Adult Survivorship: Considerations Following CAR T-Cell Therapy
Background: Significant improvement in overall survival observed in patients on clinical trials and U.S. Food and Drug Administration approval of two chimeric antigen receptor (CAR) T-cell therapies have resulted in an increasing population of survivors who have undergone this therapy. Although adult survivors may experience similar physiologic and psychosocial sequelae to traditional cancer therapies, unique late effects and considerations are related to CAR T-cell therapy.
Objectives: This article reviews survivorship considerations, with particular attention paid to the physical, psychosocial, and financial effects for adults who have undergone CAR T-cell therapy.
Methods: A review of the physiologic and psychosocial sequelae resulting from CAR T-cell therapy is presented, with a focus on late effects and financial toxicities of treatment. Physiologic concerns include B-cell aplasia and resulting hypogammaglobulinemia, as well as prolonged cytopenias and associated risk for infection.
Findings: To date, adult CAR T-cell therapy survivorship data are limited. However, data from clinical trials suggest expected late effects from treatment. As this survivor population grows, research can identify physiologic and psychosocial needs unique to adult survivors and evaluate evidence-based interventions.
Jump to a section
A growing population of cancer survivors have undergone chimeric antigen receptor (CAR) T-cell therapy. For individuals with CD19-positive B-cell hematologic malignancies, disease-specific findings include best overall response rates (ORRs) of 52%–83% and complete response rates of 40%–59% in adults with relapsed/refractory non-Hodgkin lymphoma, with durable response rates of 30%–40% seen with a median follow-up time of 6–27 months (Chavez & Locke, 2018; Schuster et al., 2019). These outcomes are notable considering the data from the international SCHOLAR-1 study of more than 600 patients with refractory diffuse large B-cell lymphoma who were treated with traditional chemotherapy showed an ORR of 26% and a complete response rate of 7%, with an overall median survival of only 6.3 months (Neelapu et al., 2017). Young adult and pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL) have also had favorable responses to this therapy. A clinical trial by Grupp et al. (2018) demonstrated an ORR of 82%, and 62% of patients had a complete response; 66% of patients who had a complete response remained in remission at 18 months. Adults with ALL with low disease burden also had promising results, showing long-term remissions with a median overall survival of 20.1 months (Park, Rivière, et al., 2018). Based on these results, expectations about the effectiveness of this therapy and its potential for application in other disease states, including solid tumors, are high (Albelda, 2019).
Studies examining survivorship concerns and patient-reported outcomes in this population are limited and have been forthcoming as individuals are monitored longer than two years post–CAR T-cell therapy. Recommendations have begun to emerge about the suggested frequency with which to collect patient-reported outcomes, including assessments of physiologic symptoms, cognitive functioning, and financial toxicity (Chakraborty et al., 2018). The majority of recommendations for survivorship management are derived from clinical experience, particularly in centers with longstanding clinical trial populations. This article presents an overview of survivorship considerations for adult patients undergoing CAR T-cell therapy, highlighting long-term and late effects and psychosocial implications, including financial toxicity. An overview of considerations for pediatric survivors is presented in this supplement by Callahan et al. (2019).
Physiologic Considerations
Late Effects
Although the acute toxicities of CAR T-cell therapy, cytokine released syndrome (CRS) and neurologic toxicity are well defined, late effect and chronic toxicity data are newly emerging as patients’ overall survival increases. Long-term and late effects of this therapy include cytopenias and infection, as well as B-cell aplasia and hypogammaglobulinemia (Cordeiro et al., 2018). Other late effects that may occur include secondary malignancies, potential neurologic toxicities, fatigue, and infertility (Cordeiro et al., 2018). To ensure long-term safety after CAR T-cell therapy, patients should continue to be monitored because they may require growth factors, transfusions, immunoglobulin infusions, and antimicrobial prophylaxis.
