PD-1 Inhibitors: Safety of Use and Management of Immune-Mediated Adverse Reactions in Patients With Head and Neck Cancer
Background: Treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) with programmed cell death protein 1 (PD-1) inhibitors has been associated with risk of developing immune-mediated adverse reactions (IMARs).
Objectives: This review provides nurses with an overview of the safety of PD-1 inhibitors approved by the U.S. Food and Drug Administration for recurrent or metastatic SCCHN following platinum chemotherapy, as well as recommendations for assisting with the diagnosis and management of IMARs.
Methods: PubMed® searches were conducted to identify relevant articles to support this review. Algorithms from drug manufacturers for IMAR management were also reviewed.
Findings: Before treatment with PD-1 inhibitors, nurses should map patients’ baseline profiles, which play a key role in aiding the healthcare team in the timely diagnosis and treatment of IMARs. Nurses should educate patients and caregivers on identifying signs and symptoms of IMARs and their progression. Within the interprofessional healthcare team, nurses play an important role in ensuring efficient management of IMARs to minimize withholding or discontinuation of PD-1 immunotherapy.
Jump to a section
Immunotherapy with programmed cell death protein 1 (pd-1) inhibitors has become the standard of care for numerous malignancies (Constantinidou, Alifieris, & Trafalis, 2019). Nivolumab and pembrolizumab are two PD-1 inhibitors that have been approved by the U.S. Food and Drug Administration (FDA) for use in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) following platinum chemotherapy; both have demonstrated efficacy benefit and have a favorable safety profile compared with standard single-agent chemotherapy (Bristol-Myers Squibb, 2019a; Merck Sharp & Dohme Corp., 2019a). Nivolumab significantly improved overall survival (OS) compared with the investigator’s choice of single-agent chemotherapy at primary analysis in a phase 3 trial (CheckMate 141) (Ferris et al., 2016), and OS benefit was maintained at two-year follow-up (Ferris et al., 2018). Pembrolizumab resulted in durable tumor responses in phase 1b (KEYNOTE-012) (Chow et al., 2016; Mehra et al., 2018; Seiwert et al., 2016) and phase 2 (KEYNOTE-055) (Bauml et al., 2017) studies. In addition, OS benefit with pembrolizumab versus standard-of-care, single-agent chemotherapy was noted in a post hoc analysis of a phase 3 study (KEYNOTE-040) (Cohen et al., 2019). In randomized phase 3 studies, nivolumab and pembrolizumab treatment stabilized health-related quality of life, whereas deterioration of health-related quality of life was noted in the chemotherapy arms of the respective studies (Cohen et al., 2018; Harrington et al., 2017). In June 2019, pembrolizumab was also approved by the FDA for first-line treatment of patients with recurrent or metastatic SCCHN in combination with platinum and fluorouracil, and as a single agent in patients with tumors that express programmed cell death-ligand 1 (PD-L1) (combined positive score [the number of PD-L1 staining cells, including tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells, multiplied by 100] of 1 or greater) (Merck Sharp & Dohme Corp., 2019a).
PD-1 inhibitors demonstrated a more favorable safety profile than chemotherapy, with a lower incidence of grade 3–4 treatment-related adverse events (TRAEs) (Cohen et al., 2019; Ferris et al., 2016, 2018). However, because of their mechanism of action, patients treated with PD-1 inhibitors may experience immune-mediated adverse reactions (IMARs). Tumor cells can upregulate PD-L1 and PD-L2, which bind to PD-1 receptors on T cells, suppressing the immune system and allowing the cancer cells to evade elimination (Topalian, Drake, & Pardoll, 2015). PD-1 inhibitors act by blocking the interaction between PD-1 and PD-L1/PD-L2, preventing checkpoint inhibition on T cells, and enhancing their ability to target tumor cells; this increased immune activity may result in the development of IMARs (Naidoo et al., 2015).
As members of the interprofessional healthcare team, nurses contribute to the diagnosis, monitoring, and management of IMARs. This review of IMARs associated with FDA-approved PD-1 inhibitors for recurrent or metastatic SCCHN provides a foundation for management of IMARs in clinical practice.
Methods
A PubMed® search was conducted to identify safety data from clinical trials of FDA-approved PD-1 inhibitors in recurrent or metastatic SCCHN between January 1, 2015, and April 11, 2019. Relevant abstracts spanning this period were identified from meetings of the American Society of Clinical Oncology, European Society for Medical Oncology, and American Association for Cancer Research. Likewise, a PubMed search was conducted to identify published reports of management guidelines for IMARs associated with PD-1 inhibitors. Relevant references cited in articles identified by the aforementioned searches were also reviewed, as were IMAR management algorithms developed by drug manufacturers.
Overview of Adverse Events
Among patients receiving at least one dose of therapy, TRAEs occurred in 59%–64% of patients in the nivolumab and pembrolizumab arms and in 77%–84% of patients in the investigator’s choice and standard-of-care arms in clinical trials of recurrent or metastatic SCCHN (Bauml et al., 2017; Chow et al., 2016; Cohen et al., 2019; Ferris et al., 2016; Seiwert et al., 2016). Grade 3–5 TRAEs were reported in 9%–17% of patients treated with nivolumab or pembrolizumab and in 35%–36% of patients treated with the investigator’s choice or standard of care. In clinical trials of recurrent or metastatic SCCHN, the most common TRAEs (with incidence of 5% or greater) associated with nivolumab and pembrolizumab were fatigue, pruritus, hypothyroidism, nausea, decreased appetite, diarrhea, rash, asthenia, anemia, and increased aspartate transaminase. Treatment discontinuation because of TRAEs was reported in 4%–6% of patients (Bauml et al., 2017; Chow et al., 2016; Cohen et al., 2019; Ferris et al., 2018).
