Glycosylated Hemoglobin A1c and Lack of Association With Symptom Severity in Patients Undergoing Chemotherapy for Solid Tumors
Purpose/Objectives: To assess the effects of high blood sugar at the levels of diabetic or prediabetic states during cancer treatment because patients undergoing chemotherapy (CTX) experience multiple symptoms that vary among individuals and may be affected by glucose levels.
Design: Descriptive, cross-sectional.
Setting: Two comprehensive cancer centers, one Veterans Affairs hospital, and four community-based oncology programs.
Sample: 244 outpatients with breast, gastrointestinal, gynecologic, and lung cancers.
Methods: Patients completed demographic and symptom questionnaires. Glycosylated hemoglobin A1c (HbA1c) was evaluated to determine diabetic state. Descriptive statistics and one-way analyses of variance were used in the analyses.
Main Research Variables: HbA1c, symptom severity scores, patient and clinical characteristics (e.g., age, gender, comorbidities, sociodemographic information, body mass index [BMI], lifestyle factors).
Findings: HbA1c results showed 9% of the sample in the diabetic and 26% in the prediabetic state. Patients in the diabetic state reported a higher number of comorbid conditions and were more likely to be African American. Patients in the prediabetic state were older aged. Patients in the diabetic and prediabetic states had a higher BMI compared to nondiabetic patients. No differences in symptom severity or quality-of-life (QOL) scores were found among the three diabetic states.
Conclusions: This study is the first to evaluate for associations between diabetic states and symptom severity and QOL scores in patients receiving CTX. This study confirmed that older age, as well as having higher BMI and having multiple comorbidities, were associated with increased mean glycemic levels.
Implications for Nursing: Clinicians should assess and identify patients with diabetes or prediabetes undergoing treatment for cancer. Patients who are older aged, those with a high BMI, and those with multiple comorbid conditions may be at increased risk for higher glycemic states.
Jump to a section
The American Cancer Society and American Diabetes Association (ADA) issued a joint statement in 2010 that provided evidence that diabetes was a risk factor for cancer (Giovannucci et al., 2010). Compared to patients without diabetes, patients with diabetes are at greater risk for pancreatic (Huxley, Ansary-Moghaddam, Berrington de Gonzàlez, Barzi, & Woodward, 2005), hepatocellular (El-Serag, Hampel, & Javadi, 2006), breast (Boyle et al., 2012), ovarian (Lee et al., 2013; Shah et al., 2014), endometrial (Zhang, Su, Hao, & Sun, 2013), kidney (Larsson & Wolk, 2011), colorectal (Luo, Cao, Liao, & Gao, 2012), gastric (Yoon, Son, Eom, Durrance, & Park, 2013), thyroid (Schmid, Behrens, Jochem, Keimling, & Leitzmann, 2013), and bladder (Xu et al., 2013) cancers, as well as hematologic malignancies (e.g., non-Hodgkin lymphoma, leukemia, myeloma) (Castillo, Mull, Reagan, Nemr, & Mitri, 2012). About 18% of patients diagnosed with cancer have preexisting diabetes (Barone et al., 2008) compared to only 11% of the general population (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], 2015).
Hyperglycemia is the hallmark sign of diabetes, and having preexisting diabetes further increases the risk for hyperglycemic events while undergoing treatments for cancer. In addition, hyperglycemia can occur in patients with cancer independent of a diabetic history. Older age (Kezerle, Shalev, & Barski, 2014), higher body mass index (BMI) (Roumen, Blaak, & Corpeleijn, 2009), nutritional imbalances (Butler, Btaiche, & Alaniz, 2005; Jenkins et al., 2002; Martin-Salces, de Paz, Canales, Mesejo, & Hernandez-Navarro, 2008), lower levels of physical activity (Katz, 2007; Moien-Afshari et al., 2008), higher stress levels (Butler et al., 2005; Godbout & Glaser, 2006), administration of glucocorticoids (Butler et al., 2005; Mazali, Lalli, Alves-Filho, & Mazzali, 2008; Willi et al., 2002), some chemotherapy (CTX) regimens (Mazali et al., 2008; Ramos-Cebrián, Torregrosa, Gutiérrez-Dalmau, Oppenheimer, & Campistol, 2007; Willi et al., 2002), and infections (Turina, Miller, Tucker, & Polk, 2006) can contribute to hyperglycemic episodes during cancer treatment. In turn, hyperglycemia can increase patients’ risk for infection and non–cancer-related mortality (Fuji et al., 2007; Hammer et al., 2009).
Although the association between diabetes and increased risk for cancer is well established, the underlying mechanisms for this association are not well understood. The increased inflammatory responses associated with diabetes may contribute to the development of cancer. For example, hyperglycemia stimulates increased levels of cytosolic calcium, which induce mitochondrial fragmentation and increased levels of reactive oxygen species (ROS) (Yu, Jhun, & Yoon, 2011). These increased levels of ROS result in oxidative stress, which alters the ability of innate immune cells to detect and destroy aberrant cells. This lack of recognition of aberrant cells may lead to the development of cancer (Mantovani, Allavena, Sica, & Balkwill, 2008; Pickup, 2004).
Investigations of the clinical outcomes of hyperglycemic-associated inflammation are lacking. Only 15 studies have investigated the effects of glycemic status during cancer treatment on a variety of patient outcomes (Ali et al., 2007; Bhatnagar et al., 2014; Brunello, Kapoor, & Extermann, 2011; Derr, Hsiao, & Saudek, 2008; Derr et al., 2009; Fuji et al., 2007, 2009; Garg, Bhutani, Alyea, & Pendergrass, 2007; Hammer et al., 2009; Hardy, Nowacki, Bertin, & Weil, 2010; Kondo, Kondo, & Kondo, 2013; Lu et al., 2014; Sheean, Freels, Helton, & Braunschweig, 2006; Srokowski, Fang, Hortobagyi, & Giordano, 2009; Weiser et al., 2004). Glycemic status is categorized as nondiabetic (fasting blood glucose [FBG] of less than 100 mg/dl and glycosylated hemoglobin A1c [HbA1c] of less than 5.7%), prediabetic (FBG of 100–125 mg/dl and HbA1c from 5.7%–6.4%), and diabetic (FBG of 126 mg/dl or greater and HbA1c of 6.5% or greater) (ADA, 2014).