Cytopenias and Infection
The lymphodepleting chemotherapies most commonly used prior to CAR T-cell infusion, cyclophosphamide and fludarabine, are known to cause cytopenias that can persist for months after treatment (Strati et al., 2013). It is not unusual for patients who have received CAR T-cell therapy to develop grade 3 or 4 cytopenias, including anemia, thrombocytopenia, neutropenia, leukopenia, and lymphopenia. Patients who did not receive conditioning chemotherapy prior to CAR T-cell infusion have also developed cytopenias, indicating that the CAR T cells may cause myelosuppression, possibly because of an immune-mediated mechanism (Brudno & Kochenderfer, 2016). In a report of 59 patients monitored from 13 to 57 months post–CAR T-cell therapy, cytopenias were observed at 90 days postinfusion in 8% of patients (n = 5), requiring growth–colony-stimulating factor or transfusion support (Cordeiro et al., 2018).
Cytopenias increase the risk for infection. A review of 133 patients with ALL, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma showed that 23% of patients developed infections within 28 days, and 14% developed infections within 29–90 days after receiving the CAR T-cell infusion (Hill et al., 2018). In that study, bacterial infections were most common within the first 28 days, whereas viral infections were most common 29 days or later after CAR T-cell infusion. By day 90, only 21% of the patients had recovered their B cells. Patients found to be at higher risk of infection included those who had received four or greater lines of prior therapy or higher CAR T-cell therapy doses or experienced more severe CRS (Hill et al., 2018). In a study of 22 patients with ALL who received anti-CD19 CAR T-cell therapy, 42% of patients developed infections (17 bacterial, 5 viral, and 4 fungal) within the first 30 days after CAR T-cell infusion, and 31% of patients experienced one or more infections from day 31 to 180 (Park, Romero, et al., 2018). The median time frame for the occurrence of infection was on day 18 for bacterial infections, day 23 for fungal infections, and day 48 for viral infections. Although there was an identified association between CRS (grade 3 or greater) and infection, the infection was not found to be a result of tocilizumab or steroid administration (Park, Romero, et al., 2018).
The CD4 lymphocytes, which function as helper T cells, play an important role in the immune system. A low CD4 count (less than 200 cells/mcl) can increase the risk of common and opportunistic viral, fungal, parasitic, and bacterial infections. Decreased CD4 counts have been associated with late-onset (median onset of 6 months, with a range of 2.4–24 months) Pneumocystis jiroveci pneumonia following fludarabine, cyclophosphamide, and rituximab conditioning for CAR T-cell therapy in 18.4% of patients in a clinical trial by Haeusler et al. (2013).
No current guidelines exist regarding revaccination following CAR T-cell therapy. However, patients have often undergone previous stem cell transplantation and have not yet received their post-transplantation vaccinations. The National Comprehensive Cancer Network (2016) recommends revaccination 6–12 months after stem cell transplantation for inactivated vaccines and 24 months or longer for live vaccines (as long as the patient is no longer immunosuppressed). Vaccine-related immunity is attributed to B cells, which are responsible for forming antibodies (plasma cells). In patients with B-cell aplasia, post–CAR T-cell therapy antibody formation may not occur; therefore, it is advisable that vaccination should occur after B-cell recovery.
Ljungman and Avetisyan (2008) noted that, in patients who had undergone hematopoietic stem cell transplantation and were given the influenza vaccine, T-cell responses could be elicited and provided some protection from the illness. Some centers are recommending flu vaccination 30 days or more post–CAR T-cell infusion in light of the minimal risk of the vaccine and the potential benefit of a T-cell response, particularly in patients who are often neutropenic (Chong et al., 2018). The importance of vaccination should be emphasized to family and caregivers—who will be in frequent, close proximity to the patient—to reduce risk of exposure.
Blood counts should be monitored weekly for the first 60 days after anti-CD19 CAR T-cell therapy and thereafter as indicated until blood counts recover. Patients may require blood and platelet transfusions and growth factor support. However, the prescribing information for tisagenlecleucel (Kymriah®) advises avoiding granulocyte macrophage–colony-stimulating factor during the first three weeks after infusion or until CRS has resolved because of concern for potential worsening of CRS (Novartis Pharmaceuticals, 2018b). To date, no standardized antimicrobial prophylaxis recommendations have been established for the post–CAR T-cell population. However, treatment guidelines for patients with cancer-related immunosuppression have been used to guide bacterial, viral, fungal, and Pneumocystis jiroveci pneumonia prophylaxis (Taplitz et al., 2018; University of Texas MD Anderson Cancer Center, 2017).
