Novel Intervention With Acupuncture for Anorexia and Cachexia in Patients With Gastrointestinal Tract Cancers: A Feasibility Study
Purpose/Objectives: To investigate the feasibility of using acupuncture as a complementary intervention to existing treatments and to evaluate the efficacy of acupuncture in improving appetite and slowing weight loss with patients with gastrointestinal (GI) tract cancers.
Design: One-group pre- and postintervention feasibility study.
Setting: Outpatient clinic for patients with cancer and a community setting, both in Florida.
Sample: A convenience sample of seven adults with GI cancer.
Methods: Eight acupuncture sessions were provided during eight weeks. Data were collected using the visual analog scale (VAS) for appetite, Simplified Nutritional Appetite Questionnaire (SNAQ), Karnofsky Performance Status, and bioelectrical impedance analysis.
Main Research Variables: Appetite, weight, attrition rate.
Findings: Seven patients with a mean age of 61 years completed the intervention. Acupuncture was well accepted, feasible, and safe without any reported side effects. Appetite showed improvement, with an average score of 3.04 on the VAS and 4.14 on SNAQ compared to the preintervention scores. The average weight loss was 1.32% compared to the baseline during an eight-week period.
Conclusions: The acupuncture intervention was feasible and indicated positive outcomes. Because of the small sample size and lack of a control group, statistical significance of effectiveness was not determined. Acupuncture seemed to improve appetite and slow weight loss in patients with GI cancers, so additional studies with a larger sample size and a variety of cancers are warranted.
Implications for Nursing: Oncology nurses are uniquely able to equip patients with information about complementary therapy modalities, such as acupuncture, which is a promising way to improve appetite and slow weight loss in patients with GI cancers.
Jump to a section
Despite advancements in the management of side effects and supportive care, many patients with cancer still suffer from side effects related to treatment interventions and the progression of disease. Most significantly, patients struggle to maintain optimal nutrition and a healthy diet and weight. More than half of patients undergoing treatment experience malnutrition, anorexia, and weight loss (Smith, Malinauskas, Garner, & Barber-Heidal, 2008), which are independent factors that contributes to a lower survival rate, decreased quality of life, and functional impairment (Andreyev, Norman, Oates, & Cunningham, 1998; Fearon et al., 2011; Tisdale, 2009). More than 15% of weight loss leads to impaired physiologic function, and weight loss of more than 30% body mass can predict death in patients with cancer (Tisdale, 2002). In addition, studies have shown that patients with solid tumors, particularly gastrointestinal (GI) cancers, experience severe weight loss (Dewys et al., 1980; Tan & Fearon, 2008), and another study revealed that participants who lost at least 2.5 kg (about 3% of baseline body weight loss) during six to eight weeks demonstrated lower Karnofsky Performance Status scores and triceps skinfold thickness compared to patients who maintained a stable weight (O’Gorman, McMillan, & McArdle, 1999). Therefore, the evidence suggests that food intake and weight loss may help to predict functioning and prognosis of patients with GI cancers (Fearon, Voss, & Hustead, 2006).
Clinicians have emphasized the importance of nutritional support for patients with cancer (Burden, Hill, Shaffer, & Todd, 2010). For example, clinicians often use pharmacologic therapies, such as progesterone (megestrol acetate [MA]) and corticosteroids, to improve appetite, and more powerful drugs are continually under investigation (Mantovani & Madeddu, 2010; Mantovani, Madeddu, & Macciò, 2013). In addition to pharmacologic therapies, clinicians frequently use nutritional interventions with patients, which may include oral nutritional supplements, enteral tube feeding, parenteral nutritional support (Paccagnella, Morassutti, & Rosti, 2011), or nutritional support through a surgically placed tube (gastrostomy tube) to enhance nutrient intake. These therapies appear to be somewhat effective in the early stages of cancer, but efficacy varies according to type of cancer, presence of comorbidities, and rate of weight loss prior to treatment (Aapro et al., 2014).
However, these treatment modalities are often complicated by chemotherapy interventions and significantly increased somatic stress levels because of metabolic changes (Graca Pereira, Figueiredo, & Fincham, 2012) and may have adverse effects (Tuca, Jimenez-Fonseca, & Gascòn, 2013). For example, pharmacologic approaches work for only a short time, cause side effects, and cannot be tolerated if patients are nauseated. MA is the most frequently prescribed therapy, and it is considered effective for patients with cancer who lack appetite. Clinical trials (Jatoi et al., 2002, 2004) demonstrated that MA was more effective than dronabinol or eicosapentanoic acid (EPA) in improving appetite, but findings showed it did not improve lean body mass (Mantovani et al., 2013). In addition, MA has serious side effects, which may include fluid retention, edema, thrombosis, and hyperglycemia (Yeh & Schuster, 2006), and these should be considered limitations when treating patients. Studies of an alternative to MA, dronabinol, had conflicting results, highlighting a need for more clinical trials, particularly in the treatment of cancer-related anorexia (Tuca et al., 2013). Like MA, dronabinol has side effects, such as cannabinoid dose-related elation, drowsiness, and central nervous system adverse events. Taken together, these studies show that more effective therapies with fewer side effects are needed to improve appetite and reduce unintentional weight loss in patients with GI cancers.
