The Experiences of Young Adults With Hodgkin Lymphoma Transitioning to Survivorship: A Grounded Theory Study
Purpose/Objectives: To explore the experiences of young adults with Hodgkin lymphoma during the first year following the end of initial treatment.
Research Approach: A qualitative grounded theory study.
Setting: Interviews with patients recruited from three cancer centers in England.
Participants: 10 Hodgkin lymphoma survivors (four men and six women aged 21–39 years) recruited as part of a larger study of 28 young adult cancer survivors.
Methodologic Approach: Semistructured interviews were conducted about two months after treatment completion, and follow-up interviews were conducted seven months later. The authors’ grounded theory of positive psychosocial adjustment to cancer provided the conceptual framework.
Findings: Positive reframing, informal peer support, acceptance, and normalization helped young adults dismantle the threats of Hodgkin lymphoma during the course of treatment. However, they described losing a sense of security following treatment completion. Greater age-specific information to enable better preparation for the future was desired regarding body image, fertility, sexual relationships, work, and socializing.
Conclusions: Informal support mechanisms, like peer support and patient navigator interventions, may be useful ways to further support young adults after treatment completion.
Intepretation: Positive psychosocial adjustment to cancer survivorship in young adults is facilitated by having informal peer support; being able to positively reframe, accept, and normalize their experience; and being prepared for the future.
Jump to a section
Young adult cancer survivors have unique experiences, needs, and challenges dependent on their age and diagnosis (Hall et al., 2012; Zebrack, 2009; Zebrack & Isaacson, 2012). Cancer can disrupt normal life stages, such as finding a partner, establishing independence, finishing education, starting a family, and gaining employment (Grinyer, 2009; Zebrack, 2011). Young adult cancer survivors may also have specific concerns and needs regarding appearance and body image, identity development, and long-term future health (Bellizzi et al., 2012; Kelly & Gibson, 2008; Zebrack, 2011; Zebrack & Isaacson, 2012). Greater levels of psychological distress have been shown in young adult survivors compared to older survivors (Costanzo et al., 2007; Costanzo, Ryff, & Singer, 2009; Kwak et al., 2013; Parker, Baile, de Moor, & Cohen, 2003); therefore, young adults require specific interventions from the oncology nurse (Adams et al., 2011; Hall et al., 2012; Zebrack, 2009; Zebrack & Isaacson, 2012).
The transition from initial treatment completion—commonly a course of 4–6 monthly cycles of cytotoxic chemotherapy treatment—to follow-up care can be a difficult period of adjustment for cancer survivors involving heightened distress, uncertainty, and lost confidence (Foster & Fenlon, 2011; Thompson, Palmer, & Dyson, 2009). Adjustment has previously been conceptualized as a dynamic and ongoing process (Brennan, 2001; National Cancer Institute [NCI], 2015). Understanding the processes involved in adjustment will help explain why some young adults adjust well, whereas others do not. Policy makers have emphasized the importance of addressing psychosocial concerns and of comprehensive cancer care planning (Adler & Page, 2008; National Coalition for Cancer Survivorship, n.d.). In the United Kingdom, key priorities also include identifying ways of promoting and sustaining recovery following treatment (Department of Health, Macmillan Cancer Support, & NHS Improvement, 2013). However, few studies have examined how young adults experience the transition at the end of treatment (Kelly & Gibson, 2008; Thompson et al., 2009), and few interventions have been developed to support young adults to adjust to survivorship (Zebrack & Isaacson, 2012). Although definitions of survivorship vary, in the United Kingdom, a cancer survivor has been defined as an individual who has completed initial treatment, regardless of his or her prognosis (Department of Health, Macmillan Cancer Support, & NHS Improvement, 2010).
Hodgkin lymphoma is one of the most common cancers found in young adults (Bleyer, Viny, & Barr, 2006) with 9,050 individuals being diagnosed annually in the United States (NCI, 2016) and 1,800 in the United Kingdom (Cancer Research UK, n.d.). Although five-year survival rates are more than 85% (Cancer Research UK, n.d.; NCI, 2016), survivors are at an increased risk of late effects of treatment and psychosocial issues, including fear of recurrence, social and financial difficulties, and sexual and fertility issues (Daniëls, Oerlemans, Krol, van de Poll-Franse, & Creutzberg, 2013; Heutte et al., 2009; Roper, McDermott, Cooley, Daley, & Fawcett, 2009). Only two qualitative studies have been published about this population (Bober, Park, Schmookler, Medeiros Nancarrow, & Diller, 2007; Grinyer, 2010), and very little research has considered the psychosocial aspects of nursing care. This article will report the experiences of young adults with Hodgkin lymphoma during the first year following initial treatment completion using the conceptual framework of the authors’ previously reported theory of positive adjustment to cancer (Matheson et al., 2016).