B-Cell Aplasia and Hypogammaglobulinemia
CAR T cells are designed to target malignant B cells, but in the process, they can also destroy healthy B-cells (National Cancer Institute [NCI], 2017a). This recognition of target antigens in normal tissue is described as an on-target/off-tumor effect, causing lineage depletion or B-cell aplasia and, therefore, chronic immunodeficiency (Bonifant, Jackson, Brentjens, & Curran, 2016; Kochenderfer & Rosenberg, 2013; Neelapu et al., 2017). In some instances, B-cell aplasia results in hypogammaglobulinemia because activated B cells produce antibody-secreting plasma cells (Janeway, Travers, Walport, & Shlomchik, 2001).
Hypogammoglobulinemia, often requiring immunoglobulin G (IgG) replacement, occurred in 41% of patients 90 days after CAR T-cell infusion in one study cohort (Cordeiro et al., 2018). B cells and immunoglobulins play a role in humoral immune response. Humoral immunity is part of the adaptive immune system that is antibody-mediated (as opposed to cell-mediated) and involves B cells that recognize antigens or pathogens circulating in the lymph fluid or blood. Patients with defects in humoral immunity are particularly susceptible to recurrent upper and lower respiratory infections (Orange, 2017). B-cell aplasia and the resulting hypogammaglobulinemia can last months to years after treatment (Porter, Levine, Kalos, Bagg, & June, 2011), resulting in the potential need for long-term IV or subcutaneous IgG replacement. However, studies of CD19-negative plasma cells show that they can contribute to long-lasting humoral immunity. These CD19-negative plasma cells do not appear to require replenishment by CD19-positive precursors, making it likely that patients will continue to have some humoral response (Bhoj et al., 2016).
Patients who develop frequent infections after CAR T-cell therapy may require monthly infusions of IgG, particularly if the IgG level is less than 400 mg/dl (Brudno & Kochenderfer, 2016). Although the frequency and duration of IgG replacement is not yet known, a study by Cordeiro et al. (2018) indicated that 41% of patients received replacement beyond 90 days post–CAR T-cell therapy.
Other Long-Term Physiologic Sequelae
Secondary malignancies: Because CAR T cells are a genetically altered product, there is an unlikely possibility of insertional mutagenesis resulting in secondary malignancies (Maus & Levine, 2016). For this reason, the FDA mandated in 2018 that healthcare providers need to follow patients who have received this therapy for 15 years.
Cordeiro et al. (2018) reported that eight patients (14%) on study experienced a subsequent malignancy, including myelodysplasia, noninvasive bladder cancer, and nonmelanoma skin cancer. However, the authors did not specify whether these were believed to be secondary to treatment or new primary cancers. All but one of these patients had undergone a previous autologous or allogeneic stem cell transplantation prior to receiving CAR T-cell therapy.
Fertility considerations: To date, no studies have evaluated the effects of CAR T-cell therapy on fertility or child-bearing outcomes. However, lymphodepleting conditioning chemotherapy may affect the reproductive capacity in individuals of childbearing potential (Johnson & June, 2017). Therefore, it is recommended that adults of childbearing age be given the opportunity to consult a fertility preservation specialist prior to conditioning chemotherapy. Fertility counseling is best scheduled prior to the start of first-line therapy, with follow-up throughout the treatment trajectory.
Fatigue: Fatigue occurred in 51% of patients on the ZUMA-1 trial and can be one of the most frequent and difficult-to-manage symptoms (Neelapu et al., 2017). Fortunately, patients reported that fatigue resolved within four to six weeks after the CAR T cells were given. Nonpharmacologic interventions to manage fatigue may include exercise, yoga, meditation, Pilates, and massage therapy (Mullen & Mistry, 2018). Steroids, which have been shown to reduce fatigue in patients, may be contraindicated because of concern for T-cell suppression (Neelapu et al., 2018).
Neurologic: Neurologic toxicities, such as seizures, weakness, confusion, aphasia, and coordination problems, may occur following treatment for weeks postinfusion (NCI, 2017b). Cordeiro et al. (2018) reported that 5% of patients in their study experienced neuropsychiatric disorders, including depression, suicide attempts, myoclonic seizures, and transient ischemic attacks. Seizure prophylaxis, including levetiracetam, may be prescribed to prevent seizure-like activity. Because of these risks, patients should not drive for at least eight weeks postinfusion (Novartis Pharmaceuticals, 2018b). In addition, the patient’s caregiver can check in regularly, monitoring for any neurologic changes, so they can be reported immediately to the healthcare team.