No pharmacologic intervention currently exists that can prevent or treat cancer-related malnutrition, anorexia, weight loss, or cachexia (Bosaeus, 2008). Because a patient’s survival is reduced in accordance with total weight loss (fat plus lean muscle mass) and rate of weight loss (Tisdale, 2002), nonpharmacologic interventions that do not require the intake of food or medication, such as acupuncture, may satisfy a large, clinical unmet need. Acupuncture has been used for a wide range of medical indications from pain to functional bowel disorders (Deng, Vickers, Simon Yeung, & Cassileth, 2006). Although the exact mechanisms of acupuncture remain unknown, it has been shown to reduce nausea and vomiting (Collins & Thomas, 2004), decrease circulating cortisol levels (Ahsin, Saleem, Bhatti, Iles, & Aslam, 2009), and prevent loss of appetite through inhibition of alpha–melanocyte-stimulating hormone (a-MSH) secretion (Shi et al., 2010). Based on these outcomes, acupuncture may benefit patients with digestive tract cancers undergoing chemotherapy treatment by reducing stress levels and proinflammatory cytokines as well as increasing appetite and nutrient status (see Figure 1). It may also reduce the risk of developing cancer-related anorexia and cachexia and improve overall quality of life.
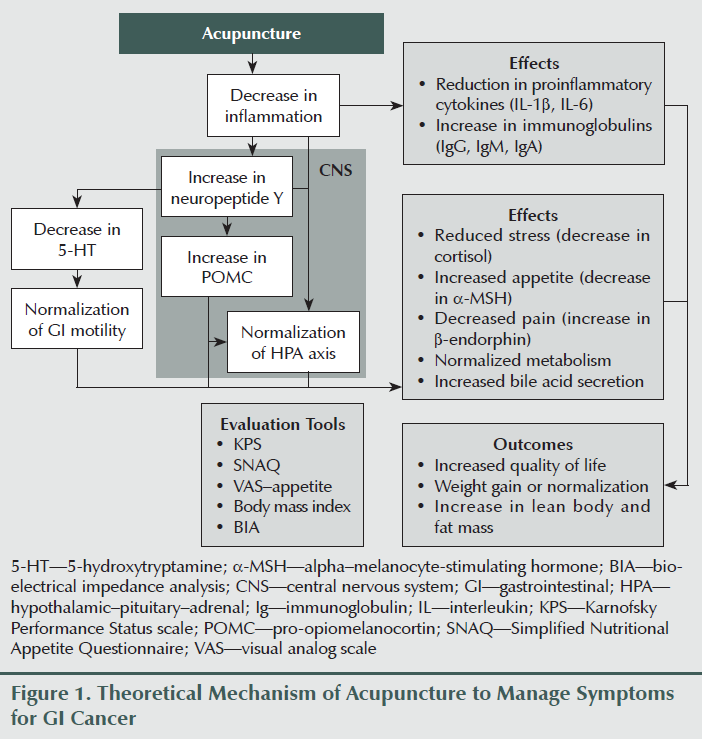
Although the evidence is promising, no clinical trials have been conducted to date that determine the direct effect of acupuncture on patient appetite. Previous and ongoing clinical trials revealed that studies were limited to the effect of acupuncture on the management of nausea and vomiting, fatigue, and pain. Therefore, the purpose of this study was to investigate the feasibility of using acupuncture as a complementary intervention and to evaluate the efficacy of acupuncture in improving appetite and slowing weight loss in patients with cancers of the GI tract.
Methods
A one-group pre- and postintervention design was used to conduct this feasibility study. All participants received the acupuncture treatment when they completed a baseline assessment. The study was approved by the institutional review board at the University of Florida in Gainesville prior to commencement.
