Heart and Lung Complications: Assessment and Prevention of Venous Thromboembolism and Cardiovascular Disease in Patients With Multiple Myeloma
Background: Venous thromboembolism (VTE) and cardiovascular (CV) disease can occur in patients with multiple myeloma. Although VTE and CV disease are separate medical conditions, they can be serious and even life-threatening.
Objectives: The objectives of this article are to describe risk factors for cancer-associated VTE, describe the influence of CV disease on patients with multiple myeloma, and review the approaches to VTE and CV disease identification and treatment.
Methods: PubMed and CINAHL® databases were used to identify literature to describe VTE and CV in patients diagnosed with multiple myeloma.
Findings: When present in patients with multiple myeloma, VTE and CV disease can limit patient tolerance for myeloma treatment and, therefore, decrease therapeutic options.
Jump to a section
Venous thromboembolism (VTE) and cardiovascular (CV) disease commonly occur in patients with cancer, specifically multiple myeloma (MM) (Ritts, Lenihan, & Cornell, 2016). Although VTE and CV disease are separate medical conditions, they are equally serious, each with the potential to be life-threatening. Like the general American population, patients with MM have a high prevalence of preexisting comorbid CV conditions, including hyperlipidemia and hypertension, and non-CV conditions such as diabetes (National Heart Lung and Blood Institute, 2012; Palumbo et al., 2008). In patients with MM, VTE is six times more likely to occur with dexamethasone combined with immunomodulatory agents, such as lenalidomide, thalidomide, or pomalidomide (Lyman et al., 2015; Palumbo et al., 2008). When present in patients with MM, VTE and CV disease can limit patient tolerance for antimyeloma treatment and, therefore, decrease therapeutic options. This article aims to (a) describe risk factors for cancer-associated VTE, (b) describe the influence of CV disease on patients with MM, and (c) review the approaches to VTE and CV disease identification and treatment. As new agents become available for MM, the prevention and management of VTE and CV complications should remain a priority for the long-term health of patients.
Venous Thromboembolism
Although thrombosis can occur anywhere in the venous system, VTE most frequently occurs in lower extremities as a deep vein thrombosis (DVT). DVT treatment is intended to prevent circulation of the VTE to the lung, where it becomes a pulmonary embolism (PE). The cause of VTE in MM is often multifactorial, involving the interaction of clinical risk factors, such as immobility and hypercoagulability from treatment, and inherent risk factors for thrombosis, such as activation of procoagulant factors and inflammation (Kristinsson, 2010). In several clinical trials, the use of immunomodulatory drugs in MM has been associated with an increased risk of VTE (Rajkumar et al., 2010; Weber et al., 2007). For patients with VTE in the first 10 years of a MM diagnosis, mortality is two times higher than in patients who do not have VTE. Patients who have VTE in the first year of a MM diagnosis have a three times higher mortality rate (Kristinsson, Pfeiffer, Bjorkholm, Schulman, & Landgren, 2012).
Pathophysiology of Venous Thromboembolism
Specific systemic conditions predispose individuals with cancer to VTE development. These conditions were first described by Rudolph Virchow in 1859 and are known as Virchow’s Triad, which consists of hypercoagulability, vessel wall or endothelial injury, and stasis (Kesieme, Kesieme, Jebbin, Irekpita, & Dongo, 2011).
Prothrombotic mechanisms respond to the presence of a tumor, causing inflammation, necrosis, and hemodynamic changes, which can also be exacerbated by MM therapy (Kristinsson, 2010). In addition, clot-promoting molecules with procoagulant and fibrinolytic activities are secreted by tumor cells, causing an interaction with endothelial cells. This plays an important role in pathogenesis. In addition, venous stasis reduces the clearance of activating coagulation factors, causing endothelial cell damage and increasing the risk for VTE (Elyamany, Alzahrani, & Bukhary, 2014; Kesieme et al., 2011).
Immunomodulalatory drugs have various anti-angiogenic and anti-inflammatory effects that can alter the interaction between the tumor cells and the bone marrow microenvironment. Although the exact reason why these drugs increase the risk of VTE is unknown, it is thought that they can enhance the expression of tissue factor and vascular endothelial growth factor and, by downregulating thrombospondin, cause cytokine-mediated activated protein C resistance (Kristinsson, Bjorkholm, Schulman, & Landgren, 2011).
Risk Factors for Venous Thromboembolism in Multiple Myeloma
VTE incidence is higher among patients diagnosed with monoclonal gammopathy of undetermined significance (an asymptomatic premalignant clonal plasma cell or lymphoplasmacytic proliferative disorder associated with an increased risk of developing MM or lymphoma) and MM than in the general population (Gregersen et al., 2011; Kristinsson et al., 2011). Risk factors for VTE are listed in Figure 1. The risk of VTE is also increased in inherited and noninherited thrombophilia conditions, such as factor V Leiden, prothrombin G20210A mutation, protein C or protein S deficiency, and antithrombin deficiency (Fahrni, Husmann, Gretener, & Keo, 2015; Heit, Spencer, & White, 2016).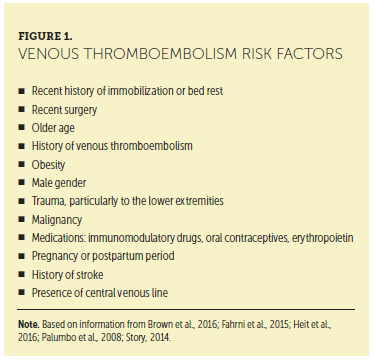
Venous Thromboembolism Risk Factors: Medication
Several medications increase the risk of VTE. Patients treated with immunomodulatory drugs (IMiDs), such as thalidomide, lenalidomide, or pomalidomide, or with steroids and/or chemotherapy are also at significant risk of developing VTE. When higher doses of dexamethasone are used, the risk of VTE increases significantly. Another medication that is associated with increased incidence of VTE is erythropoietin, which may be used in the setting of anemia in patients with MM (Anaissie et al., 2012).