Patients with recurrent or metastatic SCCHN can experience TRAEs associated with prior multimodality treatment for locally advanced disease. Fatigue is the most common TRAE associated with PD-1 inhibitors in recurrent or metastatic SCCHN (Bauml et al., 2017; Chow et al., 2016; Cohen et al., 2019; Ferris et al., 2016; Seiwert et al., 2016). The high incidence of fatigue can be attributed, in part, to hypothyroidism, which can result from prior radiation exposure during treatment for locally advanced SCCHN (Chaker, Bianco, Jonklaas, & Peeters, 2017; Cohen et al., 2016). Malnutrition is associated with SCCHN because of the disease itself or treatment (e.g., surgery, radiotherapy) (Gorenc, Kozjek, & Strojan, 2015). Treatment-related facial edema has been reported in a small proportion of patients receiving pembrolizumab for recurrent or metastatic SCCHN (Mehra et al., 2018). Although its incidence is low, treatment-related facial edema can potentially affect nutrition intake in conjunction with secondary lymphedema, which has been reported as a common late side effect of multimodality treatment received by patients for locally advanced SCCHN (incidence of approximately 75% at three or more months post-treatment) (Deng et al., 2012; Smith & Lewin, 2010).
In clinical trials, treatment-related deaths with nivolumab were attributed to pneumonitis and hypercalcemia; treatment-related deaths with pembrolizumab were attributed to pneumonitis, large intestine perforation, malignant neoplasm progression, Stevens-Johnson syndrome, and an unspecified cause (Bauml et al., 2017; Cohen et al., 2019; Ferris et al., 2016). Treatment-related deaths in the control (chemotherapy) arms occurred in patients with lung infection, pneumonia, and disease progression (Cohen et al., 2019; Ferris et al., 2016).
Treatment with PD-1 inhibitors is associated with the development of IMARs, which can affect almost any organ system (Naidoo et al., 2015). IMARs occurring in more than 1% of patients and reported in clinical trials of nivolumab and pembrolizumab in recurrent or metastatic SCCHN are listed in Table 1; the most common IMARs were skin-related, endocrine, gastrointestinal, or respiratory events. A summary of IMARs reported in less than 10% of patients treated with immune checkpoint inhibitors, including PD-1 inhibitors, is presented in Figure 1. 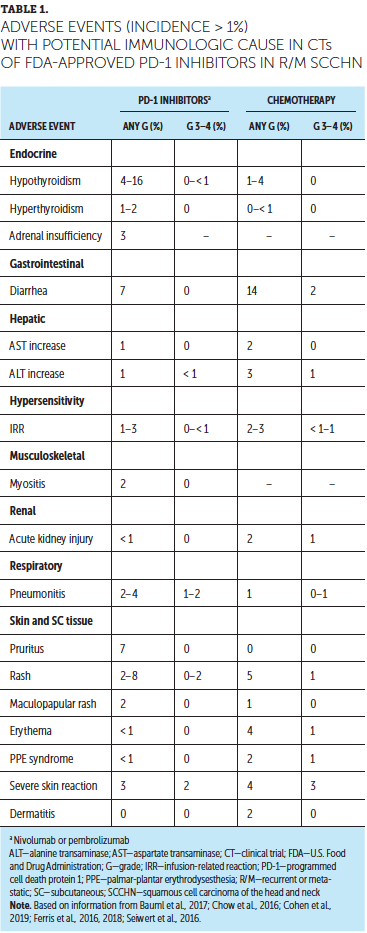
After initiation of treatment, IMARs can occur at any time, including after stopping treatment (Champiat et al., 2016). For patients with recurrent or metastatic SCCHN treated with nivolumab or pembrolizumab, no data have been reported about the onset of IMARs. In an analysis of 1,994 patients receiving single-agent nivolumab across multiple malignancies, median time to onset was 1.5 months for hyperthyroidism; 2.8–3.5 months for skin-related events, hypothyroidism or thyroiditis, hepatitis, and pneumonitis; and 4.3–5.3 months for adrenal insufficiency, type 1 diabetes mellitus, hypophysitis, and colitis (Bristol-Myers Squibb, 2019b). In an analysis of 2,799 patients receiving single-agent pembrolizumab for melanoma and non-small cell lung cancer, the median time to onset of IMARs was 1.2–1.4 months for hepatitis, thyroiditis, and hyperthyroidism and 3.3–3.7 months for pneumonitis, colitis, hypophysitis, and hypothyroidism (Merck Sharp & Dohme Corp., 2019a). 
Diagnosing, Monitoring, and Grading IMARs
Early diagnosis of IMARs is critical for their effective management and reversal (Michot et al., 2016). Timely assessment of IMARs by the interprofessional healthcare team contributes to accurate diagnoses, coordination of care, and prompt referrals; other potential etiologies of symptoms, such as infection or cancer progression, may complicate an accurate IMAR diagnosis (Champiat et al., 2016).
