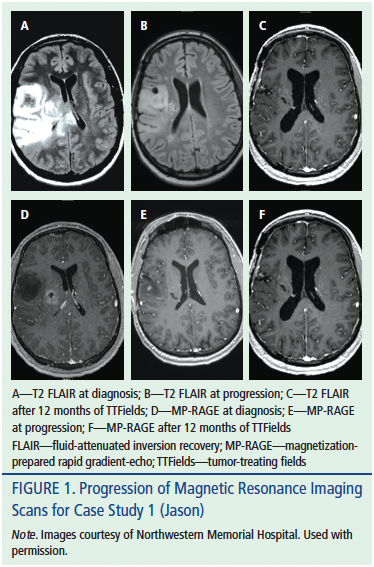
A month after surgery, Jason began a course of chemoradiotherapy, which he completed with ease. Teresa and Jason left for their honeymoon during the month-long break from treatment. Upon their return, Jason began a year of adjuvant chemotherapy cycles with temozolomide (TMZ) (Temodar®). His course was remarkable only for the occasional day of constipation. After a year off treatment and with stable scans, Jason and Teresa started trying to conceive. They were surprised to find themselves expecting triplets. Teresa reported that their lives were not what she envisioned they would be, but that she was thankful every day for their relationship and their family.
When the triplets were six years old, Jason complained to his team of intermittent right-hand shaking. The neuro-oncology nurse practitioner confirmed that his episodes were seizures and ordered a follow-up MRI scan. The scan demonstrated a 2.2 cm by 1.5 cm area of enhancing tissue growing within the original tumor bed. The lesion was felt to represent a tumor regrowth. Jason and Teresa agreed that an aggressive course of action was appropriate. Following a second craniotomy for resection, the pathologist confirmed the resected tissue was a recurrent GBM tumor with expected postradiation changes. Jason and his neuro-oncology team discussed a variety of treatment options, including clinical trials; he ultimately opted to pursue TTFields treatment. Jason is doing well seven months after beginning treatment with Optune.
Benefits of Tumor-Treating Fields Therapy
TTFields therapy generates an intermediate-frequency electrical field within tissues and does not affect quiescent (nondividing) cells. TTFields therapy exerts its effects on dividing cells by moving intracellular macromolecules and organelles toward the neck of the dividing parent cell. As the intracellular contents move toward the neck of the cell, they are trapped and the cell divides into abnormal daughter cells. Application of TTFields also inhibits proper formation of spindles during spindle tubulin polymerization. These effects on dividing cells ultimately result in apoptosis (programmed cell death) (Gera, Yang, Holtzman, Lee, & Swanson, 2015).
Systemic adverse effects are minimized with TTFields because the local administration of therapy is delivered via four transducer arrays placed directly on the scalp. The ceramic, disposable arrays are connected to the delivery device, Optune. When turned on, Optune delivers TTFields at a frequency of 200 kHz for treatment of GBM (Stupp et al., 2005). This frequency is too low to generate local tissue heating and too high to stimulate nerve or muscle tissue.
Traditional treatments for GBM expose patients to numerous risks. Surgical risks are well established. In the perioperative period, patients undergoing craniotomy are exposed to the myriad risks of anesthesia. Craniotomy risks are well defined and include mortality, hydrocephalus, venous thromboembolism, infection, permanent neurologic deficit, diminished functional status, stroke, or hemorrhage (Chang et al., 2003; Fadul et al., 1988). Likewise, radiation is not without risks. Particular common effects of cranial radiation for GBM are fatigue, headache, nausea and vomiting, radiation skin changes, and alopecia. Additional short-term risks of cranial radiation include headache, vomiting, increased intracranial edema, acute encephalopathy, cranial nerve injury, worsening of preexisting neurologic symptoms, and transient neurocognitive impairment. In longer-term GBM survivors, risks of radiation include neurocognitive decline or dementia, cerebral radionecrosis or atrophy, accelerated intracranial vascular disease, SMART (stroke-like migraine after radiation therapy) syndrome, intracranial carvernomas or hemorrhage, and secondary malignancy (Behin & Delattre, 2003; DeAngelis & Posner, 2009).