In a study comparing low- and high-dose dexamethasone in combination with lenalidomide, the incidence of VTE was 12% in the low-dose dexamethasone group and 26% in the high-dose dexamethasone group. The low-dose dexamethasone dose was 40 mg weekly on days 1, 8, and 15. The high-dose dexamethasone was 40 mg on days 1–4, 9–12, and 17–20 (Rajkumar et al., 2010). In a study by Bat et al. (2005), the incidence of VTE was 58% in patients with MM who were treated with thalidomide and an anthracycline but did not receive thrombosis prophylaxis medication. Anticogulant therapy can also reduce VTE incidence rates. When aspirin was added at the start of the thalidomide-containing regimen, the incidence rate of VTE decreased to about 19% (Baz et al., 2008).
A retrospective chart review reported that the overall incidence of VTE in patients on IMiD therapy was 9.7% (Dede & Pruemer, 2016). One goal of the study by Dede and Pruemer (2016) was to compare the rate of VTE when national VTE prophylaxis guidelines were and were not followed. The national guidelines include the use of aspirin in patients with less than two VTE risk factors, and low-molecular-weight heparin (LMWH) for patients with two or more VTE risk factors (Palumbo et al., 2008). When national guidelines were followed, VTE occurred at a rate of 4.8%. When the guidelines were not followed, VTE occurred in 12.2% of the patients. The rate of VTE in patients not receiving thromboprophylaxis was 23.1%. Of note, no patients taking LMWH or warfarin developed VTE. Of the patients taking aspirin, 7.9% developed VTE (Dede & Pruemer, 2016).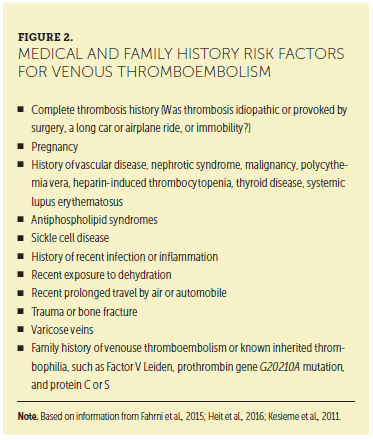
Assessment of Venous Thromboembolism Risk
Signs and Symptoms
Information in the patient’s medical and family history is vital for identifying risk factors for VTE (see Figure 2). Careful review of a patient’s medical history, elicitation of his or her subjective review of systems, and completion of a comprehensive physical examination are all necessary to identify the subtle findings that may lead to the diagnosis of VTE. Combining the subjective and objective data in the patient encounter with laboratory and radiologic assessment is key to a prompt diagnosis. For example, the physical examination may reveal a thrombosed vein or hard palpable cord in the calf, posterior knee, or thigh that is erythematous, painful, and consistent with DVT. The respiratory assessment may reveal the inability to take deep breaths, tachypnea, and shallow breaths, which are consistent with PE. Signs and symptoms of DVT and PE are presented in Figure 3.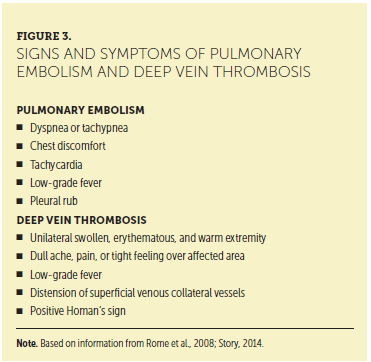
Laboratory Tests
On completion of the history and physical examination, further diagnostic testing, including laboratory tests and imaging, may be indicated. Laboratory tests that should be considered include electrolytes, D-dimer, prothrombin time, international normalized ratio, partial thromboplastin time, and antithrombin level. Of note, although an increased D-dimer level is a sensitive indicator of an acute thrombotic event, the level is also increased in nonthrombotic clinical situations, such as pregnancy, infection, malignancy, atrial fibrillation, stroke, and other inflammatory conditions (Bates et al,. 2012; Pulivarthi & Gurram, 2014). Testing for inherited thrombophilia conditions may be indicated, but the results will not change the initial treatment for a patient who is diagnosed with VTE. Figure 4 lists laboratory testing for specific inherited thrombophilia conditions. A hematologist may need to evaluate the patient before ordering laboratory tests.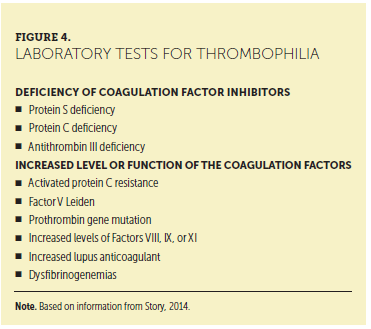
Diagnosis of Venous Thromboembolism