Inclusion criteria included adults who (a) were aged 21 years or older with a diagnosis of gastric or colorectal cancer, (b) had weight loss of 5% or more compared to prediagnosis weight, (c) had no surgery in the past month, (d) were not scheduled for radiation therapy for the next three months (or during the study period), (e) were able to speak or understand English, and (f) were willing to follow the research protocol during the study period. Exclusion criteria were adults with GI cancer who (a) were scheduled for surgical procedures at the time of recruitment, (b) were not scheduled for postsurgery chemotherapy, (c) were scheduled for radiation therapy alone or in addition to chemotherapy, (d) had any comorbidities that may affect the interpretation of study findings (e.g., HIV, AIDS, Alzheimer disease, movement disorders, acute myocardial infarction within the past three months, hepatitis), (e) had open burn site or infected wounds, or (f) had a life expectancy of less than three months.
Intervention
The acupuncture intervention was provided to participants once prior to chemotherapy, once per week for one hour between chemotherapy cycles, and once after chemotherapy. The total number of acupuncture treatments per participant was eight sessions during about eight weeks, which covered up to two chemotherapy cycles. The Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) recommendations (MacPherson et al., 2002, 2010) were used as guidelines to improve appraisal, interpretations, and challenges unique to acupuncture research. Acupuncture needles used were single-use, sterile, stainless steel, and disposable. The dimensions of the full-body acupuncture needles were either 0.16 mm by 13 mm or 0.22 mm by 25 mm, and auricular needles were 0.2 mm by 13 mm.
Acupuncture points were predetermined, founded on general principles of traditional Chinese medicine, and focused on the improvement of appetite and other symptoms of cancer treatment (Deadman, al-Khafaji, & Baker, 2007; Strittmatter, 2003, 2011; Xinnong, 1999). Acupuncture points were categorized into two major sets: primary and secondary. Primary acupuncture points were the same for all participants and aimed to increase appetite and improve GI functioning. Secondary acupuncture points consisted of several sets of predetermined points that aimed to relieve common symptoms of patients with GI cancer. These secondary sets were used to manage the secondary nutritional impact symptoms (Blum et al., 2010), such as fatigue, depression, pain, dry mouth, nausea or vomiting, constipation, and diarrhea, that were specific to each patient. These secondary sets were applied on an individual basis according to the patient’s specific symptoms at the time of the intervention session.
A U.S.-trained and licensed acupuncturist with several years of experience as an independent clinician in the management of cancer-related symptoms and women’s health provided all acupuncture treatments. The acupuncturist administered the study intervention in a private practice setting.
All participants received the same primary acupuncture points, including auricular and body acupuncture points. In addition to the primary acupuncture points, each participant received one set of the predetermined secondary acupuncture points to address any specific symptoms that the participants described during the study that affected weight loss and appetite. For example, if nausea and vomiting and appetite loss were main symptoms of the previous week, participants were treated for the management of nausea and vomiting in addition to the primary acupuncture points for appetite.
Instruments
The visual analog scale was used to evaluate appetite using a 10 cm analog scale ranging from poor appetite to good appetite (O’Gorman, McMillan, & McArdle, 2000; Raben, Tagliabue, & Astrup, 1995).
The four-item Simplified Nutritional Appetite Questionnaire (SNAQ) (Wilson et al., 2005) was derived from the eight-item Council on Nutrition Appetite Questionnaire. Sensitivity and specificity in predicting weight loss among community-dwelling patients were evaluated, and reliability of SNAQ was in an acceptable range. One example of a four-item SNAQ question was “My appetite is (a) very poor, (b) poor, (c) average, (d) good, (e) very good.” The results (range = 4–20) were then tallied based on the numerical scales (a = 1, b = 2, c = 3, d = 4, e = 5).
Body composition was measured with multifrequency bioelectrical impedance analysis (MF-BIA) using the ImpediMed Imp™ SFB7, which had a single-channel tetra polar configuration with a touch screen. The SFB7 is an objective tool that evaluates total body water, fat mass, fat free mass, intracellular water, extracellular water, body mass index, lean body mass, and phase angle (Barbosa-Silva & Barros, 2005; Buchholz, Bartok, & Schoeller, 2004; Gupta et al., 2004; Norman et al., 2010). The SFB7 is a reproducible, noninvasive, easy-to-use tool that assesses nutritional status and changes in body composition, and it has been validated in various populations, including patients with cancer (Gupta et al., 2004). BIA was measured every two weeks at the acupuncturist’s office.
Karnofsky Performance Status (Mor, Laliberte, Morris, & Weimann, 1984; O’Gorman et al., 1999) measures functional ability of patients using an 11-point numerical scale in percentages with a 10% increment for each division. A lower Karnofsky Performance Status score indicates less functional ability (0% = dead; 100% = normal functioning). Reliability (0.89–0.97 in patients with cancer) and validity have been well established in various populations.
Demographic information (e.g., age, gender, education, marital status), medical history, and current treatment with medication use, including over-the-counter (OTC) drugs and herbal supplements, were collected.