Documentation of a comprehensive baseline profile of the patient prior to initiating treatment with PD-1 inhibitors is key to ensuring that all health-related changes occurring during treatment are correctly assessed and that IMARs are diagnosed appropriately (Champiat et al., 2016). Nurses play a crucial role in this regard. In particular, they must capture any history of chronic or autoimmune diseases and long-term exposure to cortisone, which may affect susceptibility to IMARs. Chronic infections may flare up during treatment because of immune reactivation, leading to a heightened antipathogen response. Therefore, a history of any previous infections should be gathered and risk factors for viral infections, such as viral hepatitis, evaluated. For patients with altered nutritional status because of prior surgical or radiation treatment, IMARs like colitis, enteritis, and thyroiditis may also be of particular concern. Regular medications, such as nonsteroidal anti-inflammatory drugs and antibiotics, may interfere with treatment and should also be recorded. Given the high percentage of older adult patients with SCCHN, comorbidities are common (Champiat et al., 2016; Hartmann & Grandis, 2016). For example, patients with recurrent or metastatic SCCHN may have a high incidence of thyroid-related conditions or toxicities because of previous radiation exposure (Bhandare, Kennedy, Malyapa, Morris, & Mendenhall, 2007). As such, physical examination, oxygen saturation levels, laboratory tests, and imaging should be performed before starting immunotherapy with PD-1 inhibitors to provide a reference point for subsequent test results (Champiat et al., 2016; Gordon et al., 2017; Haanen et al., 2017) (see Figure 2). 
To diagnose IMARs promptly, nurses are advised to regularly assess and monitor signs and symptoms of inflammation and possible IMARs during treatment (before every treatment session or at least once a month). Nurses should also discuss changes noted on assessments with advanced practice providers to ensure that the appropriate course of action is followed. Patients should continue to be monitored for at least one year after stopping treatment (Champiat et al., 2016).