Appropriate and prompt assessment of the patient with suspected VTE is vital in making a rapid diagnosis. Radiologic studies that can identify DVT or PE include Doppler ultrasonography, contrast venography, computed tomography angiography, ventilation perfusion lung scan, and magnetic resonance venography. The standard approach to diagnosing for suspected DVT is Doppler ultrasound (Bates et al., 2012). Although computerized tomography angiography can accurately diagnose PE and pelvic vein or inferior vena cava thrombosis, it requires the use of contrast. Magnetic resonance venography may be performed without contrast to detect PE, can differentiate chronic from acute thrombus, and can be used to avoid radiation in pregnant women (Gaitini, 2007). Ventilation perfusion lung scan is useful only in patients with normal chest radiographs and is typically used when computed tomography angiography is contraindicated or inconclusive (Thompson & Kabrhel, 2017)
The Wells criteria is a scoring system used to determine the risk of PE or DVT before radiologic testing is done (see Table 1), and to determine subsequent treatment or follow-up imaging. A trial of 501 patients (78 with cancer) was conducted in Canada from 1994–1996 to determine the need for serial ultrasound testing of patients suspected of having DVT. Patients with a Wells score greater than 3 were at a high risk for DVT, whereas patients with a score of 0 had a low likelihood of DVT (Wells et al., 1997).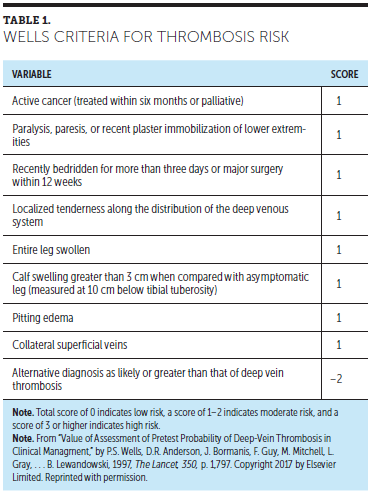
Prevention and Treatment Strategies
Prevention of VTE is essential for patients at risk for thrombosis. A heightened awareness of VTE prevention has occurred and many standards of care for VTE prevention have been implemented. Figure 5 presents several of the available strategies for VTE prevention.
Anticoagulation medications, such as LMWH, warfarin, and fondaparinux, can be used to prevent VTE (Kearon et al., 2012). Patients with MM who take IMiDs should take anticoagulation medication to prevent VTE. The use of oral anticoagulants, such as dabigatran, rivaroxaban, and apixaban, is controversial for VTE prevention in malignancy and is currently under investigation (Streiff et al., 2016).
The treatment of VTE is changing, with many options now available for patients. In the setting of acute VTE, LMWH and unfractionated heparin (UFH) should be considered. Oral anticoagulation with medications, such as warfarin, can be used in the patient with MM but should be used with LMWH (enoxaparin, dalteparin), UFH, or fondaparinux until the international normalized ratio is in a therapeutic range that is within the range of 2–3 (normal range is 0.9–1.3). Direct oral anticoagulant (DOAC) medications are not well established for VTE in malignancy and are currently under investigation (ClinicalTrials.gov, 2014; Streff et al., 2016). Rivaroxaban has been studied in a large, randomized clinical trial of patients with symptomatic VTE, and could be considered for long-term anticoagulation of patients with MM (Einstein Investigators, 2010). Dabigatran is a IIa (thrombin) inhibitor and rivaroxaban and apixaban are Xa inhibitors. Patients often prefer the new oral agents because laboratory monitoring and dietary restriction are not necessary. However, a drawback to the new oral anticoagulants is their long half-life compared to heparin or LMWH, which can lead to prolonged uncontrolled bleeding (Babilonia & Trujillo, 2014). No commercially available medications are available to reverse the anticoagulation effects of the new oral anticoagulants, although activated prothrombin complex concentrate medications have shown promise in initial studies (Awad & Cocchio, 2013). Table 2 reviews anticoagulation medications used to treat or prevent VTE.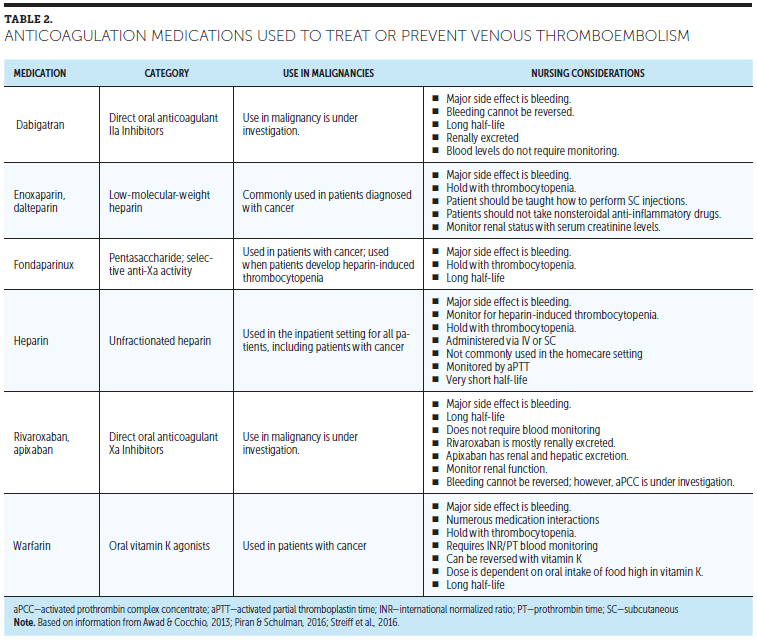
Anticoagulation medication should continue while patients with MM are treated with IMiDs, such as lenalidomide, thalidomide, or pomalidomide (Lyman et al., 2015; Palumbo et al., 2008). Patients should be prescribed anticoagulation for six months and then reevaluated for risk of recurrent VTE and/or bleeding (Lyman et al., 2015). Patients with MM and VTE should be monitored by an anticoagulation service if they receive warfarin or are seen by a vascular medicine specialist, as indicated. Blood monitoring is necessary for patients on warfarin for accurate dosing. The schedule of blood work is determined by the specialist(s) managing the warfarin dosing.