Most of the outcomes studies of hyperglycemic status focused on infection and mortality in patients with cancer. Increased risk for infection with hyperglycemia was found in six studies (Ali et al., 2007; Derr et al., 2008; Fuji et al., 2009; Hammer et al., 2009; Weiser et al., 2004), and increased risk for mortality was found in seven studies (Ali et al., 2007; Bhatnagar et al., 2014; Derr et al., 2008, 2009; Hammer et al., 2009; Kondo et al., 2013; Weiser et al., 2004). In one large epidemiologic study that used the Surveillance, Epidemiology, and End Results–Medicare database, patients with cancer and diabetes (21%) had an increased risk for hospitalization because of CTX toxicity (odds ratio [OR] = 1.38; 95% confidence interval [CI] [1.23, 1.45]) and higher all-cause mortality (hazard ratio [HR] = 1.35; 95% CI [1.31, 1.39]) (Srokowski et al., 2009).
In particular, studies in hematopoietic cell trans-plantation (HCT) showed an increased risk for mortality with glucose levels of greater than 150 mg/dl (HR = 2, p = 0.013) (Fuji et al., 2007), an increased risk for bloodstream infection with each 10 mg/dl increase in mean preneutropenic glucose (OR = 1.15, p = 0.01) (Derr et al., 2008), and a decrease in the occurrence of infections with tight glucose control compared to standard glucose control (14% versus 46%, respectively; p < 0.001) (Fuji et al., 2009). Similarly, in a study of 1,175 recipients of allogeneic HCT, HRs of 1.93 for glucose of greater than 200 mg/dl (p = 0.0009) and 2.78 for glucose of greater than 300 mg/dl (p = 0.0004) were found for mortality (Hammer et al., 2009). Increasing levels of blood glucose in patients treated for hematologic malignancies significantly increase the occurrence of infections, as well as mortality.
Although the administration of glucocorticoids as part of CTX regimens can result in patients becoming diabetic (Vigneri, Frasca, Sciacca, Pandini, & Vigneri, 2009), only one study has evaluated patients for the prediabetic state during CTX. In the study, patients receiving CTX for breast cancer were evaluated for prediabetic and diabetic states. Compared to the first CTX cycle (51%), fewer patients were classified as prediabetic in the fifth or sixth cycle (28%). However, the percentage of patients with diabetes increased from 25% at the first cycle to 33% at the fifth cycle (Lu et al., 2014).
Among the studies cited previously, only two have included symptoms. In these studies, the association between the single symptom of peripheral neuropathy and glycemic status was evaluated (Bhatnagar et al., 2014; Weiser et al., 2004). In Bhatnagar et al. (2014), 65% of the patients with breast cancer and diabetes who received a taxane required a dose reduction compared to only 35% of patients without diabetes (p = 0.02). In contrast, Weiser et al. (2004) did not find an association between hyperglycemia and peripheral neuropathy in 278 patients with acute lymphocytic leukemia. However, compared to patients without hyperglycemia, patients with hyperglycemia were at increased risk for infection (8% versus 39%, p = 0.03) and decreased survival (52 months versus 24 months, p = 0.001).
Taken together, these findings suggest that prediabetic and diabetic states are relatively common in patients with cancer. However, no studies were identified that evaluated associations between diabetic states and the severity of common symptoms associated with cancer and its treatments. Therefore, the purposes of this study of a sample of outpatients with cancer who were receiving CTX (N = 244) were to evaluate (a) the occurrence of nondiabetic, prediabetic, and diabetic states and (b) for differences in symptom severity and quality of life (QOL) among patients with these diabetic states.
Methods
This study is part of an ongoing, longitudinal study of the symptom experience of outpatients with cancer receiving CTX. Eligible patients were aged 18 years or older; had a diagnosis of breast, gastrointestinal, gynecologic, or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two comprehensive cancer centers, one Veterans Affairs hospital, and four community-based oncology programs in urban and surrounding areas in California and New York.
Instruments
A demographic questionnaire obtained information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. The Karnofsky Performance Status (KPS) scale is widely used to evaluate functional status in patients with cancer and has well-established validity and reliability (Karnofsky, Abelmann, Craver, & Burchenal, 1948). Using the KPS scale, patients rated functional status from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms) (Karnofsky, 1977; Karnofsky et al., 1948).
The Self-Administered Comorbidity Questionnaire (SCQ) was developed to measure comorbidity and is short and easily understood (Sangha, Stucki, Liang, Fossel, & Katz, 2003). The 13-item instrument lists 13 common medical conditions that have been simplified so that they can be understood without prior medical knowledge. Patients indicate whether they have the symptom or not using a “yes” or “no” format. If they indicate that they have a condition, they are asked whether they have received treatment for it using a “yes” or “no” format with a proxy for disease severity or whether it limited their daily activities using a “yes” or “no” format with an indication of functional limitations. For each condition, patients can receive as many as three points. SCQ scores range from 0–39, with higher scores indicating more severe comorbidity. The SCQ has well-established validity and reliability and has been used in studies of patients with a variety of chronic conditions (Brunner et al., 2008; Cieza et al., 2006).
The 18-item Lee Fatigue Scale (LFS) was designed to assess physical fatigue and energy (Lee, Hicks, & Nino-Murcia, 1991). Each item is rated from 0–10, and total fatigue and energy scores are calculated as the mean of the 13 fatigue items plus the 5 energy items, with higher scores indicating greater fatigue severity and higher levels of energy. Patients are asked to rate each item based on how they feel in the moment, within 30 minutes of awakening (i.e., morning fatigue or morning energy), and prior to going to bed (i.e., evening fatigue or evening energy). Cutoff scores of 3.2 or greater and 5.6 or greater indicate high levels of morning and evening fatigue, respectively (Fletcher et al., 2008). Cutoff scores of 6 or less and 3.5 or less indicate low levels of morning and evening energy, respectively. The LFS was chosen for this study because it is relatively short, easy to administer, and has well-established validity and reliability (Gay, Lee, & Lee, 2004; Lee et al., 1991; Lee, Portillo, & Miramontes, 1999; Miaskowski et al., 2006, 2008; Miaskowski & Lee, 1999). In this study, Cronbach alphas for the evening and morning fatigue scales were 0.95 and 0.96, respectively. Cronbach alphas for the evening and morning energy scales were 0.93 and 0.95, respectively.