Psychosocial Sequelae
CAR T-cell therapy extends treatment to patients who may have previously exhausted other treatment options. For this reason, CAR T-cell therapy has been reported as a lifeline by patients who have undergone this treatment (Association of Community Cancer Centers, 2018; Symes & Schorr, 2018). To date, CAR T-cell therapy is indicated for second- and third-line therapy (Zheng, Kros, & Li, 2018), so recipients may experience an elevated fear of recurrence (Crist & Grunfeld, 2013). Similarly, changes in physical functioning can lead to altered quality of life (Bloom, Stewart, Chang, & Banks, 2004). Family-based interventions can support patients and their caregivers when family stressors and compromised coping affect the patient’s quality of life and concerns about recurrence (Mellon, Kershaw, Northouse, & Freeman-Gibb, 2007). Additional consideration of age-sensitive education for children of adult patients can address the patient and their children’s coping needs and may support positive psychosocial outcomes.
Psychosocial adaptive coping is often an indicator of how the patient will respond once treatment is completed (Black & White, 2005). According to the American Society of Clinical Oncology (2017), survivorship programs provide the following:
• Avenues for continued communication between patient and treating providers/nurses
• Supportive interventions to empower patients’ ability to recognize and manage anxiety and enhance positive coping
• Opportunities for patients to engage with other survivors, such as in the context of support groups
Engagement with social workers, chaplains, clinical psychologists/psycho-oncologists, and community-based organizations can support patients as they navigate survivorship. Patients may also seek connection through online forums, including social media and online support groups that are specific to recipients of CAR T-cell therapy (American Society of Clinical Oncology, 2017).
Financial Toxicity
Financial toxicity is defined as a negative impact on the patient’s out-of-pocket costs associated with the treatment plan (Zafar et al., 2013). In 2018, the costs of treatment with tisagenlecleucel and axicabtagene ciloleucel (Yescarta®) were $475,000 and $373,000, respectively (Cavallo, 2018; Hernandez, Prasad, & Gellad, 2018). This cost does not include hospital charges for care and services provided. Because of this, private insurance companies review patient cases for CAR T-cell therapy approval on a case-by-case basis. The Centers for Medicare and Medicaid Services reimburse from $36,000 to $186,500 for CAR T-cell products administered in the inpatient setting and $395,380 (axicabtagene ciloleucel) and $500,839 (tisagenlecleucel) administered in the ambulatory setting (Gallegos, 2018; Wong, 2018). In addition, the Centers for Medicare and Medicaid Services has employed outcomes-based pricing, in which reimbursement for tisagenlecleucel is based on a patient’s response in the first month of treatment (Tran & Zafar, 2018). Patient costs with Medicare would be limited to about $1,340 out of pocket if the patient’s Medicare Part B deductible has not been met (Wong, 2018). However, because of institutional preferences around continuous toxicity monitoring and limited ambulatory programs, the majority of patients receive treatment in the inpatient setting (Smith, 2018).
Studies of patients with cancer receiving diverse treatments suggest that about 50% of individuals report significant or catastrophic financial burden (Chino et al., 2014). Another study identified that 16% of participants report acute distress associated with financial stress from the course of their treatment that affected one-third of their income (Chino et al., 2017). These studies suggest that financial toxicity not only is an economic concern, but also can contribute to psychological sequelae that may further compound the anxieties and stressors associated with treatment. Some of the costs associated with care are not specifically related to the treatment. These ancillary expenses can be the costs of transportation, accommodations if relocating for treatment, and daily living expenses, such as food and child care (NCI, 2018).