MM is an inherently hypercoagulable state that is complicated by modifiable and nonmodifiable risk factors. The science of VTE has progressed, with newer agents and methods to stratify risk. The etiology of VTE, like many conditions, is multifactorial. Risk factors for development of VTE, thrombosis assessment, and diagnostic criteria should be carefully evaluated when a patient requires treatment for VTE.
Evidence-Based Recommendations
Level of Evidence I
• The International Myeloma Foundation Nurse Leadership Board (NLB) used Melnyk and Fineout-Overholt’s (2011) levels of evidence as a systematic framework for the appraisal and grading of the NLB’s consensus statements and evidence-based recommendations.
• Nurses should teach patients with MM about their increased risk for DVT, measures they can take to prevent DVT (ambulation to prevent venous stasis and avoidance of dehydration), the signs and symptoms of DVT (such as edema and pain in the extremity), and the need to report any DVT symptoms immediately (Lyman et al., 2015; Rome, Doss, Miller, & Westphal, 2008).
• Nurses and clinicians should use risk stratification to determine patients’ risks of developing VTE. Factors that increase VTE risk include a history of prior VTE, surgery, anthracycline chemotherapy, high-dose corticosteroids, hospitalization, and heart disease. All high-risk patients who take IMiDs should also take aspirin, LMWH, warfarin, or fondaparinux prophylaxis. Preventive treatment should be initiated only after considering each patient’s risk of bleeding versus thrombosis (Lyman et al., 2015; Rome et al., 2008).
• For all patients with a suspected VTE, a subjective history of illness, targeted physical examination, laboratory testing, and radiologic imaging are indicated. If a DVT is suspected, individuals should be screened with a Doppler ultrasound of the extremity. If a PE is suspected, a ventilation perfusion lung scan is safer than contrast computed tomography angiography scan in patients with MM. In patients with evolving DVT or suspected PE, anticoagulation is recommended with preference for LMWH, if creatinine clearance greater than 30 ml per minute, or with warfarin, which carries a higher bleeding risk (Lyman et al., 2015; Palumbo et al., 2008; Rome et al., 2008).
• All patients should be taught to rapidly identify the signs and symptoms of bleeding. Clinicians should advise patients to stop taking aspirin or anticoagulation when their platelets are below a clinically significant level and prior to surgery. Patients with platelet counts less than 100 x 109/L should be monitored closely, and if the count falls below 50 x 109/L, thromboprophylaxis should be held until the platelet count recovers to above 50 x 109/L, except in very high-risk cases or during acute VTE episodes. The need for anticoagulation in patients with thrombocytopenia should be considered on a case-by-case basis (Carrier, Khorana, Zwicker, Noble, & Lee, 2013).
Level of Evidence II
• DOACs provide an oral option for anticoagulation delivery, but currently little evidence supports their use in VTE prevention and treatment in patients with MM (Lyman et al., 2015; Man, Morris, Brown, Palkimas, & Davidson, 2016).
Cardiotoxicity
CV disease and cancer are the two leading causes of death in the United States, creating a clinically challenging scenario when these two diseases intersect. Cardiovascular toxicity, defined by the National Cancer Institute as direct damage to heart tissue, is a potential short- or long-term complication from anticancer therapy (Shelburne et al., 2014). Figure 6 presents cardiovascular risk factors in patients with MM.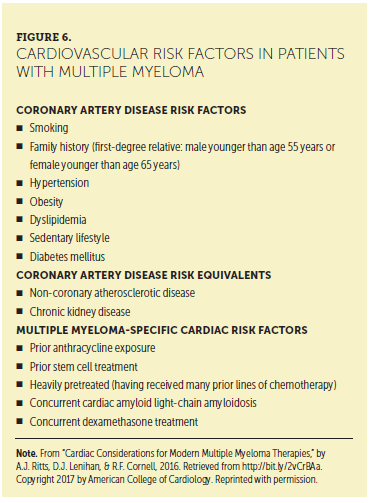
The evolution of new therapies for the treatment of MM has increased the overall survival of patients. However, longer survival exposes patients to adverse side effects of cytotoxic agents and may lead to lethal side effects. Exposure to chemotherapy medications can increase the risk of irreversible, clinically significant cardiac dysfunction. For this reason, many patients with MM now face an increase in treatment-related comorbidities, including cardiac toxicities. Of note, the gain in life expectancy because of anticancer therapy might be countered by an increase in mortality from cardiac problems, such as heart failure, myocardial ischemia, arrhythmias, hypertension, and thromboembolism (Shelburne et al., 2014).