Procedures
After institutional review board approval, participants were recruited by placing flyers at the Cancer Center of North Florida Regional Healthcare and Florida Cancer Specialists and Research Institute, both in Gainesville. The cancer care coordinator screened the potential participants, and research assistants contacted them to validate eligibility and ensure their willingness to participate in the study. If potential participants were eligible and agreed to participate, then an initial visit was scheduled at the acupuncturist’s office. During the first visit, participants were asked to sign the informed consent, and baseline information was collected. Participants were then provided with individualized schedules for the eight sessions of acupuncture. The schedules were arranged by the acupuncturist and research assistants according to patient availability and chemotherapy treatments. Throughout the intervention sessions, research assistants contacted the participants to remind them of upcoming appointments, met the participants in the acupuncturist’s office to collect data, and were consistently available to respond to questions. Outcome measures, including weight and appetite, were collected as scheduled. Predetermined acupuncture points were used and documented by the acupuncturist at each visit.
Data Analysis
Because this was a feasibility study, a sample size was not calculated. Instead, descriptive statistics (e.g., mean, percentages, frequencies) were used.
Results
A convenience sample of seven participants (four females and three males) enrolled and completed the study. No participants withdrew from the study, and no deaths occurred during the study period. Each participant completed all eight sessions of the acupuncture intervention (see Table 1).
All seven participants completed the study, and none missed the treatment sessions during the eight-session intervention period. Therefore, the intervention was feasible for chronically ill patients. In addition, no participants reported any side effects or adverse reactions from receiving the acupuncture intervention.
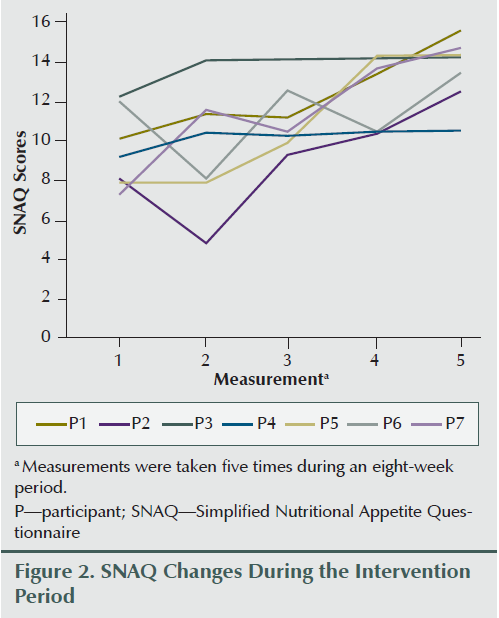
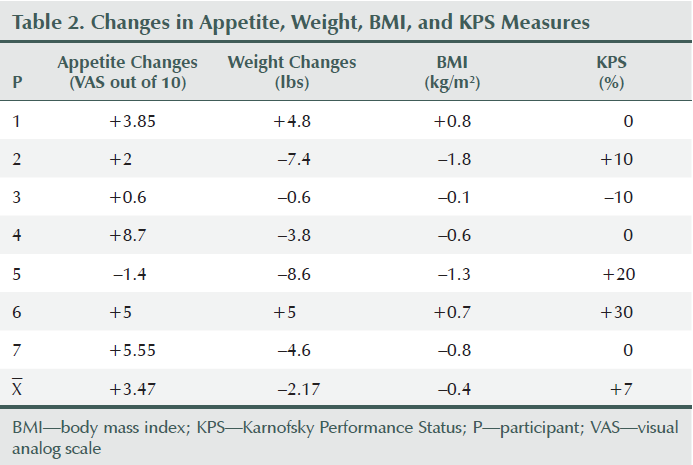
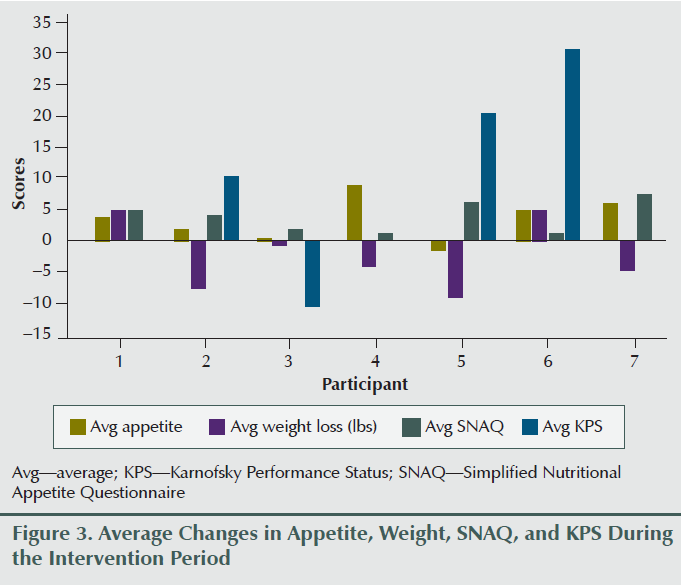
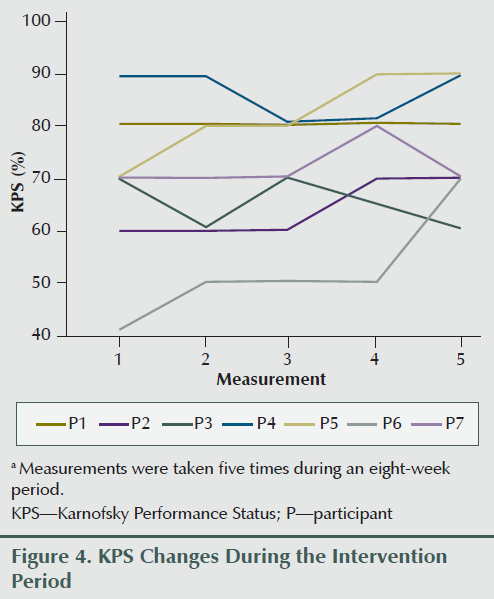
Two participants gained weight (2.65% and 3.62% of baseline body weight), two lost more than 5% of their baseline body weight (6.7% and 5.5%), and others lost less than 5% of their weight during the eight weeks of the study period (0.2%, 1.9%, and 3.8%). Overall weight decreased by an average of 2.17 lbs (1.3%) during the study period; however, average appetite and SNAQ scores improved (3.04 and 4.14, respectively) (see Figure 2) upon completion of the intervention compared to the preintervention report (see Table 2 and Figure 3). Karnofsky Performance Status was stable and did not decline during eight sessions of treatment. Average change of Karnofsky Performance Status score was an increase of 7.13 points (within 10-point increments). Six of seven participants maintained or improved their Karnofsky Performance Status score (see Figure 4). The BIA results have been published separately (Grundmann, Yoon, & Williams, 2015).