Programs exist that provide financial assistance for patients undergoing cancer treatment (see Figure 1). Because CAR T-cell therapy is limited to about 160 programs in the United States as of February 2019, many patients incur travel and housing costs to receive treatment (Kite Pharma, 2018; Novartis Pharmaceuticals, 2018a). The FDA (2018) requires that patients stay within a two-hour drive for at least four weeks during treatment, and they are unable to drive for at least eight weeks post-treatment (Novartis Pharmaceuticals, 2018b). After the patient’s hospitalization, he or she will need to have their laboratories monitored, and they are followed at the treating center for at least four weeks postinfusion. 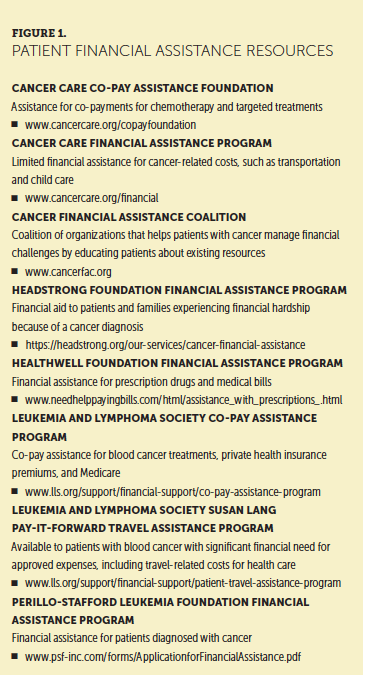
After discharge, patients may require emergency care or hospitalization that can increase the cost of care. During the first year after treatment, patients follow up with providers for additional blood laboratory evaluations and imaging every three months or as clinically necessary. Typically, these follow-up appointments are scheduled at the facility where the patient received therapy.
Few community-based grants or financial assistance programs provide aid for these additional costs associated with treatment. Most patients who have received CAR T-cell therapy have undergone at least one line of previous therapy. For many patients, the increased financial stress can be compounded over long periods of time. It is important to note that, when facing refractory and recurrent illness, patients may be willing to do whatever it takes, including mortgaging a home, taking on debt, or pursuing other avenues to secure treatment (Association of Community Cancer Centers, 2018). Transparent conversations with patients and caregivers at the outset of treatment, coupled with assessment of financial toxicity in survivorship, can best support this need for patients.
Implications for Nursing
Nursing care of patients receiving CAR T-cell therapy includes an understanding of potential long-term and late effects that may occur from therapy. For these patients, oncology nurses contribute to the promotion and preparation of survivorship care plans and ensure compliance based on national standards and institutional policy. Studies show that survivorship care plans improve communication among patients and oncologists or other healthcare providers in the community setting (Mayer, Birken, Check, & Chen, 2014). In addition, nurses educate their patients and caregivers, supporting successful transitions post-treatment. Nurses can support transitions by guiding patients to community-based resources.
To identify long-term effects associated with CAR T-cell therapy, nurses are equipped to conduct quantitative and qualitative studies. These studies can determine cognitive effects from therapy, anticipated symptomatology, the experience of survival, and quality of life. 
Conclusion
The benefit of increased survival for recipients of CAR T-cell therapy is met with the challenge of the healthcare community not yet fully grasping the long-term physiologic and psychosocial sequelae of treatment. Established late effects include cytopenias and infection, B-cell aplasia and hypogammaglobulinemia, and other physiologic sequelae, including fatigue and potential infertility. Management recommendations are still evolving for these late effects. Of greatest psychosocial concern is the financial toxicity of treatment coupled with potential feelings of isolation while undergoing a new treatment. Nurses’ support for adult survivors of CAR T-cell therapy should focus on care coordination and development of survivorship treatment plans that can guide these patients as they transition among care settings and through immunotherapy treatments.
About the Author(s)
Joaquin Buitrago, MS, RN, OCN®, is a nurse educator, Sherry Adkins, MSN, RN, ANP-C, is an advanced practice nurse supervisor, Misha Hawkins, MSN, RN, OCN®, is a cell therapy coordinator, and Kharington Iyamu, BSN, RN, is a clinical nurse, all in the Department of Lymphoma Melanoma; and Teresa van Oort, MHA, MSSW, LCSW, OSW-C, is a senior social work counselor in the Department of Social Work, all at the University of Texas MD Anderson Cancer Center in Houston. The authors take full responsibility for this content. This supplement was supported by an independent educational grant from Bristol-Myers Squibb. Adkins has previously served on advisory panels for Kite Pharma and Juno Therapeutics. Hawkins has previously served on advisory panels for Novartis Pharmaceuticals. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Buitrago can be reached at jabuitra@mdanderson.org and Adkins can be reached at sadkins@mdanderson.org, with copy to CJONEditor@ons.org. (Submitted January 2019. Accepted January 31, 2019.)