To establish the severity of IMARs, providers can use the Common Terminology Criteria for Adverse Events (CTCAE) (National Cancer Institute, 2017). However, the CTCAE guidelines were established before the advent of immunotherapies. Therefore, the CTCAE guidelines may not be sufficiently sensitive to grade IMARs without providers also offering their clinical judgment to determine the treatment plan (Kumar et al., 2017). Patient monitoring checklists are available for use by nurses and other healthcare providers to ensure that signs and symptoms of IMARs are accurately captured (Bristol-Myers Squibb, 2019c). During follow-up visits, nurses’ use of checklists as a source of accurate data allows advanced practice providers to act on that data to grade IMARs.
Patient Education
Nurses play a pivotal role in educating patients and caregivers about the unique spectrum of IMARs associated with PD-1 inhibitors and in addressing patient questions and concerns. Patients should be encouraged to promptly report apparently general symptoms, such as fatigue or nausea, and avoid self-management because the symptoms may be indicative of IMARs (Champiat et al., 2016) (see Figure 3). When visiting healthcare providers other than their regular doctor, patients should have a card containing information regarding the immunotherapies they are receiving, details of IMARs associated with treatment, and instructions to call their oncologist prior to prescribing any medications, barring instances warranting urgent care (Champiat et al., 2016).
Management of IMARs