The 20-item State-Trait Anxiety Inventories (STAI-T and STAI-S) are rated from 1–4. The scores for each scale are summed and can range from 20–80. Cutoff scores of 31.8 or greater and 32.2 or greater indicate high levels of trait and state anxiety, respectively. The STAI-S and STAI-T inventories have well-established validity and reliability (Bieling, Antony, & Swinson, 1998; Kennedy, Schwab, Morris, & Beldia, 2001; Spielberger, Gorsuch, Suchene, Vagg, & Jacobs, 1983). In the current study, the Cronbach alphas for the STAI-S and STAI-T were 0.96 and 0.92, respectively.
The 20-item Center for Epidemiological Studies–Depression scale (CES-D) consists of items representing the major symptoms for depression. Scores can range from 0–60, with scores of 16 or greater indicating a need for clinical evaluation for major depression. The CES-D has well-established validity and reliability (Carpenter et al., 1998; Radloff, 1977; Sheehan, Fifield, Reisine, & Tennen, 1995). In the current study, the Cronbach alpha for the CES-D total score was 0.89.
The 21-item General Sleep Disturbance Scale (GSDS) was designed to assess quality of sleep in the past week on a scale of 0 (never) to 7 (every day). The GSDS total score is the sum of the seven subscale scores, ranging from 0 (no disturbance) to 147 (extreme disturbance). A GSDS total score of 43 or greater indicates a significant level of sleep disturbance (Fletcher et al., 2008). The GSDS has well-established validity and reliability (Lee, 1992; Lee & DeJoseph, 1992; Miaskowski & Lee, 1999). In the current study, the Cronbach alpha for the GSDS total score was 0.83.
The 16-item Attentional Function Index (AFI) was designed to measure attentional function (Cimprich, Visovatti, & Ronis, 2011). Scores range from 0–10, and a higher mean score indicates greater capacity to direct attention (Cimprich et al., 2011). Scores of less than 5 indicate low attentional function, scores from 5–7.5 indicate moderate attentional function, and scores of greater than 7.5 indicate high attentional function (Cimprich et al., 2005). The AFI has well-established validity and reliability (Cimprich et al., 2011). In the current study, the Cronbach alpha was 0.93.
Occurrence of pain was evaluated using the Brief Pain Inventory (Daut, Cleeland, & Flanery, 1983). Patients who responded “yes” to the question about having pain were asked to indicate whether their pain was related to cancer treatments. Patients were categorized into one of four groups (no pain, only noncancer pain, only cancer pain, or both cancer and noncancer pain). Patients rated the intensity of the pain (now, average, and worst) using a scale ranging from 0 (none) to 10 (excruciating).
The Memorial Symptom Assessment Scale (MSAS) was used to evaluate the occurrence, severity, and distress of 32 symptoms commonly associated with cancer and its treatment. The MSAS is a self-report questionnaire designed to measure the multidimensional experience of symptoms. Patients were asked to indicate whether or not they had experienced each symptom in the past week (symptom occurrence). If they had experienced the symptom, they were asked to rate its severity and distress. In the current article, the total number of MSAS symptoms is reported. The validity and reliability of the MSAS is well established in studies of inpatients and outpatients with cancer (Portenoy et al., 1994).
The QOL Scale–Patient Version (QOL-PV) is a 41-item instrument that measures four dimensions of QOL (physical, psychological, social, and spiritual well-being) in patients with cancer, as well as a total QOL score. Each item is rated on a 0–10 numeric rating scale, with higher scores indicating better QOL. The QOL-PV has established validity and reliability (Ferrell, 1995; Ferrell, Dow, & Grant, 1995; Padilla, Ferrell, Grant, & Rhiner, 1990; Padilla et al., 1983). In the current study, the Cronbach alpha for the QOL-PV total score was 0.92.
The SF-12® consists of 12 questions about physical and mental health, as well as overall health status. The individual items on the SF-12 are evaluated, and the instrument is scored into two components that measure physical component summary (PCS) and mental component summary (MCS) scores, which can range from 0–100. Higher PCS and MCS scores indicate a better QOL. The SF-12 has well-established validity and reliability (Ware, Kosinski, & Keller, 1996).
Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the institutional review board at each of the study sites. Eligible patients were approached by a research staff member in the infusion unit to discuss participation in the study. Written informed consent was obtained from all patients. Patients completed study questionnaires in their homes for a total of six times during two cycles of CTX (i.e., prior to CTX administration and recovery from previous cycle). The timing per patient varied by their cycle length; about one week after CTX administration related to acute symptoms, and about two weeks after CTX administration related to potential nadir. For the current article, symptom severity scores from the enrollment booklet that asked patients to report on their symptom experience for the week prior to the administration of the next cycle of CTX were analyzed. Medical records were reviewed for disease and treatment information.
Blood glucose was assessed during the same clinical visit when the enrollment booklets were administered. HbA1c provides a 2–3 month assessment of an individual’s blood glucose status (Triplitt, 2010; U.S. Department of Health and Human Services, 2006). HbA1c was measured using one droplet of blood that was processed through the Bio-Rad in2it analyzer (Petersen et al., 2010). Samples were processed within minutes of collection. Control samples were run periodically per guidelines to ensure proper calibration of the instrument. For analyses, HbA1c percentages were used. Per the ADA (2014) guidelines, the authors categorized HbA1c levels of 6.5% or greater as diabetic, 5.7%–6.4% as prediabetic, and less than 5.7% as nondiabetic.
Data Analysis
Data were analyzed using SPSS®, version 22.0. Descriptive statistics and frequency distributions were generated on the sample characteristics. One-way analyses of variance (ANOVAs) were used to evaluate for differences in demographic and clinical characteristics, symptom severity scores, and QOL scores among the three diabetic states (i.e., nondiabetic, prediabetic, and diabetic). Post-hoc contrasts were done using the Bonferroni procedure to control the overall family alpha level. Differences among the diabetic states were considered statistically significant at the p < 0.05 level.