The blood–brain barrier prohibits dose efficacy for many available systemic chemotherapies. Since 1990, several chemotherapeutic agents have demonstrated efficacy against GBM. Current chemotherapies with FDA approval for treatment of GBM include TMZ, bevacizumab (Avastin®), lomustine (Gleostine®), and carmustine (BiCNU® for IV use, Gliadel® wafers for intraoperative implantation). Each of these chemotherapies has an established adverse effect profile (see Table 1). Patients may experience hematologic toxicities, particularly with TMZ, an alkylating agent. In longer-surviving patients, there is a risk of developing a secondary malignancy, such as leukemia or myelodysplastic syndrome. 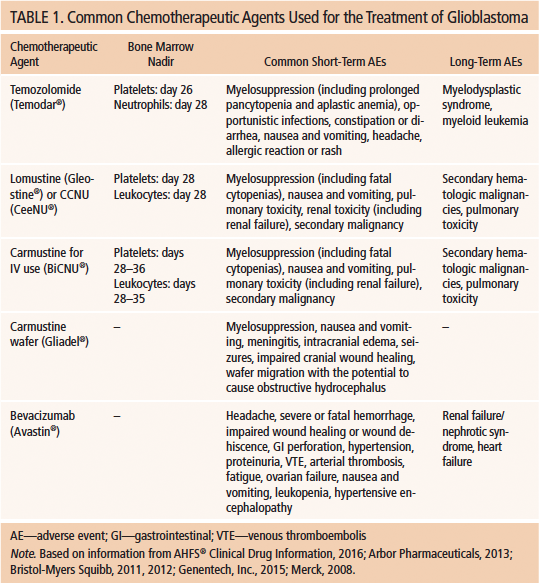
The localized, focal therapy delivered by TTFields provides for a lack of systemic adverse effects. Safety data from the EF-14 trial demonstrated notably lacking systemic adverse effects in TTFields therapy compared to chemotherapeutic agents (Stupp et al., 2015). The reasons for this are varied. The delivery of TTFields ceases when the device is turned off. This is in stark contrast to alkylating chemotherapies or anti–vascular endothelial growth factor therapies. The half-life of bevacizumab is 21 days; TMZ, lomustine, and carmustine all have prolonged expected nadirs for hematologic toxicities (Merck, 2008). In addition, TTFields therapy is anatomically limited to the brain tissue.
When TTFields therapy was approved by the FDA in 2011, the approval was limited to patients with recurrent GBM. The studies leading to FDA approval were the EF-07 and EF-11 trials (DeAngelis & Posner, 2009). The EF-07 trial was a pilot trial designed to evaluate safety and feasibility in 10 patients with newly diagnosed GBM after resection and chemoradiotherapy. The EF-11 trial was a phase III randomized trial in which 237 patients with recurrent GBM were treated with either chemotherapy alone or with TTFields therapy alone (FDA, 2011). In this trial, patients receiving TTFields experienced about the same six-month progression-free survival (PFS-6) and overall survival (OS) as the cohort receiving chemotherapy. However, evaluation of toxicities and quality of life clearly favored TTFields. In the follow-up study to EF-11, 695 patients with newly diagnosed GBM were enrolled in the EF-14 clinical trial. After completing maximal safe surgical resection and chemoradiotherapy, patients were randomized (2:1) to treatment with either maintenance TMZ plus TTFields or to maintenance TMZ alone. Patients in the TMZ plus TTFields arm had a statistically significant survival benefit in PFS-6 and OS in the interim analysis (Stupp et al., 2015). Data evaluated at this interim point demonstrated a 3.2-month increase in PFS-6 and OS. These data led to FDA approval for crossover of patients in the control arm to the TMZ plus TTFields arm (Stupp et al., 2015).