Timely treatment of IMARs is crucial to reduce their duration and severity (Champiat et al., 2016). Unlike adverse events associated with chemotherapy, IMARs with PD-1 inhibitors do not appear to be dose-dependent, and dose reductions are not recommended for their management. Instead, treatment may be withheld or discontinued. Resources for management of IMARs include recommendations provided by the manufacturers of PD-1 inhibitors (Bristol-Myers Squibb, 2019b; Merck Sharp & Dohme Corp., 2019b) that are based on established algorithms used in clinical trials (Bristol-Myers Squibb, 2019b) and guidelines developed by oncology societies that are based on clinical consensus and experience (Brahmer et al., 2018; Haanen et al., 2017; Puzanov et al., 2017). Detailed recommendations for the management of the most common IMARs associated with PD-1 inhibitors, which include skin-related IMARs, endocrinopathies, pneumonitis, gastrointestinal IMARs, and hepatitis, are summarized in Table 2. 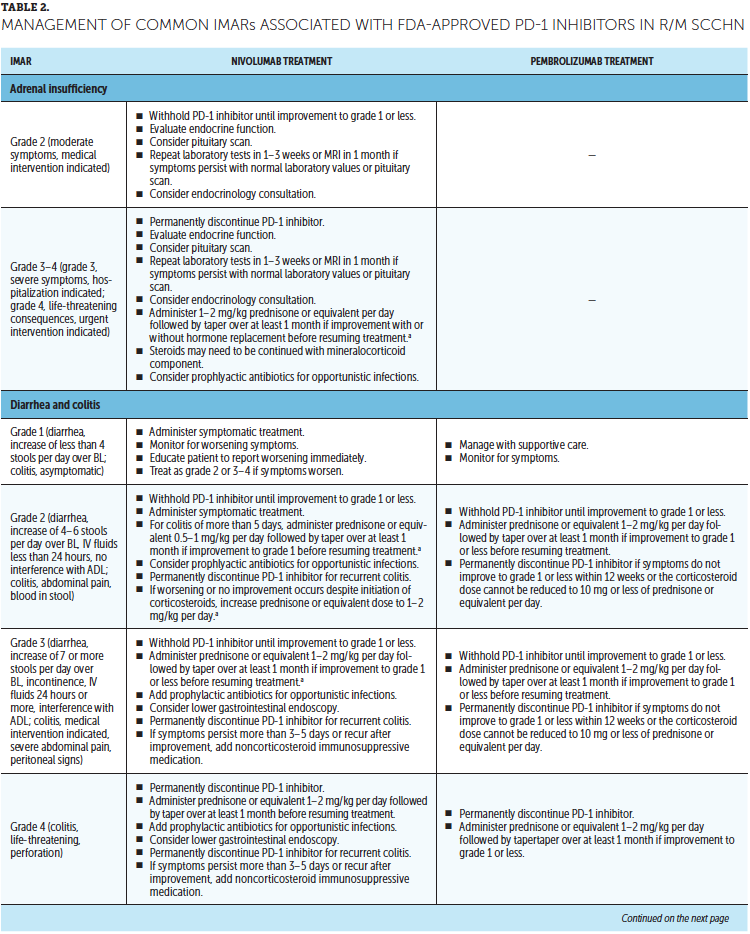
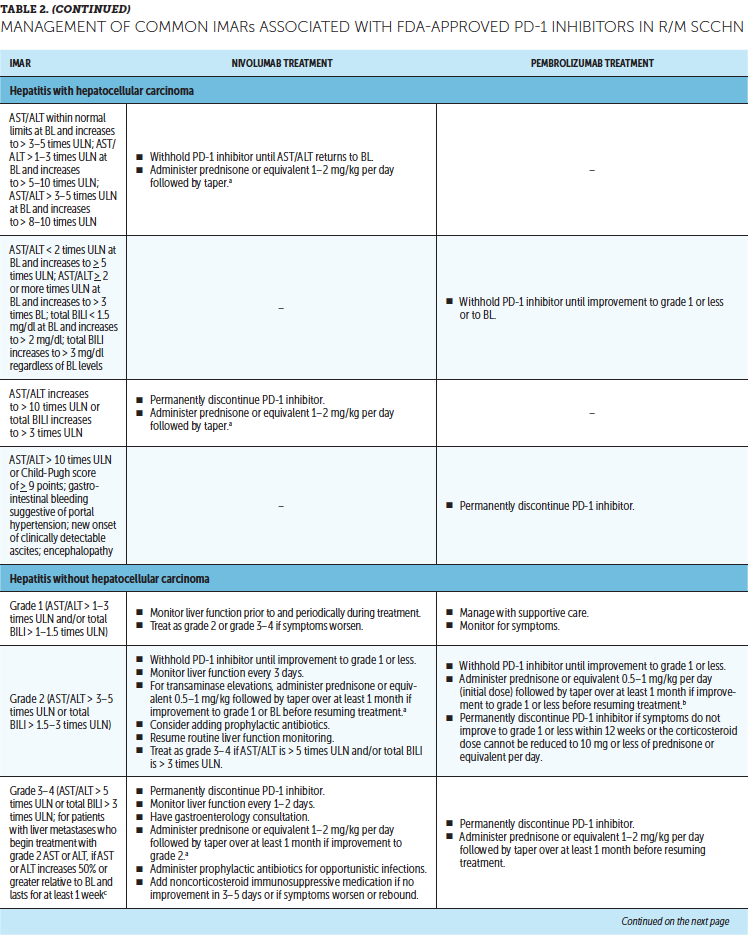
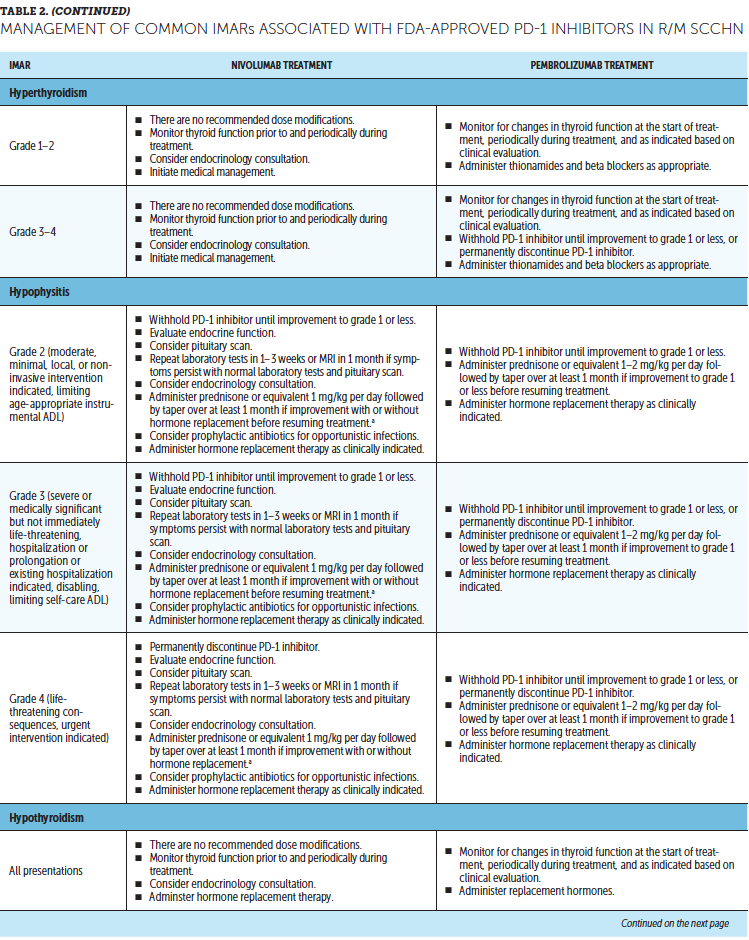
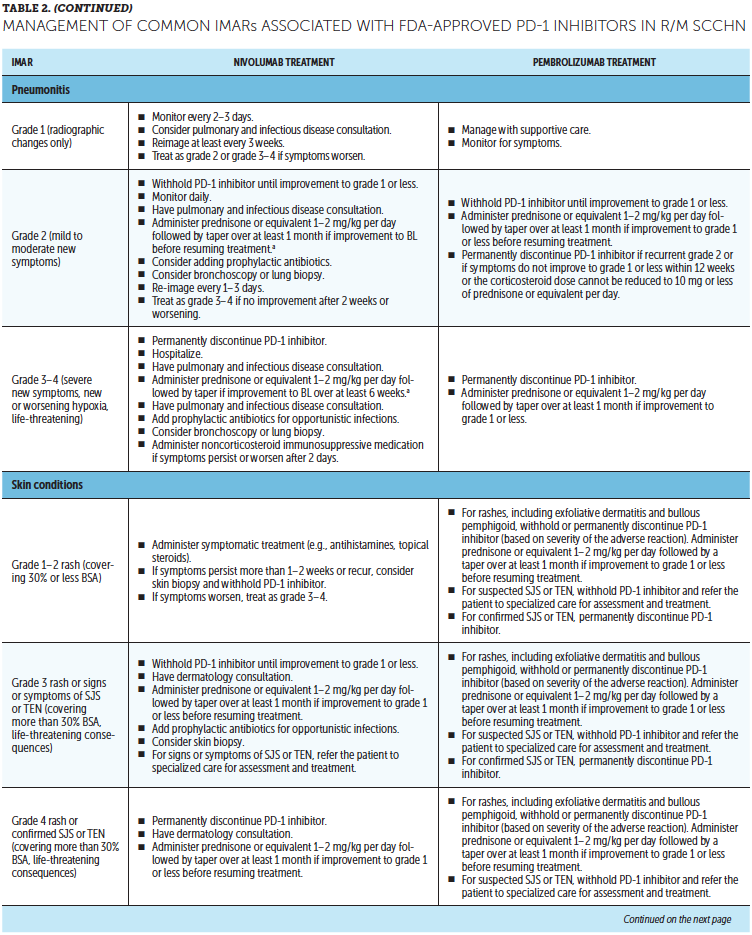
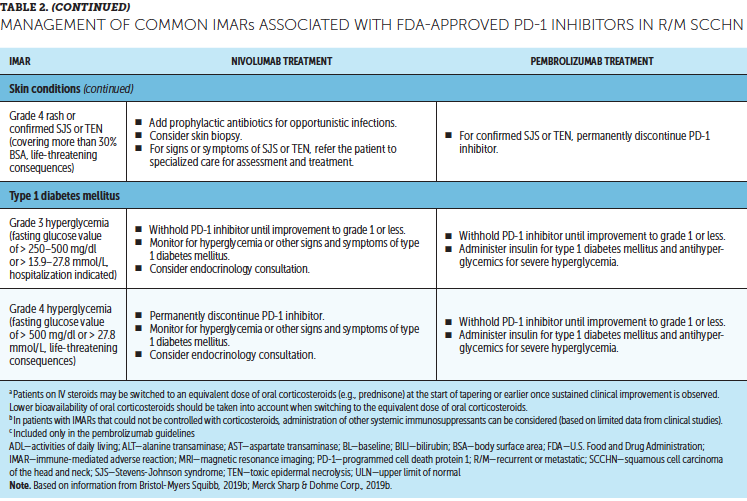
Corticosteroids are the primary treatment for IMARs and are administered when there is a need to reduce inflammation (Champiat et al., 2016). Because of their immunosuppressive effects, potential infectious etiologies should be ruled out prior to administration of corticosteroids. Clinical judgment should play an important role, in addition to CTCAE grading, in determining the appropriateness of initiating corticosteroid treatment (Horvat et al., 2015). Prolonged use of corticosteroids may leave the patient vulnerable to opportunistic infections (Postow, Sidlow, & Hellmann, 2018); to prevent this, prophylactic antibiotics may be warranted. Extended corticosteroid use could also be associated with hyperglycemia (Williams, Grauer, Henry, & Rockey, 2017). Nurses should monitor blood sugar levels regularly, particularly in patients with an increased risk of diabetes, such as those with high body mass index and baseline blood glucose levels. Supportive antacid medication may be used to reduce corticosteroid-associated heartburn (Spain, Diem, & Larkin, 2016).