References
Albelda, S.M. (2019, January 2). NCI grant to fund study of CAR T-cell therapy for solid tumors. HemOnc Today. Retrieved from https://www.healio.com/hematology-oncology/lung-cancer/news/online/%7Be…
American Society of Clinical Oncology. (2017). Coping with fear of recurrence. Retrieved from https://www.cancer.net/survivorship/life-after-cancer/coping-with-fear-…
Association of Community Cancer Centers. (2018). CAR T-cell therapy gave me back my life: Robyn Stacy-Humphries’ cancer odyssey. Retrieved from https://www.accc-cancer.org/home/learn/immunotherapy/resource-detail/ca…
Bhoj, V.G., Arhontoulis, D., Wertheim, G., Capobianchi, J., Callahan, C.A., Ellebrecht, C.T., . . . Milone, M.C. (2016). Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood, 128, 360–370. https://doi.org/10.1182/blood-2016-01-694356
Black, E.K., & White, C.A. (2005). Fear of recurrence, sense of coherence and posttraumatic stress disorder in haematological cancer survivors. Psycho-Oncology, 14, 510–515. https://doi.org/10.1002/pon.894
Bloom, J.R., Stewart, S.L., Chang, S., & Banks, P.J. (2004). Then and now: Quality of life of young breast cancer survivors. Psycho-Oncology, 13, 147–160. https://doi.org/10.1002/pon.794
Bonifant, C.L., Jackson, H.J., Brentjens, R.J., & Curran, K.J. (2016). Toxicity and management in CAR T-cell therapy. Molecular Therapy—Oncolytics, 3, 16011. https://doi.org/10.1038/mto.2016.11
Brudno, J.N., & Kochenderfer, J.N. (2016). Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood, 127, 3321–3330. https://doi.org/10.1182/blood-2016-04-703751
Callahan, C., Barry, A., Fooks-Parker, S., Smith, L., Baniewicz, D., & Hobbie, W. (2019). Pediatric survivorship: Considerations following CAR T-cell therapy. Clinical Journal of Oncology Nursing, 23(Suppl. 1), 35–41. https://doi.org/10.1188/19.CJON.S1.35-41
Cavallo, J. (2018, May 25). Weighing the cost and value of CAR T-cell therapy. The ASCO Post. Retrieved from http://www.ascopost.com/issues/may-25-2018/weighing-the-cost-and-value-…
Chakraborty, R., Sidana, S., Shah, G.L., Scordo, M., Hamilton, B.K., & Majhail, N.S. (2018). Patient-reported outcomes with chimeric antigen receptor-T cell therapy: Challenges and opportunities. Biology of Blood and Marrow Transplantation. Advance online publication. https://doi.org/10.1016/j.bbmt.2018.11.025
Chavez, J.C., & Locke, F.L. (2018). CAR T cell therapy for B-cell lymphomas. Best Practice and Research. Clinical Haematology, 31, 135–146. https://doi.org/10.1016/j.beha.2018.04.001
Chino, F., Peppercorn, J., Taylor, D.H., Jr., Lu, Y., Samsa, G., Abernethy, A.P., & Zafar, S.Y. (2014). Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist, 19, 414–420. https://doi.org/10.1634/theoncologist.2013-0374
Chino, F., Peppercorn, J.M., Rushing, C., Kamal, A.H., Altomare, I., Samsa, G., & Zafar, S.Y. (2017). Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncology, 3, 1582–1584. https://doi.org/10.1001/jamaoncol.2017.2148
Chong, C.R., Park, V.,Harding, J.J., Brite, J.,Wolchok, J.D., & Kamboj, M. (2018). Safety of influenza vaccination in patients undergoing immunotherapy treatment for advanced cancer. Journal of Clinical Oncology, 36(15, Suppl.), e15073. https://doi.org/10.1200/JCO.2018.36.15_suppl.e15073
Cordeiro, A., Bezerra, E.D., Hill, J.A., Turtle, C.J., Maloney, D.G., & Bar, M. (2018, December). Late effects of CD19-targeted CAR-T cell therapy. Poster presented at the American Society of Hematology annual meeting, San Diego, CA. Retrieved from https://ash.confex.com/ash/2018/webprogram/Paper112023.html
Crist, J.V., & Grunfeld, E.A. (2013). Factors reported to influence fear of recurrence in cancer patients: A systematic review. Psycho-Oncology, 22, 978–986. https://doi.org/10.1002/pon.3114
Gallegos, A. (2018, August 21). CMS finalizes CAR T-cell therapy inpatient payments. Oncology Practice. Retrieved from https://www.mdedge.com/oncologypractice/article/173086/practice-managem…
Grupp, S.A., Maude, S.L., Rives, S., Baruchel, A., Boyer, M.W., Bittencourt, H., . . . Hiramatsu, H. (2018, December). Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia [895]. Poster presented at the American Society of Hematology annual meeting, San Diego, CA.