Findings
Of the 244 patients in the study, 16% self-reported having diabetes on the SCQ. However, based on the HbA1c measurements, 65% (n = 159) of the patients were nondiabetic, 26% (n = 64) were prediabetic, and 9% (n = 21) were diabetic.
As summarized in Table 1, a number of demographic and clinical characteristics differed among the diabetic states. Compared to the nondiabetic and prediabetic states, patients in the diabetic state had a higher SCQ score and were more likely to be African American compared to the other three ethnic groups. Compared to the nondiabetic state, patients in the prediabetic state were significantly older. Patients in the diabetic state reported a higher number of comorbid conditions and high blood pressure than nondiabetic patients. In addition, patients in the prediabetic and diabetic states had a higher BMI than patients in the nondiabetic state. The self-reported occurrence of diabetes was in the expected direction, with the fewest reporting being nondiabetic and the most reporting diabetic. 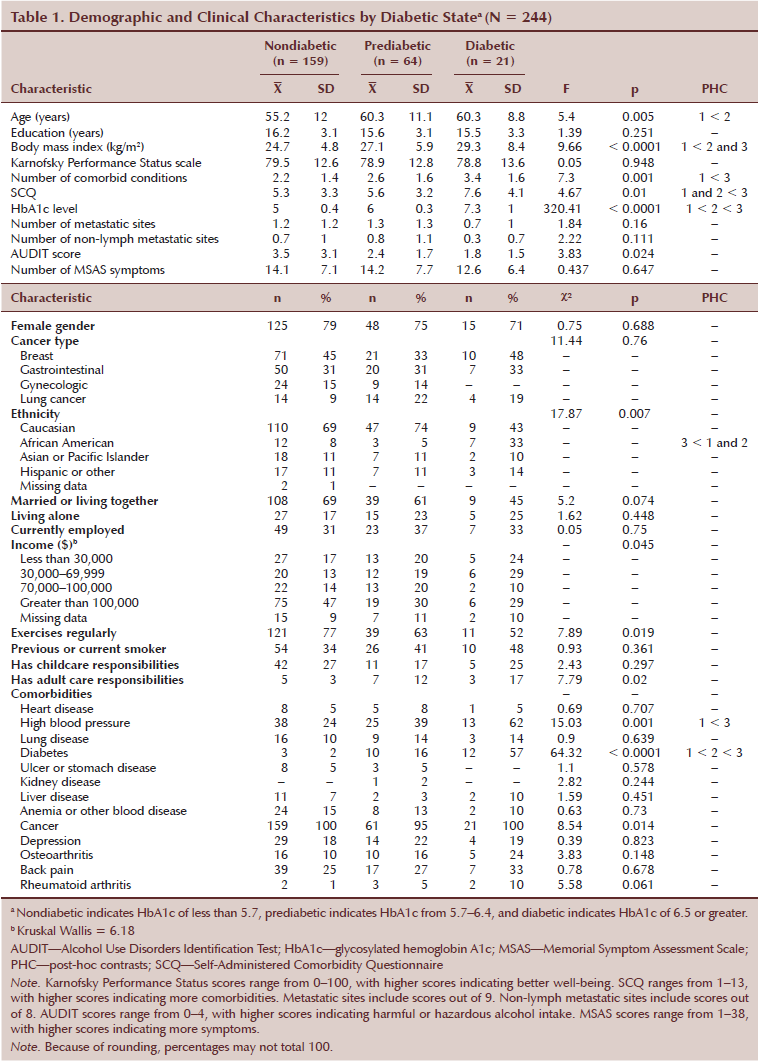
As shown in Table 2, no differences in any of the symptom severity scores were found among the three diabetic states. All of the patients reported clinically meaningful levels of state and trait anxiety, as well as sleep disturbance, and they reported low levels of morning energy. Patients in the prediabetic and diabetic states reported clinically meaningful levels of morning fatigue. Patients in the diabetic state reported clinically meaningful levels of evening fatigue.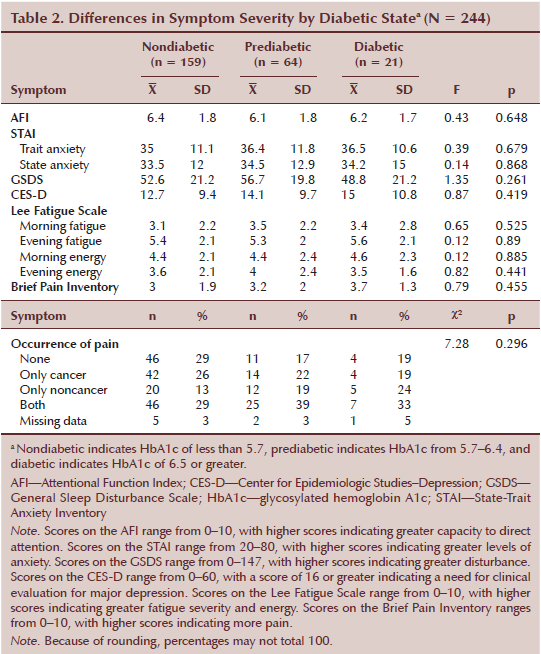
No differences in the SF-12 subscale and PCS and MCS scores were found among the three diabetic state groups. No differences in MQOLS-PV subscale and total scores were found among the three diabetic states.
Discussion
This study is the first to evaluate the occurrence of diabetes and the association between diabetic states and symptom severity and QOL scores in patients receiving CTX. Among the diabetic states, 63% of the patients were nondiabetic, 26% were prediabetic, and 9% were diabetic. These percentages are lower than previous studies, which found that 44% of patients with cancer had prediabetes, and from 19% (Bhatnagar et al., 2014) to 22% (Ji et al., 2013) of patients had diabetes. Reasons for these differences are not readily apparent and warrant additional investigation.
In terms of demographic and clinical characteristics, compared to the nondiabetic state, patients in the diabetic state had a higher BMI, reported a higher number of comorbidities, had a higher SCQ score, were more likely to be African American, and self-reported a higher occurrence of hypertension. However, no differences in symptom severity and QOL scores were found among the three diabetic states. Of note, no differences were found among the diabetic states in terms of cancer diagnoses or number of metastatic sites.