The incidence of cardiotoxicity depends on various features of oncologic therapies. The type of drug, dose administered during each chemotherapy cycle, cumulative dose, schedule of administration, route of administration, combination with other cardiotoxic drugs or association with radiation therapy, and patient health history (age, presence of CV risk factors, previous CV disease, and prior mediastinal radiation therapy) all play an integral role in causing potential cardiotoxicity (Hahn, Lenihan, & Ky, 2014; Yeh & Bickford, 2009).
Anticancer drugs influence or disrupt pathways that are centrally involved in cell survival, cell growth, inflammatory activation, and angiogenesis (Minami, Matsumoto, & Horiuchi, 2010). Cardiac toxicity may be a dose-limiting factor throughout a patient’s clinical course and treatment. Healthcare providers (RNs, advanced practitioners, physician assistants) need to understand the possible risks for cardiac toxicity and the signs and symptoms of cardiac events (see Figure 7).
Definition of Chemotherapy-Induced Cardiotoxicity
Chemotherapy may induce cardiotoxicity through several mechanisms, including direct injury to myocardial cells and indirect injury to cardiac tissue through effects on the coagulation system, changes in cardiac rhythm, induction of hypertension, and myocardial and/or pericardial inflammation (Florescu, Cinteza, & Vinereanu, 2013). Cardiotoxicity is a general term used for toxicity that affects the heart but is without a clear definition. Although the National Comprehensive Cancer Network (2017) defines cardiac toxicity as damage to the heart by harmful chemicals, Florescu et al. (2013) defines chemotherapy-induced cardiotoxicity according to the presence of reduced left-ventricular ejection faction (LVEF) and/or signs or symptoms of heart failure. Specifically, cardiotoxicity is defined by Florescu et al. (2013) in the presence of at least one of the following: (a) global or specific interventricular reduction in LVEF; (b) symptoms or signs of heart failure; or (c) LVEF reduction to 5%–55% of baseline with heart failure signs or symptoms, or LVEF reduction to 10%–55% of baseline without heart failure signs or symptoms.
Chemotherapy and Immunotherapy Agents and Toxicities
MM treatments include chemotherapeutic and immunotherapy agents that have the potential to cause cardiac toxicities. Table 3 presents immunotherapy- and chemotherapy-associated cardiac toxicities and nursing considerations for patients with cardiac abnormalities. The following sections highlight classifications of these agents and treatments that may prompt cardiac toxicities.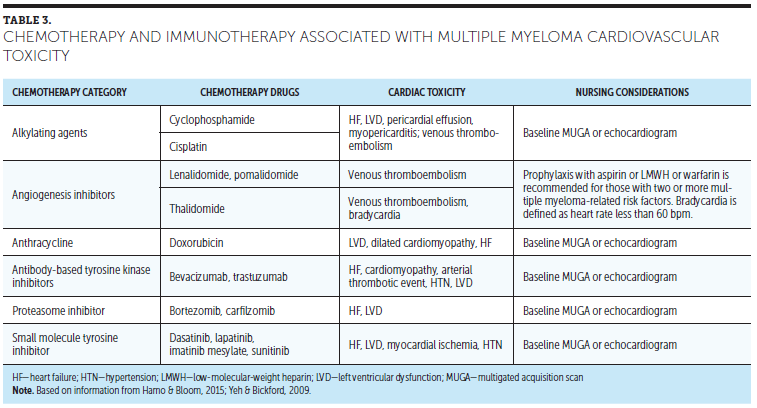
Bortezomib
Bortezomib is a proteasome inhibitor approved for the treatment of MM, and carfilzomib is a proteasome inhibitor used in the treatment of refractory or relapsing MM. Proteasome inhibitors cause cell death by interfering with the breakdown of cell cycle proteins and coronary vasospasm by impairing endothelial nitric oxide synthase activity. In several case reports, clinical heart failure has been reported in 2%–5% of proteasome inhibitor recipients (Hamo & Bloom, 2015).
Alkylating Agents
Cyclophosphamide is an alkylating agent that kills rapidly dividing cells. Depending on the dose of cyclophosphamide, it may be associated with the development of acute myopericarditis and left ventricular dysfunction in 7%–22% of patients (Hamo & Bloom, 2015; Yeh & Bickford, 2009).
Anthracyclines
Anthracyclines, such as doxorubicin, act as antitumor agents by interfering with protein synthesis, producing reactive oxygen, and inhibiting DNA repair. Doxorubicin causes myocardial damage by interacting with topoisomerase II, an enzyme that is present on cardiac myocytes. Anthracyclines cause left ventricular dysfunction, which occurs with lifetime exposure to more than 450 mg/m2 (Hamo & Bloom, 2015; Yeh & Bickford, 2009).