With close monitoring, therapy may continue despite grade 1 IMARs (Brahmer et al., 2018; Bristol-Myers Squibb, 2019b; Champiat et al., 2016; Merck Sharp & Dohme Corp., 2019b). Treatment should be suspended in patients with grade 2 IMARs, with the exception of skin-related and endocrine IMARs, until they improve to grade 1. Grade 1–2 IMARs may be treated in the ambulatory setting, whereas more severe IMARs may require hospitalization. For grade 3 IMARs in any organ category, suspension of treatment is recommended along with a course of corticosteroids, typically administered via IV. Immunosuppressive therapy, such as anti-tumor necrosis factor-a (infliximab) or anti-interleukin-6 (tocilizumab), may be added for some IMARs that have not improved after corticosteroid use. Importantly, the continuation of treatment should be considered only if IMAR severity has reduced to grade 1, if corticosteroid (prednisone) dosage is 10 mg or less per day, and if treatment includes no other immunosuppressive drugs. For grade 4 toxicities, discontinue treatment. An exception to discontinuing treatment is if certain endocrinopathies can be controlled by medical management, such as hormone replacement therapy.
After IMAR severity reduces to grade 1, providers can gradually taper corticosteroids over a period of at least 30 days to avoid relapse or worsening of symptoms (Brahmer et al., 2018). Although most IMARs can be managed and do resolve, endocrine IMARs, such as hypophysitis and diabetes, may require lifelong hormonal treatment (Michot et al., 2016).
The baseline profile of patients developed by nurses prior to initiation of PD-1 immunotherapy can be helpful in ensuring that patients are eligible to receive corticosteroids. Nurses play a significant role in the management of IMARs by coordinating care among members of the interprofessional healthcare team, including specialists for the affected organs.
Case Studies
Case Study 1
A White female nonsmoker in her late 50s was initially diagnosed with biopsy-proven, poorly differentiated stage IVA human papillomavirus (HPV)–positive SCC of the left tonsil. No distant metastases were identified by computed tomography (CT) or positron-emission tomography (PET). She underwent left pharyngectomy and node dissection, and was subsequently treated with adjuvant cisplatin (weekly) and radiation therapy, which was well tolerated.
At her six-month follow-up, CT and PET imaging identified the presence of pulmonary nodules and metastatic disease. Biopsy confirmed the diagnosis of metastatic SCC. Treatment with nivolumab (240 mg every two weeks) was started. After eight treatments, she developed grade 2 diarrhea and grade 2 hyponatremia. She was treated with 1 mg/kg prednisone and conventional antidiarrheal agents. She received fluids and underwent comprehensive metabolic panel checks on a weekly basis. Nivolumab treatment was held for six weeks until resolution of hyponatremia and diarrhea and completion of steroid tapering. She has since been treated with nivolumab for three months and has stable disease with no recurrence of diarrhea or worsening of hyponatremia.
Case Study 2
A White male nonsmoker in his late 40s was diagnosed initially with HPV-positive SCC of the base of the tongue; according to the TNM system, which measures tumor (T), lymph node (N), and metastasis (M), his cancer stage was described as T2N2a. He underwent concurrent chemotherapy and radiation therapy with weekly cisplatin for three months. After seven months, he developed lesions within the liver, consistent with biopsy-proven metastatic SCC. He underwent segmental resection and radio-frequency ablation of two liver lesions, which were positive for disease. At that point, he had no detectable disease.
He subsequently developed progressive liver and bone metastases and was treated with the EXTREME regimen (cetuximab with platinum-based chemotherapy and 5-fluorouracil). He responded well and continued on maintenance cetuximab for eight months. However, progression of liver and bone metastases was diagnosed, as well as new metastases to the neck, peripancreatic, and precaval lymph nodes. He underwent palliative radiation therapy, and treatment with nivolumab at 240 mg was started. He received eight biweekly doses, and imaging data indicated stable disease.
About five months after the initiation of nivolumab treatment, he developed left eye pain, particularly with eye movement, and a small distortion in his central vision, followed by a halo that worsened over a four-day period. Nivolumab treatment was stopped, and he was referred for ophthalmology consultation. Based on a magnetic resonance imaging scan, retrobulbar optic neuritis was diagnosed, and it was attributed to nivolumab. He was initially treated with three doses of 1,000 mg of methylprednisolone and transitioned to prednisone 1 mg/kg in divided doses of 120 mg every 12 hours. His decreased vision resolved over three days. After this, steroids were tapered over a period of at least 30 days. One month after treatment was stopped, he was doing well, with normal vision. Because of his history of optic neuritis, nivolumab treatment was not resumed.
Implications for Practice
The provided case studies serve as examples of IMAR management using the recommendations outlined in this article. Nurses are significantly involved in the early detection and monitoring of IMARs. As members of the interprofessional healthcare team, nurses contribute their assessment and monitoring skills so that potential IMARs can be promptly diagnosed and managed. Nurses hold a pivotal role in educating patients and caregivers in identifying signs and symptoms of IMARs and seeking timely treatment. 