Older age is associated with higher glucose levels (Kezerle et al., 2014). Twenty-seven percent of individuals aged 65 years or older have diabetes (NIDDK, 2015). In older adults, increases in blood sugar may be caused by the normal process of cellular senescence (Campisi & d’Adda di Fagagna, 2007; Campisi & Yaswen, 2009) and related increases in plasma hypertonicity, which result in increased insulin resistance and impaired glucose use (Stookey, Pieper, & Cohen, 2004). Although the current age-related findings are consistent with the general population, other studies of patients with cancer failed to find an association between age and diabetic state (Bhatnagar et al., 2014; Shah et al., 2014).
Higher BMI increases the risk for hyperglycemia (Giovannucci et al., 2010; Makki, Froguel, & Wolowczuk, 2013; Meigs, Hu, Rifai, & Manson, 2004; Roumen et al., 2009). A higher BMI increases insulin resistance (Giovannucci et al., 2010; Makki et al., 2013) and, in obese individuals, induces metabolic inflammation, which leads to impaired glucose metabolism (Makki et al., 2013). Findings from the current study are consistent with previous reports in that the nondiabetic patients had a BMI of 24.7 kg/m2, which borders on being overweight. Patients in the prediabetic range had a mean BMI of 27.1 kg/m2, which is considered overweight, and those in the diabetic range had a mean BMI of 29.3 kg/m2, which borders on obesity (ADA, 2014).
In addition, a higher BMI is associated with increased risk for comorbidities. In general, obese individuals have higher rates of cancer, diabetes, and cardiovascular disease (Ligibel et al., 2014). In this study, patients in the diabetic state had a higher number of comorbidities and a higher SCQ score. The most common comorbidities in this group were hypertension (n = 13), self-reported diabetes (n = 12), back pain (n = 7), and osteoarthritis (n = 5).
The primary goal of this study was to evaluate associations between HbA1c levels and symptom severity. Because hyperglycemia induces systemic inflammation (Esposito et al., 2002) and increased inflammatory responses are associated with higher symptom burden (Bower & Lamkin, 2013; Esposito et al., 2002; Irwin, Olmstead, Ganz, & Hague, 2013), the authors hypothesized that patients with higher HbA1c would report higher symptom severity scores. However, the findings do not support this hypothesis. In a previous study (Miaskowski et al., 2014), no associations were found between any disease characteristic (e.g., cancer diagnosis, presence of metastatic disease) and symptom burden.
In hindsight, the most likely reason for this lack of association is that HbA1c was used as a measure of glycemic status. HbA1c was chosen as the measure for this study to avoid the need for patients to have to fast for a blood glucose determination. HbA1c is a reflection of circulating blood glucose that adheres to hemoglobin. Therefore, HbA1c is a mean level of glucose, which is dependent on the lifespan of the red blood cell (RBC). Various conditions, including cancer, can impair normal RBC production and destruction, yielding a potentially altered HbA1c level (Wright & Hirsch, 2012). In addition, in a study of patients with hematologic malignancies who received allogeneic HCT, glucose variability was more likely to be associated with adverse outcomes than hyperglycemia (Hammer et al., 2009). Although this sample of patients with solid tumors was quite different, glucose fluctuations, which were not captured by the HbA1c measurement, may be associated with patients’ symptom experiences.
Another limitation was that the associations between HbA1c and symptom severity were done at a single time point. Measurements over time of symptom severity scores and HbA1c levels may have shown significant associations. In addition, exercise is an established regulator of blood glucose (Katz, 2007; Moien-Afshari et al., 2008), and more than 70% of patients in the current study reported exercising regularly. This finding may be indicative of the type of individual who was willing to enroll in the study, which impaired the authors’ ability to evaluate patients with cancer who are more sedentary.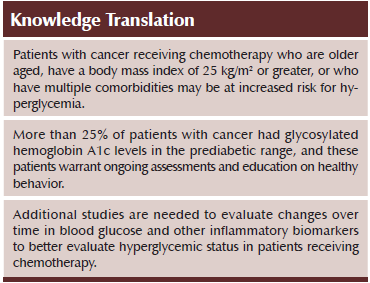
Conclusion
This study confirmed that older age, higher BMI, and having multiple comorbidities are associated with increased mean glycemic levels. This information has important implications for practice. Patients diagnosed with cancer who are older aged, have a BMI of greater than 25 kg/m2, or who have multiple comorbidities should be closely monitored for perturbations in glycemic status, whether or not they have preexisting diabetes. In addition, these patients warrant education about diet and exercise. Oncology nurses should consider whether or not these patients with prediabetes should be referred to a dietitian.
Numerous areas for research warrant consideration. Longitudinal studies are needed to describe fluctuations in blood glucose and other inflammatory biomarkers in patients receiving CTX. Studies that determine modifiable risk factors that contribute to interindividual differences in these fluctuations are warranted to identify patients at higher risk for prediabetic and diabetic states. Future longitudinal studies also need to evaluate the associations between changes in blood glucose and symptom severity in patients with solid tumors.
References
Ali, N.A., O’Brien, J.M., Jr., Blum, W., Byrd, J.C., Klisovic, R.B., Marcucci, G., . . . Grever, M.R. (2007). Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer, 110, 96–102.
American Diabetes Association. (2014). Standards of medical care in diabetes—2014. Diabetes Care, 37(Suppl. 1), S14–S80. doi:10.2337/dc14-S014
Barone, B.B., Yeh, H.C., Snyder, C.F., Peairs, K.S., Stein, K.B., Derr, R.L., . . . Brancati, F.L. (2008). Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA, 300, 2754–2764. doi:10.1001/jama.2008.824
Bhatnagar, B., Gilmore, S., Goloubeva, O., Pelser, C., Medeiros, M., Chumsri, S., . . . Bao, T. (2014). Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single-center experience. SpringerPlus, 3, 366. doi:10.1186/2193-1801-3-366
Bieling, P.J., Antony, M.M., & Swinson, R.P. (1998). The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behaviour Research and Therapy, 36, 777–788.
Bower, J.E., & Lamkin, D.M. (2013). Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, Behavior, and Immunity, 30(Suppl.), S48–S57.