With regard to medication (e.g., prescription, OTC, herbal or dietary supplement) use, it is important to note that a total of 36 prescription medications, eight OTC medications, and three herbal or dietary supplements were reported by the seven participants. All but one participant reported use of at least one opioid analgesic (e.g., oxycodone with acetaminophen, oxycodone) or muscle relaxant (e.g., cyclobenzaprine, skelaxin). An average of five prescription medications and one OTC medication were used among the seven participants. One participant reported receiving three chemotherapy drugs during the questionnaire evaluation; however, these three chemotherapy drugs were excluded when prescription drugs were counted because no other participants included their chemotherapy drugs in the questionnaire. Only one participant reported use of all three dietary supplements, and the other six participants did not report any supplement use. According to the self-reported medications provided by participants, there was no indication of MA, dronabinol, or any other types of appetite stimulant being prescribed.
Participants were also asked about their expectations of the study. All participants were very optimistic about the acupuncture intervention, and expected outcomes, which were noted prior to the intervention, included the following: “[It may be] easier to swallow and eat,” “I like holistic medicine,” “I hope it helps with the pain and appetite,” “[I would like] comfort and reduction of symptoms due to chemotherapy,” “[I hope to acquire a better appetite, gain weight, [and] feel better,” “I expect to increase [my] energy and appetite,” “[I want to] maintain weight,” and “[I want to] improve appetite and minimize nausea and vomiting.” These answers indicated that the participants were positive toward the acupuncture intervention and were ready to try complementary interventions for symptom reduction.
Discussion
To the authors’ knowledge, this feasibility study is the first to investigate the potential use of acupuncture for appetite improvement in patients with GI tract cancer, and results indicated that acupuncture was well accepted and favorably perceived. The acupuncture intervention was designed based on the STRICTA guidelines (MacPherson et al., 2002), which means it can be accurately interpreted and easily replicated in future studies. Results indicated that the intervention successfully increased appetite in participants. Despite the fact that participants had an average weight loss of 1.3%, this was not considered clinically significant and may have been because of the choice of acupuncture points; in addition, two participants presented with greater weight loss. It is possible that these participants did not respond well to the intervention because the acupuncture points focused on appetite improvement instead of inflammation reduction, which would have accounted for tumor-mediated metabolic changes in cancer-related cachexia (Fearon, Arends, & Baracos, 2013; Fearon et al., 2006; O’Gorman et al., 1999; Tisdale, 2009). It is also likely that weight loss would have been greater if appetite had not improved. Lack of appetite is a major risk factor for anorexia and the eventual development of cachexia in patients receiving chemotherapy for GI cancers. Therefore, these results demonstrate that acupuncture may be considered a promising symptom management modality for patients with cancer undergoing treatment, and further studies should be conducted to validate these findings.