Haeusler, G.M., Slavin, M.A., Seymour, J.F., Lingaratnam, S., Teh, B.W., Tam, C.S., . . . Worth, L.J. (2013). Late-onset Pneumocystis jirovecii pneumonia post-fludarabine, cyclophosphamide and rituximab: Implications for prophylaxis. European Journal of Haematology, 91, 157–163. https://doi.org/10.1111/ejh.12135
Hernandez, I., Prasad, V., & Gellad, W.F. (2018). Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncology, 4, 994–996. https://doi.org/10.1001/jamaoncol.2018.0977
Hill, J.A., Li, D., Hay, K.A., Green, M.L., Cherian, S., Chen, X., . . . Turtle, C.J. (2018). Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood, 131, 121–130. https://doi.org/10.1182/blood-2017-07-793760
Janeway, C.A., Jr., Travers, P., Walport, M., & Shlomchik, M.J. (Eds.). (2001). Immunobiology: The immune system in health and disease (5th edition). New York, NY: Garland Science.
Johnson, L.A., & June, C.H. (2017). Driving gene-engineered T cell immunotherapy of cancer. Cell Research, 27, 38–58. https://doi.org/10.1038/cr.2016.154
Kite Pharma. (2018). Where can Yescarta® be received? Retrieved from https://www.yescarta.com/treatment-centers
Kochenderfer, J.N., & Rosenberg, S.A. (2013). Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nature Reviews. Clinical Oncology, 10, 267–276. https://doi.org/10.1038/nrclinonc.2013.46
Ljungman, P., & Avetisyan, G. (2008). Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplantation, 42, 637–641. https://doi.org/10.1038/bmt.2008.264
Maus, M.V., & Levine, B.L. (2016). Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist, 21, 608–617. https://doi.org/10.1634/theoncologist.2015-0421
Mayer, D.K., Birken, S.A., Check, D.K., & Chen, R.C. (2015). Summing it up: An integrative review of studies of cancer survivorship care plans (2006–2013). Cancer, 121, 978–996. https://doi.org/10.1002/cncr.28884
Mellon, S., Kershaw, T.S., Northouse, L.L., & Freeman-Gibb, L. (2007). A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psycho-Oncology, 16, 214–223. https://doi.org/10.1002/pon.1074
Mullen, E., & Mistry, H. (2018). Managing cancer survivorship issues. Journal for Nurse Practitioners, 14, 337–343. https://doi.org/10.1016/j.nurpra.2017.12.022
National Cancer Institute. (2017a). CAR T-cell therapy can lead to long-lasting remissions in patients with lymphoma. Retrieved from https://ccr.cancer.gov/node/19395
National Cancer Institute. (2017b). CAR T cells: Engineering patients’ immune cells to treat their cancers. Retrieved from https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
National Cancer Institute. (2018). Financial toxicity (financial distress) and cancer treatment (PDQ®)—Health professional version. Retrieved from https://www.cancer.gov/about-cancer/managing-care/track-care-costs/fina…
National Comprehensive Cancer Network. (2016). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prevention and treatment of cancer-related infections [v.2.2016]. Retrieved from https://oralcancerfoundation.org/wp-content/uploads/2016/09/infections…
Neelapu, S.S., Locke, F.L., Bartlett, N.L., Lekakis, L.J., Miklos, D.B., Jacobson, C.A., . . . Go, W.Y. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377, 2531–2544. https://doi.org/10.1056/NEJMoa1707447
Neelapu, S.S., Tummala, S., Kebriaei, P., Wierda, W., Gutierrez, C., Locke, F.L., . . . Shpall, E.J. (2018). Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nature Reviews. Clinical Oncology, 15, 47–62. https://doi.org/10.1038/nrclinonc.2017.148