Boyle, P., Boniol, M., Koechlin, A., Robertson, C., Valentini, F., Coppens, K., . . . Autier, P. (2012). Diabetes and breast cancer risk: A meta-analysis. British Journal of Cancer, 107, 1608–1617. doi:10.1038/bjc.2012.414
Brunello, A., Kapoor, R., & Extermann, M. (2011). Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. American Journal of Clinical Oncology, 34, 292–296.
Brunner, F., Bachmann, L.M., Weber, U., Kessels, A.G., Perez, R.S., Marinus, J., & Kissling, R. (2008). Complex regional pain syndrome 1—The Swiss cohort study. BMC Musculoskeletal Disorders, 9, 92. doi:10.1186/1471-2474-9-92
Butler, S.O., Btaiche, I.F., & Alaniz, C. (2005). Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy, 25, 963–976.
Campisi, J., & d’Adda di Fagagna, F. (2007). Cellular senescence: When bad things happen to good cells. Nature Reviews. Molecular Cell Biology, 8, 729–740.
Campisi, J., & Yaswen, P. (2009). Aging and cancer cell biology, 2009. Aging Cell, 8, 221–225.
Carpenter, J.S., Andrykowski, M.A., Wilson, J., Hall, L.A., Rayens, M.K., Sachs, B., & Cunningham, L.L. (1998). Psychometrics for two short forms of the Center for Epidemiologic Studies–Depression Scale. Issues in Mental Health Nursing, 19, 481–494.
Castillo, J.J., Mull, N., Reagan, J.L., Nemr, S., & Mitri, J. (2012). Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: A meta-analysis of observational studies. Blood, 119, 4845–4850. doi:10.1182/blood-2011-06-362830
Cieza, A., Geyh, S., Chatterji, S., Kostanjsek, N., Ustün, B. T., & Stucki, G. (2006). Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Medical Research Methodology, 6, 36. doi:10.1186/1471-2288-6-36
Cimprich, B., Visovatti, M., & Ronis, D. L. (2011). The Attentional Function Index—A self-report cognitive measure. Psycho-Oncology, 20, 194–202. doi:10.1002/pon.1729
Daut, R.L., Cleeland, C.S., & Flanery, R.C. (1983). Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain, 17, 197–210.
Derr, R.L., Hsiao, V.C., & Saudek, C.D. (2008). Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care, 31, 1972–1977.
Derr, R.L., Ye, X., Islas, M.U., Desideri, S., Saudek, C.D., & Grossman, S.A. (2009). Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. Journal of Clinical Oncology, 27, 1082–1086. doi:10.1200/jco.2008.19.1098
El-Serag, H.B., Hampel, H., & Javadi, F. (2006). The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clinical Gastroenterology and Hepatology, 4, 369–380. doi:10.1016/j.cgh.2005.12.007
Esposito, K., Nappo, F., Marfella, R., Giugliano, G., Giugliano, F., Ciotola, M., . . . Giugliano, D. (2002). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation, 106, 2067–2072.
Ferrell, B.R. (1995). The impact of pain on quality of life. A decade of research. Nursing Clinics of North America, 30, 609–624.
Ferrell, B.R., Dow, K.H., & Grant, M. (1995). Measurement of the quality of life in cancer survivors. Quality of Life Research, 4, 523–531.
Fletcher, B.S., Paul, S.M., Dodd, M.J., Schumacher, K., West, C., Cooper, B., . . . Miaskowski, C.A. (2008). Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology, 26, 599–605. doi:10.1200/jco.2007.12.2838
Fuji, S., Kim, S.W., Mori, S., Fukuda, T., Kamiya, S., Yamasaki, S., . . . Takaue, Y. (2007). Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation, 84, 814–820.
Fuji, S., Kim, S.W., Mori, S., Kamiya, S., Yoshimura, K., Yokoyama, H., . . . Fukuda, T. (2009). Intensive glucose control after allogeneic hematopoietic stem cell transplantation: A retrospective matched-cohort study. Bone Marrow Transplantation, 44, 105–111. doi:10.1038/bmt.2008.431
Garg, R., Bhutani, H., Alyea, E., & Pendergrass, M. (2007). Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation. Diabetes Care, 30, 993–994. doi:10.2337/dc06-2563
Gay, C.L., Lee, K.A., & Lee, S.Y. (2004). Sleep patterns and fatigue in new mothers and fathers. Biological Research for Nursing, 5, 311–318. doi:10.1177/1099800403262142.
Giovannucci, E., Harlan, D.M., Archer, M.C., Bergenstal, R.M., Gapstur, S.M., Habel, L.A., . . . Yee, D. (2010). Diabetes and cancer: A consensus report. Diabetes Care, 33, 1674–1685.
Godbout, J.P., & Glaser, R. (2006). Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. Journal of Neuroimmune Pharmacology, 1, 421–427.
Hammer, M.J., Casper, C., Gooley, T.A., O’Donnell, P.V., Boeckh, M., & Hirsch, I.B. (2009). The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation, 15, 344–351.
Hardy, S.J., Nowacki, A.S., Bertin, M., & Weil, R.J. (2010). Absence of an association between glucose levels and surgical site infections in patients undergoing craniotomies for brain tumors. Journal of Neurosurgery, 113, 161–166. doi:10.3171/2010.2.jns09950
Huxley, R., Ansary-Moghaddam, A., Berrington de Gonzàlez, A., Barzi, F., & Woodward, M. (2005). Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. British Journal of Cancer, 92, 2076–2083. doi:10.1038/sj.bjc.6602619
Irwin, M.R., Olmstead, R.E., Ganz, P.A., & Haque, R. (2013). Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior, and Immunity, 30(Suppl.), S58–S67.
Jenkins, D.J., Kendall, C.W., Augustin, L.S., Franceschi, S., Hamidi, M., Marchie, A., . . . Axelsen, M. (2002). Glycemic index: Overview of implications in health and disease. American Journal of Clinical Nutrition, 76, 266S–273S.
Ji, G.Y., Jin, L.B., Wang, R.J., Bai, Y., Yao, Z.X., Lu, L.J., . . . Kong, L.Q. (2013). Incidences of diabetes and prediabetes among female adult breast cancer patients after systemic treatment. Medical Oncology, 30, 687. doi:10.1007/s12032-013-0687-4
Karnofsky, D., Abelmann, W.H., Craver, L.V., & Burchenal, J.H. (1948). The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer, 1, 634–656.