Case Study: Newly Diagnosed Glioblastoma
Lisa was 63 years old when she traveled to her hometown to care for her dying sister. She found herself dragging her left foot and bumping into the wall during the few weeks that she was home. After Lisa’s sister passed away, she finally agreed to see her physician. The physician ordered an MRI brain scan, which demonstrated a large ring-enhancing right parieto-occipital lesion with central necrosis and associated vasogenic edema (see Figure 2). Lisa went to surgery the same day for a decompressive craniotomy. Pathologic analysis confirmed the surgeon’s suspicion of GBM. After a near-total resection, she consented to standard-of-care treatment with chemoradiotherapy for her diagnosis of GBM. At her appointment after completing radiation, Lisa reported recurrence of left-leg weakness. Neuroimaging demonstrated a large cyst within Lisa’s tumor cavity with mass effect in the left precentral gyrus, consistent with her neurologic symptoms. She underwent a repeat craniotomy for cyst decompression. The neuro-oncology tumor board reviewed Lisa’s imaging and pathology specimens. The consensus of the tumor board was that Lisa’s tumor had not recurred. Lisa was advised to proceed with standard-of-care treatment. Lisa recovered well and began adjuvant TMZ for 5 of 28 days in combination with TTFields therapy. 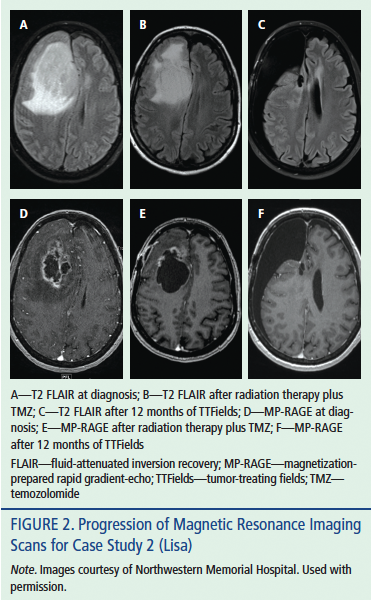
Lisa is nearing completion of one year of adjuvant therapy. She continues to work full-time, reporting that her quality of life remains high. Lisa finds herself needing naps on the few days of each TMZ week, as well as coping with significant chemotherapy-induced constipation. On her second cycle of adjuvant TMZ, Lisa forgot to premedicate with ondansetron (Zofran®) and was “violently ill.” With scheduled ondansetron dosing and rescue doses of metoclopramide (Reglan®), Lisa is doing well. At a recent visit with the neuro-oncologist, Lisa and her team discussed the risks and benefits of continuing on TTFields therapy. She said, “Out of the very few things I can control during treatment, Optune is fully in my control.” Lisa relates that her sense of self-empowerment and overall lack of side effects have led her to conclude she will likely continue on TTFields therapy for the foreseeable future, even past completion of adjuvant chemotherapy. 
Conclusion
After years without advances in treatment for GBM, TTFields therapy offers a welcome treatment option for patients with a dire diagnosis. The lack of side effects makes TTFields an appealing choice for many patients. The self-administered nature of treatment with TTFields translates to a high level of educational needs for the patient and family at initiation of treatment; therefore, the oncology nurse must be prepared with appropriate knowledge. As the case studies in this article illustrate, TTFields therapy can be an empowering experience for patients with GBM.
References
AHFS® Clinical Drug Information. (2016). Lomustine. In AHFS® Clinical Drug Information (Ed.), AHFS drug information, 2016 (pp. 1094–1096). Bethesda, MD: American Society of Health-System Pharmacists.
Arbor Pharmaceuticals. (2013). Gliadel wafer (carmustine wafer) [Package insert]. Retrieved from http://gliadel.com/hcp/media/_pdfs/prescribing-information-gliadel.pdf
Bailey, P., & Cushing, H. (1926). A classification of the tumours of the glioma group on a histogenetic basis with a correlated study of prognosis. Philadelphia, PA: J.B. Lippincott.