Radiation Therapy
In radiation therapy, high-energy particles interrupt cell growth and kill cells, including cancer cells and cardiac myocytes. When radiation is directed at the mediastinum, it can cause pericarditis, atherosclerosis, valve dysfunction, heart failure, and fatal cardiovascular events. The risk of myocardial damage is increased with high radiation doses, long exposure times, and the use of cytotoxic therapy (Hamo & Bloom, 2015).
Immunomodulator Drugs
The use of thalidomide, lenalidomide, and pomalidomide in patients with cancer is commonly associated with the development of thromboembolic complications, including thrombotic coronary artery disease (Kristinsson et al., 2012; Palumbo & Palladino, 2012).
Clinical Follow Up and Monitoring of Patients
Healthcare providers should monitor patients for side effects of any treatment and keep accurate records of cumulative treatments given over time. All patients undergoing chemotherapy should have prior evaluation and assessment of CV risk factors or comorbidities. CV side effects of chemotherapy may vary from mild, transient, asymptomatic reduction in LVEF to cardiac death.
Chemotherapy-induced cardiotoxicity can be monitored using plasma concentrations of troponin I and N-terminal pro-B-type natriuretic peptide, which are released from overloaded myocardial tissue (Minami et al., 2010). Echocardiography is commonly used before cancer therapy is started to determine baseline cardiac function and may be used to detect cardiotoxicity. Patients undergoing cancer therapy should be routinely evaluated for echocardiography abnormalities that could indicate cardiotoxity (Bovelli, Plataniotis, & Roila, 2010).
General Recommendations for Cardiovascular Health
The American Heart Association has set forth goals for the CV health of people in the United States. These health goals should apply to patients with MM to minimize morbidities, particularly as patients live longer. In general, smoking, physical activity, blood pressure, cholesterol, glucose, and body mass index are all modifiable risk factors (Lloyd-Jones et al., 2010; Ritts et al., 2016).
Evidence-Based Recommendations
Level of Evidence I
• Because patients with MM are at risk of developing CV disease, they should be evaluated by a cardiologist early in the course of their disease if they have a history of CV disease or develop cardiac events during therapy (Ritts et al., 2016).
• All healthcare providers should assess symptoms of chemotherapy- and radiation-induced vascular and cardiac changes. Dysrhythmias, angina, dyspnea at rest and with exertion, acute tachypnea, lower extremity pain, hypotension, diaphoresis, venous jugular distention, peripheral edema, and echocardiography changes should be evaluated promptly (Loerzel & Dow, 2003).
• Patients should be educated about healthy behaviors, such as smoking cessation, weight loss, and diet. Modifiable risk factors for CV disease should be addressed (Lloyd-Jones et al., 2010).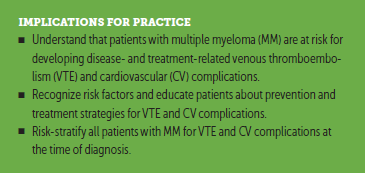
Conclusion
Traditional and novel anticancer agents have the potential to induce VTE and CV toxicity. The dose and duration of certain types of chemotherapy may have to be limited if cardiac toxicity develops during treatment (Loerzel & Dow, 2003). Thorough patient assessments must be performed at intervals throughout the disease course and should focus on prior cardiac history, potential cardiotoxicity, and thromboembolic risk.
The authors gratefully acknowledge Rafat Abonour, MD, Brian G.M. Durie, MD, and Diane P. Moran, RN, MA, EdM, at the International Myeloma Foundation for their review of this manuscript.
About the Author(s)
Kimberly Noonan, RN, MS, CNP, AOCN®, is a nurse practitioner at the Dana-Faber Cancer Institute in Boston, MA; Sandra Rome, RN, MN, AOCN®, CNS, is a hematology- oncology clinical nurse specialist at the Cedars-Sinai Medical Center in Los Angeles, CA; Beth Faiman, PhD, MSN, APRN-BC, AOCN®, is a nurse practitioner in the Department of Hematology and Medical Oncology at the Cleveland Clinic Taussig Cancer Institute in Ohio; and Daniel Verina, MSN, RN, ACNP, is a nurse practitioner at the Mount Sinai Hospital Medical Center in New York, NY. The authors take full responsibility for this content. This supplement was supported by the International Myeloma Foundation, with funding from Celgene Corporation, Karyopharm Therapeutics, and Takeda Oncology. Writing and editorial support was provided by Eubio Medical Communications. Faiman consults and serves on speakers bureaus for Amgen, Bristol- Myers Squibb, Celgene Corporation, and Takeda Oncology, and has received support from Celgene Corporation and Takeda Oncology. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Noonan can be reached at kimberly_noonan@dfci.harvard.edu, with copy to CJONEditor@ons.org. (Submitted June 2017. Accepted July 27, 2017.)
References
Anaissie, E.J., Coleman, E.A., Goodwin, J.A., Kennedy, R.L., Lockhart, K.D., Stewart, C.B., . . . Barlogie, B. (2012). Prophylactic recombinant erythropoietin therapy and thalidomide are predictors of venous thromboembolism in patients with multiple myeloma: Limited effectiveness of thromboprophylaxis. Cancer, 118, 549–557. https://doi.org/10.1002/cncr.26302
Awad, N.I., & Cocchio, C. (2013). Activated prothrombin complex concentrates for the reversal of anticoagulant-associated coagulopathy. Pharmacy and Therapeutics, 38, 696–701.