Conclusion
PD-1 inhibitors have demonstrated efficacy in patients with recurrent or metastatic SCCHN following platinum chemotherapy and have become an important part of the treatment paradigm for this cancer. Treatment with PD-1 inhibitors is generally associated with a more favorable safety profile compared with chemotherapy. However, because of their mechanism of action, treatment with PD-1 inhibitors may be associated with the development of IMARs. As the primary point of contact for patients treated with PD-1 inhibitors, nurses play an important role in the early detection and monitoring of IMARs. Nurses also aid an interprofessional team of healthcare providers in the efficient management of IMARs by providing the information necessary for timely intervention and treatment. Finally, nurses play a pivotal role in educating patients and caregivers in identifying signs and symptoms of IMARs and seeking timely treatment. This article has summarized recommendations to provide nurses with the tools to efficiently assist with the diagnosis, monitoring, and management of IMARs associated with FDA-approved PD-1 inhibitors and to minimize treatment disruption in patients with recurrent or metastatic SCCHN following platinum chemotherapy.
About the Author(s)
Rebecca L. Lewis, CRNP, is a certified RN practitioner at the University of Pittsburgh Medical Center Hillman Cancer Center in Pennsylvania; and Kristen L. Miller, APRN, AOCNP®, is an advanced practice RN at the Cancer Institute of Florida, Florida Hospital Medical Group, in Orlando. The authors take full responsibility for this content. This work was supported by Bristol-Myers Squibb. Miller has previously served on speakers bureaus for AstraZeneca and Genentech. Writing support was provided by Caroline Anderson, PhD, and Meenakshi Subramanian, PhD, CMPP, at Evidence Scientific Solutions. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Lewis can be reached at lewisrl2@upmc.edu, with copy to CJONEditor@ons.org. (Submitted May 2019. Accepted July 14, 2019.)
References
Bauml, J., Seiwert, T.Y., Pfister, D.G., Worden, F., Liu, S.V., Gilbert, J., . . . Haddad, R. (2017). Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. Journal of Clinical Oncology, 35, 1542–1549. https://doi.org/10.1200/JCO.2016.70.1524
Bhandare, N., Kennedy, L., Malyapa, R.S., Morris, C.G., & Mendenhall, W.M. (2007). Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. International Journal of Radiation Oncology, Biology, Physics, 68, 1131–1139. https://doi.org/10.1016/j.ijrobp.2007.01.029
Brahmer, J.R., Lacchetti, C., Schneider, B.J., Atkins, M.B., Brassil, K.J., Caterino, J.M., . . . Thompson, J.A. (2018). Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology, 36, 1714–1768. https://doi.org/10.1200/JCO.2017.77.6385
Bristol-Myers Squibb. (2019a). Opdivo® (nivolumab) [Package insert]. Retrieved from https://packageinserts.bms.com/pi/pi_opdivo.pdf
Bristol-Myers Squibb. (2019b). Opdivo® (nivolumab) immune-mediated adverse reactions management guide. Retrieved from http://www.opdivohcp.com/servlet/servlet.FileDownload?file=00Pi000000kL…
Bristol-Myers Squibb. (2019c). Opdivo® (nivolumab) patient monitoring checklist. Retrieved from http://www.opdivohcp.com/servlet/servlet.FileDownload?file=00P1Y00000zA…
Chaker, L., Bianco, A.C., Jonklaas, J., & Peeters, R.P. (2017). Hypothyroidism. Lancet, 390, 1550–1562. https://doi.org/10.1016/S0140-6736(17)30703-1
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., . . . Marabelle, A. (2016). Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Annals of Oncology, 27, 559–574. https://doi.org/10.1093/annonc/mdv623
Chow, L.Q.M., Haddad, R., Gupta, S., Mahipal, A., Mehra, R., Tahara, M., . . . Seiwert, T.Y. (2016). Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. Journal of Clinical Oncology, 34, 3838–3845. https://doi.org/10.1200/JCO.2016.68.1478
Cohen, E.E.W., LaMonte, S.J., Erb, N.L., Beckman, K.L., Sadeghi, N., Hutcheson, K.A., . . . Pratt-Chapman, M.L. (2016). American Cancer Society head and neck cancer survivorship care guideline. CA: A Cancer Journal for Clinicians, 66, 203–239. https://doi.org/10.3322/caac.21343
Cohen, E.E.W., Soulieres, D., Le Tourneau, C., Dinis, J., Licitra, L.F., Ahn, M.-J., . . . Harrington, K.J. (2018). Health-related quality of life (HRQoL) of pembrolizumab (pembro) vs standard of care (SOC) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) in KEYNOTE-040. Journal of Clinical Oncology, 36(Suppl.), 6013. https://doi.org/10.1200/JCO.2018.36.15_suppl.6013
Cohen, E.E.W., Soulières, D., Le Tourneau, C., Dinis, J., Licitra, L., Ahn, M.-J., . . . Harrington, K.J. (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet, 393, 156–167. https://doi.org/10.1016/S0140-6736(18)31999-8
Constantinidou, A., Alifieris, C., & Trafalis, D.T. (2019). Targeting programmed cell death -1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacology and Therapeutics, 194, 84–106. https://doi.org/10.1016/j.pharmthera.2018.09.008