Methods
A longitudinal, qualitative study using a grounded theory methodology was conducted to explore, in depth, the experiences of young adults with Hodgkin lymphoma and their psychosocial adjustment to survivorship in the year following treatment completion. This was part of a larger doctoral research project examining the experiences of young adults with both Hodgkin lymphoma and testicular cancer.
Participants
Patients with Hodgkin lymphoma aged 20–45 years and treated with curative intent who had completed initial or secondary treatment (less than six months ago) were eligible to participate in a larger study that included a sample of testicular cancer survivors (n = 18) reported previously (Matheson et al., 2016). Patients were excluded if they were unable to participate in a face-to-face interview or speak fluent English, had a major medical comorbidity, or required palliative or end-of-life care. Because the number of eligible patients was likely to be small, the authors extended the NCI’s (2014) definition of young adulthood to include patients aged 45 years or younger instead of 39 years or younger. This was also the rationale for recruiting two patient groups in the larger study.
Procedure
Following approval from a National Health Service (NHS) research ethics committee, participants were recruited from three NHS cancer centers in England from September 2012 to September 2014. Patients were identified and invited into the study by their clinician (hematologist or oncologist) or oncology or hematology nurse during a clinic appointment around the time of treatment completion. Face-to-face interviews were conducted by the principal investigator (PI) at each participant’s preferred venue and lasted an hour on average. Interviews at time 1 (T1) took place within six months of treatment completion (mean = 2 months), and time 2 (T2) interviews occurred about seven months later. A semistructured interview guide was used involving open-ended questions to elicit participants’ experiences of the psychosocial impact of cancer on different aspects of their lives, their information and support needs, and suggestions for improvements to cancer services. Questions included the following:
• “Can you tell me about your experience of completing cancer treatment?”
• “Can you tell me about the impact of cancer on different areas of your life since finishing treatment?”
• “Are there any areas where you felt you wanted more information or support?”
• “What do you think might help young adults in the future?”
As part of the larger study, patients with Hodgkin lymphoma were recruited and interviewed concurrently with the sample of patients with testicular cancer reported elsewhere (Matheson et al., 2016). The same interview topic guide was administered to all participants. Interview data were stored securely in electronic and hard copy form, in compliance with ethical protocols and Oxford Brookes University regulations. Only the PI and the study supervisory team had full access to participants’ demographic and interview data.
Data Analysis
Interviews were transcribed verbatim and anonymized (mostly by the PI, with six interviews transcribed by a professional transcriber). Data were analyzed by the PI using social constructivist grounded theory (Charmaz, 2006). A systematic and iterative line-by-line coding procedure was employed involving three stages: initial coding, focused coding, and theoretical coding as outlined by Charmaz (2006). Throughout the coding procedure, the processes involved in participants’ experiences were explored, as well as participants’ feelings, beliefs, actions, and hidden assumptions (Charmaz, 2006). Constant comparison between codes was employed, and codes were constantly revisited, which was an iterative process. Trustworthiness of the analysis was sought by regular use of a reflexive research diary and detailed memo writing (Charmaz, 2006). The analysis was discussed regularly during formal meetings with the study supervisory team, two of whom listened to a number of the interviews, read and/or analyzed a sample of transcripts, and agreed on the final themes.
The interviews at T1 and T2 were analyzed individually and then collectively as similarities and differences were explored. Little evidence showed substantial change between T1 and T2 interviews, although some participants exhibited specific challenges or issues that changed over time; however, T2 interviews added further depth to the analysis. Data collection and preliminary analysis occurred concurrently, and emerging themes were explored in greater depth in subsequent interviews.
Because of the recruitment of higher numbers of patients with testicular cancer as part of the larger study, the initial analysis of interviews, which formed the basis of the grounded theory reported previously (Matheson et al., 2016), was carried out with this group of patients. The subsequent collective analysis of interviews of patients with Hodgkin lymphoma provided a source of comparison and helped further refine the grounded theory as it developed. The main processes involved in the theory related to young adult survivors with both testicular cancer and Hodgkin lymphoma.