Karnofsky, D.A. (1977). Performance scale. In G.T. Kennealey & M.S. Mitchell (Eds.), Factors that influence the therapy response in cancer: A comprehensive treatise (pp. 97–101). New York, NY: Plenum Press.
Katz, A. (2007). Modulation of glucose transport in skeletal muscle by reactive oxygen species. Journal of Applied Physiology, 102, 1671–1676. doi:10.1152/japplphysiol.01066.2006
Kennedy, B.L., Schwab, J.J., Morris, R.L., & Beldia, G. (2001). Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatric Quarterly, 72, 263–276.
Kezerle, L., Shalev, L., & Barski, L. (2014). Treating the elderly diabetic patient: Special considerations. Journal of Diabetes, Metabolic Syndrome and Obesity, 7, 391–400. doi:10.2147/dmso.s48898
Kondo, S., Kondo, M., & Kondo, A. (2013). Glycemia control using A1C level in terminal cancer patients with preexisting type 2 diabetes. Journal of Palliative Medicine, 16, 790–793. doi:10.1089/jpm.2012.0471
Larsson, S.C., & Wolk, A. (2011). Diabetes mellitus and incidence of kidney cancer: A meta-analysis of cohort studies. Diabetologia, 54, 1013–1018. doi:10.1007/s00125-011-2051-6
Lee, J.Y., Jeon, I., Kim, J.W., Song, Y.S., Yoon, J.M., & Park, S.M. (2013). Diabetes mellitus and ovarian cancer risk: A systematic review and meta-analysis of observational studies. International Journal of Gynecological Caner, 23, 402–412. doi:10.1097/IGC.0b013e31828189b2
Lee, K.A. (1992). Self-reported sleep disturbances in employed women. Sleep, 15, 493–498.
Lee, K.A., & DeJoseph, J.F. (1992). Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth, 19, 208–213.
Lee, K.A., Hicks, G., & Nino-Murcia, G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36, 291–298.
Lee, K.A., Portillo, C.J., & Miramontes, H. (1999). The fatigue experience for women with human immunodeficiency virus. Journal of Obstetrics and Gynecological Neonatal Nursing, 28, 193–200.
Ligibel, J.A., Alfano, C.M., Courneya, K.S., Demark-Wahnefried, W., Burger, R.A., Chlebowski, R.T., . . . Hudis, C.A. (2014). American Society of Clinical Oncology position statement on obesity and cancer. Journal of Clinical Oncology, 32, 3568–3574. doi:10.1200/jco.2014.58.4680
Lu, L.J., Wang, R.J., Ran, L., Gan, L., Bai, Y., Jin, L.B., . . . Kong, L.Q. (2014). On the status and comparison of glucose intolerance in female breast cancer patients at initial diagnosis and during chemotherapy through an oral glucose tolerance test. PLOS One, 9, e93630. doi:10.1371/journal.pone.0093630
Luo, W., Cao, Y., Liao, C., & Gao, F. (2012). Diabetes mellitus and the incidence and mortality of colorectal cancer: A meta-analysis of 24 cohort studies. Colorectal Disease, 14, 1307–1312. doi:10.1111/j.1463-1318.2012.02875.x
Makki, K., Froguel, P., & Wolowczuk, I. (2013). Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflammation, 2013, 139239. doi:10.1155/2013/139239
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454, 436–444.
Martin-Salces, M., de Paz, R., Canales, M.A., Mesejo, A., & Hernandez-Navarro, F. (2008). Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition, 24, 769–775. doi:10.1016/j.nut.2008.02.021
Mazali, F.C., Lalli, C.A., Alves-Filho, G., & Mazzali, M. (2008). Posttransplant diabetes mellitus: Incidence and risk factors. Transplantation Proceedings, 40, 764–766. doi:10.1016/j.transproceed.2008.03.018
Meigs, J.B., Hu, F.B., Rifai, N., & Manson, J.E. (2004). Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA, 291, 1978–1986. doi:10.1001/jama.291.16.1978
Miaskowski, C., Cooper, B.A., Melisko, M., Chen, L.M., Mastick, J., West, C., . . . Aouizerat, B.E. (2014). Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer, 120, 2371–2378. doi:10.1002/cncr.28699
Miaskowski, C., Cooper, B.A., Paul, S.M., Dodd, M., Lee, K., Aouizerat, B.E., . . . Bank, A. (2006). Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis [Online exclusive]. Oncology Nursing Forum, 33, E79–E89. doi:10.1188/06.ONF.E79-E89
Miaskowski, C., & Lee, K.A. (1999). Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. Journal of Pain and Symptom Management, 17, 320–332.
Miaskowski, C., Paul, S.M., Cooper, B.A., Lee, K., Dodd, M., West, C., . . . Wara, W. (2008). Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. Journal of Pain and Symptom Management, 35, 632–643. doi:10.1016/j.jpainsymman.2007.07.007
Moien-Afshari, F., Ghosh, S., Elmi, S., Rahman, M.M., Sallam, N., Khazaei, M., . . . Laher, I. (2008). Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia, 51, 1327–1337. doi:10.1007/s00125-008-0996-x
National Institute of Diabetes and Digestive and Kidney Diseases. (2015). Health statistics: Diabetes. Retrieved from http://www.niddk.nih.gov/health-information/health-statistics/Pages/def…
Padilla, G.V., Ferrell, B., Grant, M.M., & Rhiner, M. (1990). Defining the content domain of quality of life for cancer patients with pain. Cancer Nursing, 13, 108–115.
Padilla, G.V., Presant, C., Grant, M.M., Metter, G., Lipsett, J., & Heide, F. (1983). Quality of life index for patients with cancer. Research in Nursing and Health, 6, 117–126.
Petersen, J.R., Omoruyi, F.O., Mohammad, A.A., Shea, T.J., Okorodudu, A.O., & Ju, H. (2010). Hemoglobin A1c: Assessment of three POC analyzers relative to a central laboratory method. Clinica Chimica Acta, 411, 2062–2066.
Pickup, J.C. (2004). Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care, 27, 813–823.
Portenoy, R.K., Thaler, H.T., Kornblith, A.B., Lepore, J.M., Friedlander-Klar, H., Kiyasu, E., . . . Norton, L. (1994). The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer, 30A, 1326–1336.