Other studies have supported these results, such as a study of patients with lung cancer (Kasymjanova et al., 2013) that found that an acupuncture intervention was safe, feasible, and tolerable, and resulted in statistically significant improvement in appetite. That study reported seven sessions as the median number of acupuncture sessions (range = 4–13), and the current study used eight sessions consistently for each participant. Similarly, Dean-Clower et al. (2010) used eight weeks of an acupuncture intervention with a total of 12 sessions for palliative purpose in patients with advanced cancer and found improvements in anxiety, fatigue, pain, and depression. Overall, results of both studies indicated positive outcomes but differed in the number of acupuncture sessions provided.
Of note, the list of medications provided by the participants did not indicate prescriptions for MA or dronabinol in spite of their weight loss, but it is common to observe the prescription of MA for patients with cancer who are in the hospital (Yeh & Schuster, 2006). It was not clear whether these participants had ever been prescribed MA or dronabinol because this question was not asked during the study. Because of the nature of a feasibility study with a small sample size, it was not possible to confirm or validate the prescription practices for MA among healthcare providers, this will be accomplished in follow-up studies in the future.
Limitations
In addition to the many strengths of the study, there were also several limitations. First, this study was limited by a small sample size and lack of control. Second, the study team used a rigorous screening process and acquired general knowledge that cancer was advanced but did not collect participants’ cancer stages. Despite these limitations, the authors’ findings still reveal that acupuncture is a worthy treatment modality that is currently underused, particularly in conjunction with chemotherapy, hospice, or palliative care (Standish, Kozak, & Congdon, 2008). This may be because of the limited number of effectiveness studies for specific symptoms and out-of-pocket expenses related to limited coverage by insurance companies. Therefore, more rigorous studies are needed to validate effectiveness of acupuncture and implement them into evidence-based practice, which may further affect insurance coverage and policy changes. Future studies should explore the management of cancer-related cachexia with acupuncture, which is a promising way to help improve quality of life and extend overall survival in patients with GI cancers.
[[{"type":"media","view_mode":"media_original","fid":"19566","attributes":{"alt":"","class":"media-image","height":"235","typeof":"foaf:Image","width":"364"}}]]
Implication for Nursing Practice and Conclusions
Cancer-related anorexia and cachexia should be assessed regardless of the patient-care settings (e.g., inpatients, outpatients, community settings) to implement early interventions for patients who are at high risk of unintentional weight loss. For patients with poor oral intake, nurses can provide information about safety and feasibility of using acupuncture if they are asked about complementary treatment options. Further research is warranted to replicate this study with a larger sample size, including a control group to confirm the initial positive findings of this feasibility study. More studies are needed to determine the intervention dosages (e.g., frequency, number of sessions) that maximize the benefit of acupuncture. In this study, weekly sessions were well tolerated and led to improvements in patients with GI cancers.
References
Aapro, M., Arends, J., Bozzetti, F., Fearon, K., Grunberg, S.M., Herrstedt, J., . . . Strasser, F. (2014). Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Annals of Oncology, 25, 1492–1499. doi:10.1093/annonc/mdu085
Ahsin, S., Saleem, S., Bhatti, A.M., Iles, R.K., & Aslam, M. (2009). Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. Pain, 147, 60–66. doi:10.1016/j.pain.2009.08.004
Andreyev, H.J., Norman, A.R., Oates, J., & Cunningham, D. (1998). Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? European Journal of Cancer, 34, 503–509. doi:10.1016/S0959-8049(97)10090-9
Barbosa-Silva, M.C., & Barros, A.J. (2005). Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Current Opinion in Clinical Nutrition and Metabolic Care, 8, 311–317. doi:10.1097/01.mco.0000165011.69943.39
Blum, D., Omlin, A., Fearon, K., Baracos, V., Radbruch, L., Kaasa, S., & Strasser, F. (2010). Evolving classification systems for cancer cachexia: Ready for clinical practice? Supportive Care in Cancer, 18, 273–279. doi:10.1007/s00520-009-0800-6
Bosaeus, I. (2008). Nutritional support in multimodal therapy for cancer cachexia. Supportive Care in Cancer, 16, 447–451. doi:10.1007/s00520-007-0388-7
Buchholz, A.C., Bartok, C., & Schoeller, D.A. (2004). The validity of bioelectrical impedance models in clinical populations. Nutrition in Clinical Practice, 19, 433–446. doi:10.1177/0115426504019005433
Burden, S.T., Hill, J. Shaffer, J.L., & Todd, C. (2010). Nutritional status of preoperative colorectal cancer patients. Journal of Human Nutrition and Dietetics, 23, 402–407. doi:10.1111/j.1365-277X.2010.01070.x
Collins, K.B., & Thomas, D.J. (2004). Acupuncture and acupressure for the management of chemotherapy-induced nausea and vomiting. Journal of the American Academy of Nurse Practitioners, 16(2), 76–80. doi:10.1111/j.1745-7599.2004.tb00376.x
Deadman, P., al-Khafaji, M., & Baker, K. (2007). A manual of acupuncture (2nd ed.). London, England: Journal of Chinese Medicine Publications.