Novartis Pharmaceuticals. (2018a). Find a Kymriah® treatment center. Retrieved from https://www.us.kymriah.com/treatment-center-locator
Novartis Pharmaceuticals. (2018b). Kymriah® (tisagenlecleucel) [Package insert]. Retrieved from https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/fil…
Orange, J.S. (2017). Primary humoral immunodeficiencies: An overview. In E. TePas, UpToDate. Retrieved from https://www.uptodate.com/contents/primary-humoral-immunodeficiencies-an…
Park, J.H., Rivière, I., Gonen, M., Wang, X., Sénéchal, B., Curran, K.J., . . . Sadelain, M. (2018). Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New England Journal of Medicine, 378, 449–459. https://doi.org/10.1056/NEJMoa1709919
Park, J.H., Romero, F.A., Taur, Y., Sadelain, M., Brentjens, R.J., Hohl, T.M., & Seo, S.K. (2018). Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clinical Infectious Diseases, 67, 533–540. https://doi.org/10.1093/cid/ciy152
Porter, D.L., Levine, B.L., Kalos, M., Bagg, A., & June, C.H. (2011). Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. New England Journal of Medicine, 365, 725–733. https://doi.org/10.1056/NEJMoa1103849
Schuster, S.J., Bishop, M.R., Tam, C.S., Waller, E.K., Borchmann, P., McGuirk, J.P., . . . Maziars, R.T. (2019). Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New England Journal of Medicine, 380, 45–56. https://doi.org/10.1056/NEJMoa1804980
Smith, S. (2018). Evolving the delivery of CAR T-cell therapies to the outpatient setting. Journal of Clinical Pathways, 4(8), 42–47. https://doi.org/10.25270/jcp.2018.10.00039
Strati, P., Wierda, W., Burger, J., Ferrajoli, A., Tam, C., Lerner, S., . . . O’Brien, S. (2013). Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: Analysis of persistent and new-onset cytopenia. Cancer, 119, 3805–3811. https://doi.org/10.1002/cncr.28318
Symes, D., & Schorr, A. (2018, January 24) Dan’s story: My experience with CAR T-cell therapy. Patient Power. Retrieved from https://www.patientpower.info/video/dans-story-my-experience-with-car-t…
Taplitz, R.A., Kennedy, E.B., Bow, E.J., Crews, J., Gleason, C., Hawley, D.K., . . . Flowers, C.R. (2018). Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. Journal of Clinical Oncology. Advance online publication. https://doi.org/10.1200/JCO.18.00374
Tran, G., & Zafar, S.Y. (2018). Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Annals of Translational Medicine, 6(9), 166. https://doi.org/10.21037/atm.2018.03.28
University of Texas MD Anderson Cancer Center. (2017). Chimeric antigen receptor (CAR) cell therapy toxicity assessment and management—Adult. Retrieved from https://www.mdanderson.org/documents/for-physicians/algorithms/clinical…
U.S. Food and Drug Administration. (2018). Long term follow-up after administration of human gene therapy products. Retrieved from https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceCompliance…
Wong, W. (2018). CAR-T reimbursement levels set by CMS, so where should patients be treated? Journal of Clinical Pathways, 4(3). Retrieved from https://www.journalofclinicalpathways.com/news/car-t-reimbursement-leve…
Zafar, S.Y., Peppercorn, J.M., Schrag, D., Taylor, D.H., Goetzinger, A.M., Zhong, X., & Abernethy, A.P. (2013). The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist, 18, 381–390. https://doi.org/10.1634/theoncologist.2012-0279
Zheng, P.P., Kros, J.M., & Li, J. (2018). Approved CAR T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discovery Today, 23, 1175–1182. https://doi.org/10.1016/j.drudis.2018.02.012