Deng, J., Ridner, S.H., Dietrich, M.S., Wells, N., Wallston, K.A., Sinard, R.J., . . . Murphy, B.A. (2012). Prevalence of secondary lymphedema in patients with head and neck cancer. Journal of Pain and Symptom Management, 43, 244–252. https://doi.org/10.1016/j.jpainsymman.2011.03.019
Ferris, R.L., Blumenschein, G., Jr., Fayette, J., Guigay, J., Colevas, A.D., Licitra, L., . . . Gillison, M.L. (2016). Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New England Journal of Medicine, 375, 1856–1867. https://doi.org/10.1056/NEJMoa1602252
Ferris, R.L., Blumenschein, G., Jr., Fayette, J., Guigay, J., Colevas, A.D., Licitra, L., . . . Gillison, M.L. (2018). Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncology, 81, 45–51. https://doi.org/10.1016/j.oraloncology.2018.04.008
Gordon, R., Kasler, M.K., Stasi, K., Shames, Y., Errante, M., Ciccolini, K., . . . Fischer-Cartlidge, E. (2017). Checkpoint inhibitors: Common immune-related adverse events and their management. Clinical Journal of Oncology Nursing, 21(Suppl.), 45–52. https://doi.org/10.1188/17.CJON.S2.45-52
Gorenc, M., Kozjek, N.R., & Strojan, P. (2015). Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Reports of Practical Oncology and Radiotherapy, 20, 249–258. https://doi.org/10.1016/j.rpor.2015.03.001
Haanen, J.B.A.G., Carbonnel, F., Robert, C., Kerr, K.M., Peters, S., Larkin, J., & Jordan, K. (2017). Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28(Suppl. 4), iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Harrington, K.J., Ferris, R.L., Blumenschein, G., Jr., Colevas, A.D., Fayette, J., Licitra, L., . . . Guigay, J. (2017). Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncology, 18, 1104–1115. https://doi.org/10.1016/S1470-2045(17)30421-7
Hartmann, S., & Grandis, J.R. (2016). Treatment of head and neck cancer in the elderly. Expert Opinion on Pharmacotherapy, 17, 1903–1921. https://doi.org/10.1080/14656566.2016.1220540
Horvat, T.Z., Adel, N.G., Dang, T.-O., Momtaz, P., Postow, M.A., Callahan, M.K., . . . Chapman, P.B. (2015). Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of Clinical Oncology, 33, 3193–3198. https://doi.org/10.1200/JCO.2015.60.8448
Kumar, V., Chaudhary, N., Garg, M., Floudas, C.S., Soni, P., & Chandra, A.B. (2017). Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Frontiers in Pharmacology, 8, 49. https://doi.org/10.3389/fphar.2017.00049
Mehra, R., Seiwert, T.Y., Gupta, S., Weiss, J., Gluck, I., Eder, J.P., . . . Haddad, R. (2018). Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. British Journal of Cancer, 119, 153–159. https://doi.org/10.1038/s41416-018-0131-9
Merck Sharp & Dohme Corp. (2019a). Keytruda® (pembrolizumab) [Package insert]. Retrieved from https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.p…
Merck Sharp & Dohme Corp. (2019b). Keytruda® (pembrolizumab): Give more patients a key: A treatment guide for Keytruda. Retrieved from https://www.keytruda.com/static/pdf/keytruda-treatment-guide.pdf
Michot, J.M., Bigenwald, C., Champiat, S., Collins, M., Carbonnel, F., Postel-Vinay, S., . . . Lambotte, O. (2016). Immune-related adverse events with immune checkpoint blockade: A comprehensive review. European Journal of Cancer, 54, 139–148. https://doi.org/10.1016/j.ejca.2015.11.016
Naidoo, J., Page, D.B., Li, B.T., Connell, L.C., Schindler, K., Lacouture, M.E., . . . Wolchok, J.D. (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of Oncology, 26, 2375–2391. https://doi.org/10.1093/annonc/mdv383
National Cancer Institute. (2017). Common Terminology Criteria for Adverse Events (CTCAE) [v.5.0]. Retrieved from https://bit.ly/2Nn0LTT
Postow, M.A., Sidlow, R., & Hellmann, M.D. (2018). Immune-related adverse effects associated with immune checkpoint blockade. New England Journal of Medicine, 378, 158–168. https://doi.org/10.1056/NEJMra1703481
Puzanov, I., Diab, A., Abdallah, K., Bingham, C.O., III, Brogdon, C., Dadu, R., . . . Ernstoff, M.S. (2017). Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for ImmunoTherapy of Cancer, 5, 95. https://doi.org/10.1186/s40425-017-0300-z
Seiwert, T.Y., Burtness, B., Mehra, R., Weiss, J., Berger, R., Eder, J.P., . . . Chow, L.Q. (2016). Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncology, 17, 956–965. https://doi.org/10.1016/S1470-2045(16)30066-3
Smith, B.G., & Lewin, J.S. (2010). The role of lymphedema management in head and neck cancer. Current Opinion in Otolaryngology and Head and Neck Surgery, 18, 153–158. https://doi.org/10.1097/MOO.0b013e32833aac21
Spain, L., Diem, S., & Larkin, J. (2016). Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews, 44, 51–60. https://doi.org/10.1016/j.ctrv.2016.02.001
Topalian, S.L., Drake, C.G., & Pardoll, D.M. (2015). Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell, 27, 450–461. https://doi.org/10.1016/j.ccell.2015.03.001 Williams, K.J., Grauer, D.W., Henry, D.W., & Rockney, M.L. (2017). Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. Journal of Oncology Pharmacy Practice, 25, 544–550. https://doi.org/10.1177/1078155217744872