Radloff, L.S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401.
Ramos-Cebrián, M., Torregrosa, J.V., Gutiérrez-Dalmau, A., Oppenheimer, F., & Campistol, J.M. (2007). Conversion from tacrolimus to cyclosporine could improve control of posttransplant diabetes mellitus after renal transplantation. Transplantation Proceedings, 39, 2251–2253. doi:10.1016/j.transproceed.2007.06.035
Roumen, C., Blaak, E.E., & Corpeleijn, E. (2009). Lifestyle intervention for prevention of diabetes: Determinants of success for future implementation. Nutrition Reviews, 67, 132–146. doi:10.1111/j.1753-4887.2009.00181.x
Sangha, O., Stucki, G., Liang, M.H., Fossel, A.H., & Katz, J.N. (2003). The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Care and Research, 49, 156–163. doi:10.1002/art.10993
Schmid, D., Behrens, G., Jochem, C., Keimling, M., & Leitzmann, M. (2013). Physical activity, diabetes, and risk of thyroid cancer: A systematic review and meta-analysis. European Journal of Epidemiology, 28, 945–958. doi:10.1007/s10654-013-9865-0
Shah, M.M., Erickson, B.K., Matin, T., McGwin, G., Jr., Martin, J.Y., Daily, L.B., . . . Leath, C.A., III. (2014). Diabetes mellitus and ovarian cancer: More complex than just increasing risk. Gynecologic Oncology, 135, 273–277. doi:10.1016/j.ygyno.2014.09.004
Sheean, P.M., Freels, S.A., Helton, W.S., & Braunschweig, C.A. (2006). Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation, 12, 656–664.
Sheehan, T.J., Fifield, J., Reisine, S., & Tennen, H. (1995). The measurement structure of the Center for Epidemiologic Studies Depression Scale. Journal of Personality Assessment, 64, 507–521. doi:10.1207/s15327752jpa6403_9
Spielberger, C.G., Gorsuch, R.L., Suchene, R., Vagg, P.R., & Jacobs, G.A. (1983). Manual for the State-Trait Anxiety Inventory (form Y): Self-evaluation questionnaire. Palo Alto, CA: Consulting Psychologists Press.
Srokowski, T.P., Fang, S., Hortobagyi, G.N., & Giordano, S.H. (2009). Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. Journal of Clinical Oncology, 27, 2170–2176. doi:10.1200/jco.2008.17.5935
Stookey, J.D., Pieper, C.F., & Cohen, H.J. (2004). Hypertonic hyperglycemia progresses to diabetes faster than normotonic hyperglycemia. European Journal of Epidemiology, 19, 935–944.
Triplitt, C. (2010). Improving treatment success rates for type 2 diabetes: Recommendations for a changing environment. American Journal of Managed Care, 16(Suppl.), S195–S200.
Turina, M., Miller, F.N., Tucker, C.F., & Polk, H.C. (2006). Short-term hyperglycemia in surgical patients and a study of related cellular mechanisms. Annals of Surgery, 243, 845–851.
U.S. Department of Health and Human Services. (2006). Report on closing the disparity between hemoglobin A1c treatment guidelines and practice. Retrieved from http://1.usa.gov/1Km2A6O
Vigneri, P., Frasca, F., Sciacca, L., Pandini, G., & Vigneri, R. (2009). Diabetes and cancer. Endocrine-Related Cancer, 16, 1103–1123. doi:10.1677/erc-09-0087
Ware, J., Jr., Kosinski, M., & Keller, S.D. (1996). A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233.
Weiser, M.A., Cabanillas, M.E., Konopleva, M., Thomas, D.A., Pierce, S.A., Escalante, C.P., . . . O’Brien, S.M. (2004). Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer, 100, 1179–1185.
Willi, S.M., Kennedy, A., Wallace, P., Ganaway, E., Rogers, N.L., & Garvey, W.T. (2002). Troglitazone antagonizes metabolic effects of glucocorticoids in humans: Effects on glucose tolerance, insulin sensitivity, suppression of free fatty acids, and leptin. Diabetes, 51, 2895–2902.
Wright, L.A.C., & Hirsch, I.B. (2012). The challenge of the use of glycemic biomarkers in diabetes: Reflecting on hemoglobin A1C, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectrum, 25, 141–148.
Xu, X., Wu, J., Mao, Y., Zhu, Y., Hu, Z., Xu, X., . . . Xie, L. (2013). Diabetes mellitus and risk of bladder cancer: A meta-analysis of cohort studies. PLOS One, 8, e58079. doi:10.1371/journal.pone.0058079
Yoon, J.M., Son, K.Y., Eom, C.S., Durrance, D., & Park, S.M. (2013). Pre-existing diabetes mellitus increases the risk of gastric cancer: A meta-analysis. World Journal of Gastroenterology, 19, 936–945.
Yu, T., Jhun, B.S., & Yoon, Y. (2011). High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxidants and Redox Signaling, 14, 425–437.
Zhang, Z.H., Su, P.Y., Hao, J.H., & Sun, Y.H. (2013). The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: A meta-analysis of prospective cohort studies. International Journal of Gynecological Cancer, 23, 294–303.
About the Author(s)
Marilyn J. Hammer, PhD, DC, RN, is an assistant professor in the College of Nursing at New York University in New York; Bradley E. Aouizerat, PhD, MAS, is a professor in the School of Nursing at the University of California, San Francisco; Brian L. Schmidt, DDS, MD, PhD, is the director of the College of Dentistry at New York University; Frances Cartwright, PhD, RN, is the system vice president of oncology and nursing clinical quality at Mount Sinai Hospital in New York; Fay Wright, RN, MS, APRN-BC, is a doctoral student in the College of Nursing at New York University; and Christine Miaskowski, RN, PhD, FAAN, is a professor in the School of Nursing at the University of California, San Francisco. The study was funded by a grant (No. CA134900) from the National Cancer Institute (NCI) and the New York University Pless Center for Nursing Research. Miaskowski is supported by a grant from the American Cancer Society and NCI (No. CA168960). Hammer can be reached at marilyn.hammer@nyu.edu, with copy to editor at ONFEditor@ons.org. (Submitted January 2015. Accepted for publication May 27, 2015.)