Behin, A., & Delattre, J-.Y. (2003). Neurologic sequelae of radiotherapy on the nervous system. In D. Schiff & P.Y. Wen (Eds.), Cancer neurology in clinical practice (pp. 173–191). Totowa, NJ: Humana.
Bristol-Myers Squibb. (2011). BiCNU® (carmustine for injection) [Package insert]. Princeton, NJ: Author.
Bristol-Myers Squibb. (2012). CeeNU® (lomustine) [Package insert]. Princeton, NJ: Author.
Chang, S.M., Parney, I.F., McDermott, M., Barker, F.G., Schmidt, M.H., Huang, W., . . . Berger, M. (2003). Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. Journal of Neurosurgery, 98, 1175–1181.
DeAngelis, L.M., & Mellinghoff, I.K. (2011). Virchow 2011 or how to ID(H) human glioblastoma. Journal of Clinical Oncology, 29, 4473–4474.
DeAngelis, L.M., & Posner, J.B. (2009). Side effects of radiation therapy. In Neurologic complications of cancer (2nd ed., pp. 511–555). New York, NY: Oxford University Press.
Fadul, C., Wood, J., Thaler, H., Galicich, J., Patterson, R.H. Jr., & Posner, J.B. (1988). Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology, 38, 1374–1379.
Genentech, Inc. (2015). Avastin® (bevacizumab) [Package insert]. Retrieved from http://www.gene.com/download/pdf/avastin_prescribing.pdf
Gera, N., Yang, A., Holtzman, T.S., Lee, S.X., & Swanson, K.D. (2015). Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLOS One, 10, e0125269.
Johnson, G. (1885). Reports of societies. British Medical Journal, 1(1272), 988–995.
Merck. (2008). Temodar® (temozolomide) [Package insert]. Retrieved from https://www.merck.com/product/usa/pi_circulars/t/temodar_capsules/temod…
Ostrom, Q.T., Gittleman, H., de Blank, P.M., Finlay, J.L., Gurney, J.G., McKean-Cowdin, R., . . . Barnholtz-Sloan, J.S. (2016). American Brain Tumor Association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology, 18(Suppl. 1), i1–i50. doi:10.1093/neuonc/nov297
Schwartzbaum, J.A., Fisher, J.L., Aldape, K.D., & Wrensch, M. (2006). Epidemiology and molecular pathology of glioma. Nature Clinical Practice Neurology, 2, 494–503.
Stupp, R., Mason, W.P., van den Bent, M.J., Weller, M., Fisher, B., Taphoorn, M.J.B., . . . Mirimanoff, R.O. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine, 352, 987–996.
Stupp, R., Taillibert, S., Kanner, A.A., Kesari, S., Steinberg, D.M., Toms, S.A., . . . Ram, Z. (2015). Maintenance therapy with tumor-treating fields plus temozolomide vs. temozolomide alone for glioblastoma: A randomized clinical trial. JAMA, 314, 2535–2543. doi:10.1001/jama.2015.16669
U.S. Food and Drug Administration. (2011). Summary of safey and effectiveness data (SSED): Tumor treatment fields, NovoTTF-100A System. Retrieved from http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100034b.pdf
About the Author(s)
Margaret A. Schwartz, MSN, APN/ANP-C, CNRN, is an advanced practice nurse and Lynnette Onuselogu, MSN, APN/FNP-C, is a nurse practitioner, both at the Northwestern Memorial Hospital Brain Tumor Institute in Chicago, IL. The authors take full responsibility for the content of the article. Schwartz has previously consulted for and received honorarium from Novocure, Inc. Editorial support was provided by Stephen Swyberius, BA, copy supervisor at PharmaHealthLabs, through support from Novocure, Inc. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers or editorial staff. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Clinical Journal of Oncology Nursing or the Oncology Nursing Society. Schwartz can be reached at meg.a.walsh@gmail.com, with copy to editor at CJONEditor@ons.org. (Submitted May 2016. Revision submitted July 2016. Accepted for publication July 21, 2016.)