Findings
Thirty-one patients with Hodgkin lymphoma were offered information packs about the study; 11 responded initially, and 10 participated (4 men and 6 women). Participant characteristics are summarized in Table 1. All participants were interviewed at T1. Seven were invited to a second interview; one failed to respond, so six were reinterviewed at T2 (two men and four women). The remaining participants (n = 3) were not invited to be re-interviewed because of the time constraints of the doctoral research studentship. In total, 16 interviews were conducted. At T1, most interviews took place in participants’ homes (n = 6), their workplaces (n = 2), or a cafe (n = 2). At T2, participants were reinterviewed in their homes (n = 6); one of these participants had received treatment since T1 for relapsed Hodgkin lymphoma.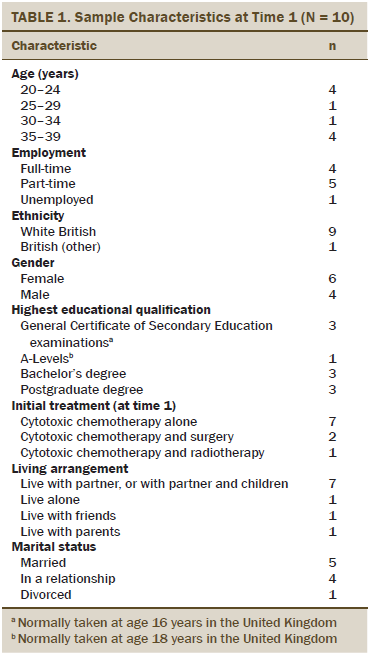
The key processes in the authors’ grounded theory of positive psychosocial adjustment to cancer in young adults with testicular cancer (Matheson et al., 2016) were found to also apply to Hodgkin lymphoma survivors, and they are used as a framework for presenting the authors’ findings (see Figure 1). Positive psychosocial adjustment was conceptualized as a process of dismantling the current and future threats of cancer involving the transitions of gaining a sense of perspective over the threats of cancer (Transition 1) and striving to get on with life and restore normality (Transition 2). Six processes were involved in these two transitions to positive adjustment.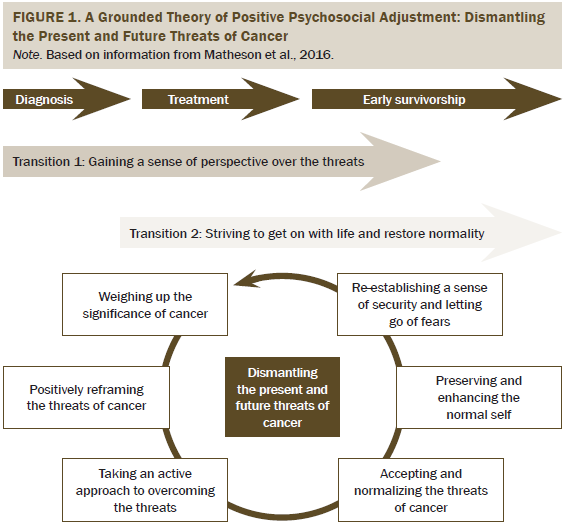
Transition 1
Process 1: Weighing up the significance of cancer—Holding on to positive gains: Hodgkin lymphoma was unexpected during the life stages of young adulthood. A male participant at T1 (Participant Number 28 [P28]) referred to his cancer as a “pretty major life event, frankly,” noting that it was his “first serious health issue, and, at the age of 24, you don’t expect serious health issues.” When participants reflected on their experience, they tended to appraise the disease in negative and positive terms. Although the disease and treatment were assessed as being threatening and disruptive toward many aspects of their lives, the illness was also perceived as having a positive impact, particularly on close relationships, their life priorities, and their sense of personal growth. A female participant at T1 (P4) said, “To be honest, nothing’s changed; if anything, it’s better. . . . Cancer can be devastating, and it can ruin lives, but also I think it’s made my life better, and it makes you find out who the true people are.” The process of holding on to the positive gains from cancer helped participants find greater meaning from the experience and better accept significant losses and threats of the disease, including lost time, threatened fertility, and lost confidence in some areas.
Process 2: Positively reframing the threats of cancer—Making social comparisons and holding on to positive illness perceptions: The process of making social comparisons to others with perceived “worse cancers” enabled participants to reframe having Hodgkin lymphoma more positively and as a “good cancer.” A male participant at T2 (P21) noted, “I’m grateful that I had Hodgkin’s lymphoma; it could have been a lot worse.” Social comparisons with long-term survivors of Hodgkin lymphoma helped participants hold on to the hope that life would be restored to normality, particularly for participants whose recovery was taking longer than they had expected. A male participant at T2 (P3) explained that “being lymphoma, you still look at it and weigh it up against, well, at least it isn’t lung cancer or bowel cancer or . . . something else; this one’s the best type to have.” Holding on to positive perceptions of Hodgkin lymphoma also facilitated positive reframing because the disease was perceived to be a specific cancer with a transient trajectory. A female participant at T1 (P22) said that because “it’s sort of a boxed cancer . . . it’s easier to cope with.” Participants who were parents sought to shape their children’s perceptions of Hodgkin lymphoma as positive to minimize distress.