Babilonia, K., & Trujillo, T. (2014). The role of prothrombin complex concentrates in reversal of target specific anticoagulants. Thrombosis Journal, 12, 8.
Bates, S.M., Jaeschke, R., Stevens, S.M., Goodacre, S., Wells, P.S., Stevenson, M.D., . . . Guyatt, G.H. (2012). Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis. Chest, 141(Suppl. 2), e351S–e418S. https://doi.org/10.1378/chest.11-2299
Bauersachs, R., Berkowitz, S.D., Brenner, B., Buller, H.R., Decousus, H., Gallus, A.S., . . . Schellong, S. (2010). Oral rivaroxaban for symptomatic venous thromboembolism. New England Journal of Medicine, 363, 2499–2510. https://doi.org/10.1056/NEJMoa1007903
Baz, R., Li, L., Kottke-Marchant, K., Srkalovic, G., McGowan, B., Yiannaki, E., . . . Hussein, M.A. (2005). The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clinic Proceedings, 80, 1568–1574. https://doi.org/10.4065/80.12.1568
Bovelli, D., Plataniotis, G., & Roila, F. (2010). Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Annals of Oncology, 21(Suppl. 5), v277–v282. https://doi.org/10.1093/annonc/mdq200
Brown, J.D., Adams, V.R., & Moga, D.C. (2016). Impact of time-varying treatment exposures on the risk of venous thromboembolism in multiple myeloma. Healthcare, 4(4), E93.
Carrier, M., Khorana, A.A., Zwicker, J., Noble, S., & Lee, A.Y. (2013). Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: Guidance from the SSC of the ISTH. Journal of Thrombosis and Haemostasis, 11, 1760–1765. https://doi.org/10.1111/jth.12338
ClinicalTrials.gov. (2014). Evaluation of the use of apixaban in prevention of thromboembolic disease in patients with myeloma treated with iMiDs (MYELAXAT) [NCT02066454]. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02066454
Dede, R.J., & Pruemer, J.M. (2016). Comparing venous thromboembolism prophylactic strategies for ambulatory multiple myeloma patients on immunomodulatory drug therapy. Journal of Oncology Pharmacy Practice, 22, 248–255.
Einstein Investigators. (2010). Oral rivaroxaban for symptomatic venous thromboembolism. New England Journal of Medicine, 363, 2499–2510. doi:10.1056/NEJMoa1007903
Elias, S., Hoffman, R., Saharov, G., Brenner, B., & Nadir, Y. (2016). Dehydration as a possible cause of monthly variation in the incidence of venous thromboembolism. Clinical and Applied Thrombosis/Hemostasis, 22, 569–574.
Elyamany, G., Alzahrani, A.M., & Bukhary, E. (2014). Cancer-associated thrombosis: An overview. Clinical Medicine Insights Oncology, 8,129–137. https://doi.org/10.4137/CMO.S18991
Fahrni, J., Husmann, M., Gretener, S.B., & Keo, H.H. (2015). Assessing the risk of recurrent venous thromboembolism. Vascular Health and Risk Management, 11, 451–459.
Florescu, M., Cinteza, M., & Vinereanu, D. (2013). Chemotherapy-induced cardiotoxicity. Maedica, 8, 59–67.
Gaitini, D. (2007). Multimodality imaging of the peripheral venous system. International Journal of Biomedical Imaging, 2007, 54616. https://doi.org/10.1155/2007/54616
Gregersen, H., Nørgaard, M., Severinsen, M.T., Engebjerg, M.C., Jensen, P., & Sørensen, H.T. (2011). Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. European Journal of Haematology, 82, 129–134.
Hahn, V.S., Lenihan, D.J., & Ky, B. (2014). Cancer therapy-induced cardiotoxicity. Journal of the American Heart Association, 3(2), e000665. https://doi.org/10.1161/JAHA.113.000665
Hamo, C.E., & Bloom, M.W. (2015). Getting to the heart of the matter: An overview of cardiac toxicity related to cancer therapy. Clinical Medicine Insights Cardiology, 9(Suppl. 2), 47–51.
Heit, J.A., Spencer, F.A., & White, R.H. (2016). The epidemiology of venous thromboembolism. Journal of Thrombosis and Thrombolysis, 41, 3–14. https://doi.org/10.1007/s11239-015-1311-6
Kearon, C., Akl, E.A., Comerota, A.J., Prandoni, P., Bounameaux, H., Goldhaber, S.Z., . . . Kahn, S.R. (2012). Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed. Chest, 141(Suppl. 2), e419S–e494S.
Kesieme, E., Kesieme, C., Jebbin, N., Irekpita, E., & Dongo, A. (2011). Deep vein thrombosis: A clinical review. Journal of Blood Medicine, 2, 59–69. https://doi.org/10.2147/JBM.S19009
Kristinsson, S.Y. (2010). Thrombosis in multiple myeloma. Hematology, 2010, 437–444.
Kristinsson, S.Y., Bjorkholm, M., Schulman, S., & Landgren, O. (2011). Hypercoagulability in multiple myeloma and its precursor state, monoclonal gammopathy of undetermined significance. Seminars in Hematology, 48, 46–54.