Dean-Clower, E., Doherty-Gilman, A.M., Keshaviah, A., Baker, F., Kaw, C., Lu, W., . . . Rosenthal, D.S. (2010). Acupuncture as palliative therapy for physical symptoms and quality of life for advanced cancer patients. Integrative Cancer Therapies, 9, 158–167. doi:10.1177/1534735409360666
Deng, G., Vickers, A., Simon Yeung, K., & Cassileth, B.R. (2006). Acupuncture: Integration into cancer care. Journal of the Society for Integrative Oncology, 4, 86–92.
Dewys, W.D., Begg, C., Lavin, P.T., Band, P.R., Bennett, J.M., Bertino, J.R., . . . Tormey, D.C. (1980). Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. American Journal of Medicine, 69, 491–497. doi:10.1016/S0149-2918(05)80001-3
Fearon, K., Arends, J., & Baracos, V. (2013). Understanding the mechanisms and treatment options in cancer cachexia. Nature Reviews. Clinical Oncology, 10, 90–99.
Fearon, K.C., Strasser, F., Anker, S.D., Bosaeus, I., Bruera, E., Fainsinger, R.L., . . . Baracos, V.E. (2011). Definition and classification of cancer cachexia: An international consensus. Lancet Oncology, 12, 489–495. doi:10.1016/S1470-2045(10)70218-7
Fearon, K.C., Voss, A.C., & Hustead, D.S. (2006). Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. American Journal of Clinical Nutrition, 83, 1345–1350.
Graca Pereira, M., Figueiredo, A.P., & Fincham, F.D. (2012). Anxiety, depression, traumatic stress and quality of life in colorectal cancer after different treatments: A study with Portuguese patients and their partners. European Journal of Oncology Nursing, 16, 227–232. doi:10.1016/j.ejon.2011.06.006
Grundmann, O., Yoon, S.L., & Williams, J.J. (2015). Influence of acupuncture on bioelectrical impedance measures in patients with gastrointestinal cancer: Results of a pilot study. Acupuncture in Medicine, 33, 16–22. doi:10.1136/acupmed-2014-010667
Gupta, D., Lammersfeld, C.A., Burrows, J.L., Dahlk, S.L., Vashi, P.G., Grutsch, J., . . . Lis, C.G. (2004). Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. American Journal of Clinical Nutrition, 80, 1634–1638.
Jatoi, A., Rowland, K., Loprinzi, C.L., Sloan, J.A., Dakhil, S.R., MacDonald, N., . . . Christensen, B. (2004). An eicosapentaenoic acid supplement versus megesterol acetate versus both for patients with cancer-associated wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. Journal of Clinical Oncology, 22, 2469–2476. doi:10.1200/JCO.2004.06.024
Jatoi, A., Windschitl, H.E., Loprinzi, C.L., Sloan, J.A., Dakhil, S.R., Mailliard, J.A., . . . Christensen, B. (2002). Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. Journal of Clinical Oncology, 20, 567–573. doi:10.1200/JCO.20.2.567
Kasymjanova, G., Grossman, M., Tran, T., Jagoe, R.T., Cohen, V., Pepe, C., . . . Agulnik, J. (2013). The potential role for acupuncture in treating symptoms in patients with lung cancer: An observational longitudinal study. Current Oncology, 20, 152–157. doi:10.3747/co.20.1312
MacPherson, H., Altman, D.G., Hammerschlag, R., Youping, L., Taixiang, W., White, A., & Moher, D. (2010). Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT dtatement. Journal of Evidence-Based Medicine, 3, 140–155. doi:10.1111/j.1756-5391.2010.01086.x
MacPherson, H., White, A., Cummings, M., Jobst, K.A., Rose, K., & Niemtzow, R.C. (2002). Standards for Reporting Interventions in Controlled Trials of Acupuncture: The STRICTA recommendations. Acupuncture in Medicine, 20, 22–25. doi:10.1136/aim.20.1.22
Mantovani, G., & Madeddu, C. (2010). Cancer cachexia: Medical management. Supportive Care in Cancer, 18, 1–9.