Kristinsson, S.Y., Pfeiffer, R.M., Bjorkholm, M., Schulman, S., & Landgren, O. (2012). Thrombosis is associated with inferior survival in multiple myeloma. Haematologica, 97, 1603–1607.
Lloyd-Jones, D.M., Hong, Y., Labarthe, D., Mozaffarian, D., Appel, L.J., Van Horn, L., . . . Rosamond, W.D. (2010). Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation, 121, 586–613.
Loerzel, V.W., & Dow, K.H. (2003). Cardiac toxicity related to cancer treatment. Clinical Journal of Oncology Nursing, 7, 557–562. https://doi.org/10.1188/03.CJON.557-562
Lyman, G.H., Bohlke, K., Khorana, A.A., Kuderer, N.M., Lee, A.Y., Arcelus, J.I., . . . Falanga, A. (2015). Venous thromboembolism prophylaxis and treatment in patients with cancer. Journal of Clinical Oncology, 33, 654–656. https://doi.org/10.1200/jco.2014.59.7351
Man, L., Morris, A.L., Brown, J., Palkimas, S., & Davidson, K.M. (2016). Use of direct oral anticoagulants in patients on immunomodulatory agents. Blood, 128, 1449.
Melnyk, B.M., & Fineout-Overholt, E. (2011). Evidence-based practice in nursing and healthcare: A guide to best practice. Philadelphia, PA: Lippincott Williams and Wilkins.
Minami, M., Matsumoto, S., & Horiuchi, H. (2010). Cardiovascular side-effects of modern cancer therapy. Circulation Journal, 74, 1779–1786.
Moheimani, F., & Jackson, D.E. (2011). Venous thromboembolism: Classification, risk factors, diagnosis, and management. ISRN Hematology, 2011, 124610.
National Comprehensive Cancer Network. (2017). Cardiac toxicity. Retrieved from https://www.nccn.org/patients/resources/life_with_cancer/managing_sympt…
National Heart Lung and Blood Institute. (2012). Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. Retrieved from https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook.pdf
Palumbo, A., & Palladino, C. (2012). Venous and arterial thrombotic risks with thalidomide: Evidence and practical guidance. Therapeutic Advances in Drug Safety, 3(5), 255–266.
Palumbo, A., Rajkumar, S.V., Dimopoulos, M.A., Richardson, P.G., San Miguel, J., Barlogie, B., . . . Hussein, M.A. (2008). Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia, 22, 414–423. https://doi.org/10.1038/sj.leu.2405062
Piran, S., & Schulman, S. (2016). Management of venous thromboembolism: An update. Thrombosis Journal, 14(Suppl.), 23. https://doi.org/10.1186/s12959-016-0107-z
Pulivarthi, S., & Gurram, M.K. (2014). Effectiveness of d-dimer as a screening test for venous thromboembolism: An update. North American Journal of Medical Sciences, 6, 491–499.
Rajkumar, S.V., Jacobus, S., Callander, N.S., Fonseca, R., Vesole, D.H., Williams, M.E., . . . Greipp, P.R. (2010). Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncology, 11, 29–37.
Ritts, A.J., Lenihan, D.J., & Cornell, R.F. (2016). Cardiac considerations for modern myeloma therapies. Journal of American College of Cardiology. Retrieved from http://bit.ly/2f8Y8n5
Rome, S., Doss, D., Miller, K., & Westphal, J. (2008). Thromboembolic events associated with novel therapies in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(Suppl. 3), S21–S28.
Shelburne, N., Adhikari, B., Brell, J., Davis, M., Desvigne-Nickens, P., Freedman, A., . . . Remick, S.C. (2014). Cancer treatment-related cardiotoxicity: Current state of knowledge and future research priorities. Journal of the National Cancer Institute, 106(9), 1–9.
Story, K.T. (2014). Hypercoagulable state. In D. Camp-Sorrell, & R.A Hawkins (Eds.), Clinical manual for the oncology advanced practice nurse (3rd ed., pp. 1045–1052). Pittsburgh, PA: Oncology Nursing Society.
Streiff, M.B., Agnelli, G., Connors, J.M., Crowther, M., Eichinger, S., Lopes, R., . . . Ansell, J. (2016). Guidance for the treatment of deep vein thrombosis and pulmonary embolus. Journal of Thrombosis and Thrombolysis, 41, 32–67 https://doi.org/10.1007/s11239-015-1317-0
Thompson, B.T., & Kabrhel, C. (2017). Clinical presentation, evaluation, and diagnosis of the nonpregnant adult with suspected acute pulmonary embolism. UpToDate. Retrieved from http://bit.ly/2xTWopk
Weber, D.M., Chen, C., Niesvizsky, R., Wang, M., Belch, A., Stadtmauer, E.A., . . . Knight, R.D. (2007). Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. New England Journal of Medicine, 357, 2133–2142.
Wells, P.S., Anderson, D.R., Bormanis, J., Guy, F., Mitchell, M., Gray, L., . . . Lewandowski, B. (1997). Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet, 350, 1795–1798. https://doi.org/10.1016/S0140-6736(97)08140-3
Yeh, E.T., & Bickford, C.L. (2009). Cardiovascular complications of cancer therapy. Journal of the American College of Cardiology, 53, 2231–2247.