Mantovani, G., Madeddu, C., & Macciò, A. (2013). Drugs in development for treatment of patients with cancer-related anorexia and cachexia syndrome. Drug Design, Development and Therapy, 7, 645–656.
Mor, V., Laliberte, L., Morris, J.N., & Weimann, M. (1984). The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer, 53, 2002–2007.
Norman, K., Stobäus, N., Zocher, D., Bosy-Westphal, A., Szramek, A., Scheufele, R., . . . Pirlich, M. (2010). Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. American Journal of Clinical Nutrition, 92, 612–619. doi:10.3945/ajcn.2010.29215
O’Gorman, P., McMillan, D.C., & McArdle, C.S. (1999). Longitudinal study of weight, appetite, performance status, and inflammation in advanced gastrointestinal cancer. Nutrition and Cancer, 35, 127–129. doi:10.1207/S15327914NC352_5
O’Gorman, P., McMillan, D.C., & McArdle, C.S. (2000). Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutrition and Cancer, 37, 36–40. doi:10.1207/S15327914NC3701_4
Paccagnella, A., Morassutti, I., & Rosti, G. (2011). Nutritional intervention for improving treatment tolerance in cancer patients. Current Opinion in Oncology, 23, 322–330. doi:10.1097/CCO.0b013e3283479c66
Raben, A., Tagliabue, A., & Astrup, A. (1995). The reproducibility of subjective appetite scores. British Journal of Nutrition, 73, 517–530. doi:10.1079/BJN19950056
Shi, H.F., Xu, B., Guo, X.C., Qiu, X.W., Zhang, Y.P., & Ding, X.J. (2010). Effect of Gan-Pi regulatory needling in treating chloasma. Chinese Journal of Integrative Medicine, 16, 66–70.
Smith, J.L., Malinauskas, B.M., Garner, K.J., & Barber-Heidal, K. (2008). Factors contributing to weight loss, nutrition-related concerns and advice received by adults undergoing cancer treatment. Advances in Medical Sciences, 53, 198–204. doi:10.2478/v10039-008-0019-7
Standish, L.J., Kozak, L., & Congdon, S. (2008). Acupuncture is underutilized in hospice and palliative medicine. American Journal of Hospice and Palliative Care, 25, 298–308. doi:10.1177/1049909108315916
Strittmatter, B. (2003). Identifying and treating blockages to healing: New approaches to therapy-resistant patients. New York, NY: Thieme.
Strittmatter, B. (2011). Ear acupuncture: A precise pocket atlas based on the works of Nogier/Bahr (2nd ed.). New York, NY: Thieme.
Tan, B.H., & Fearon, K.C. (2008). Cachexia: Prevalence and impact in medicine. Current Opinion in Clinical Nutrition and Metabolic Care, 11, 400–407.
Tisdale, M.J. (2002). Cachexia in cancer patients. Nature Reviews. Cancer, 2, 862–871.
Tisdale, M.J. (2009). Mechanisms of cancer cachexia. Physiological Reviews, 89, 381–410. doi:10.1152/physrev.00016.2008
Tuca, A., Jimenez-Fonseca, P., & Gascón, P. (2013). Clinical evaluation and optimal management of cancer cachexia. Critical Reviews in Oncology/Hematology, 88, 625–636.
Wilson, M.M., Thomas, D.R., Rubenstein, L.Z., Chibnall, J.T., Anderson, S., Baxi, A., . . . Morley, J.E. (2005). Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. American Journal of Clinical Nutrition, 82, 1074–1081.
Xinnong, C. (1999). Chinese acupuncture and moxibustion. Beijing, China: Foreign Languages Press.
Yeh, S.S., & Schuster, M.W. (2006). Megestrol acetate in cachexia and anorexia. International Journal of Nanomedicine, 1, 411–416. doi:10.2147/nano.2006.1.4.411
About the Author(s)
Saunjoo L. Yoon, PhD, RN, is an associate professor in the Department of Adult and Elderly Nursing and Oliver Grundmann, PhD, is a clinical associate professor in the Department of Adult and Elderly Nursing and the Department of Medicinal Chemistry, both at the University of Florida; Joseph J. Williams, AP, DOM, is an acupuncture physician at Sunshine Integrative Health; and Gwen Carriere, ARNP, MSN, was, at the time of writing, a cancer program coordinator at North Florida Regional Medical Center, all in Gainesville, FL. This study was funded, in part, by the University of Florida Research Professorship Award to Yoon. Yoon can be reached at yoon@ufl.edu, with copy to editor at ONFEditor@ons.org. (Submitted July 2014. Accepted for publication September 14, 2014.)