Process 3: Taking an active approach to overcoming the threats—Using informal peer support and regaining control: Participants strongly valued informal sources of emotional support from existing friendships and from young cancer survivors, which helped them normalize the experience and reduce the threats of the disease. A male participant at T1 (P3) said when “you speak to people with lymphoma, it’s not that bad, but that’s only through talking to other people. . . . It gives you a bit of hope as well.” Some participants felt that peer mentoring, or having “a kind of buddy person,” said a female participant at T1 (P6), would have been helpful, particularly if this relationship was with someone of a similar age and initiated during treatment. A female participant at T1 elaborated further, saying, “If I could have been linked up with someone maybe who’d gone through treatment in a similar situation as me, with young children . . . that would have been really useful.” Participants regained a sense of control through actively using support and information. This came from organizations that provide supportive cancer care including complementary and alternative therapies, such as meditation and relaxation therapies, nutritional support and advice, specialist psychological support, and social, financial, and spiritual support and advice. Participants also sought support and information from cancer charities, patient information websites, and their families and spouses. They particularly valued being directed toward sources of support (signposting); however, some participants expressed ambivalence about seeking support from their nurse or a clinical psychologist. Instead, they valued informal opportunities to reflect on the experience with peers and health professionals. A male participant at T2 (P3) described the feeling of getting “it off [his] chest” and how “being able to talk to someone” felt “amazing.” Some participants wanted specialist counseling to be offered.
Transition 2
Process 4: Accepting and normalizing the threats of cancer—Accepting the timing of cancer in young adulthood and accepting appearance changes: Treatment was perceived as stealing precious time from what was felt to be a normally industrious period of their lives. Participants struggled with having “life on hold,” as a female participant said at T1 (P6). They sought to normalize the experience and accept the timing and threats of cancer in young adulthood. This helped them to transition toward a sense of normality, which was felt to be achieved in some participants by T2. A female participant at T1 said, “You can’t change life. You can’t say this should be happening to somebody else because one in three of us will be affected with cancer, and that’s life.” Some participants even reframed the timing of cancer in young adulthood as advantageous (e.g., higher chances of survival, their children would be too young to remember), which facilitated the process of acceptance. However, some participants who had not yet achieved parenthood or typical milestones of adulthood struggled to accept the disease because of its threat to their future life plans. A female participant at T2 described this further.
So there’s work and career—the way it influences that. There’s the whole fertility thing, there’s the appearance thing, there’s indirect things like insurance . . . and getting mortgages. I think all of that is [going to] be affected by having to say, “Yes, I have had cancer in the past.”
Participants who had not experienced initial visible symptoms of Hodgkin lymphoma, such as lumps, also found their cancer harder to accept because of the invisible nature of the disease, as one male participant at T2 (P3) described.
It still doesn’t feel like I’ve got cancer. You still imagine cancer to be something different to what I’ve got. It’s not something I can touch, where some people get it and they got lumps. . . . I’ve probably got a lump, but it’s in my chest, so I can’t feel it or see it or touch it. I get side effects from it but not directly to do with that, so sometimes it feels like I’m a cheat [because] it feels like I don’t have it.
Accepting temporary dependence and the need for support from others was challenging for male participants in particular because doing so conflicted with their perceived role of supporting and protecting others. Many female participants struggled to accept changes in their appearance, particularly alopecia; some male participants also were unexpectedly concerned about appearance changes, such as weight gain. Participants employed strategies of taking control (through shaving hair), maintaining identity (through keeping up with beauty regimens and hiding appearance changes with head scarves or hats), and using humor to minimize and normalize appearance changes, as the following quotation from a female participant at T2 (P4) illustrates:
Me and my mates used to make jokes about it. . . . Me and [my partner] was making cakes once and was having a cake fight, and he put [chocolate spread] on the top of my head and licked it off when I was bald. . . . It was just making a laugh of it.
The perceived loss of femininity and sexual attractiveness led to poorer self-confidence by T2 in some female participants who struggled to accept an altered appearance. One single female participant at T1 (P6) felt that appearance changes affected her confidence toward re-entering the dating scene: “I feel like I can’t do it [because] I haven’t got my beautiful long hair.”
Process 5: Preserving and enhancing the normal self—Defending against illness centrality and striving to regain physical health normality: Young adults rejected the idea that being a cancer survivor was central to their identity; a male participant at T1 (P11) said simply, “Cancer won’t define me as a person.” Participants sought to preserve a normal self throughout treatment and beyond, which helped defend against the perceived stigma of having cancer. Some also felt having cancer had enhanced their self-identity, including a female participant at T2 (P4).
It takes a big chunk of you away. . . . It changed me for the better. I’m obviously not glad that it happened, but, in a way, I suppose I am a little bit [because] it makes you appreciate things [and] it makes you less insecure. . . . I feel more confident now than I did before cancer.
Preservation of the normal self for some participants involved avoiding talking about lymphoma as a cancer. A male participant at T2 (P3) noted, “I can’t say ‘cancer’; I’ve got to say ‘lymphoma.’” Participants also attempted to keep the disease in the background of their lives. However, this led to risk-taking and nonadherence to advice in one female participant at T1 (P4): “I wasn’t [going to] let [cancer] stop me doing what I wanted to do. . . . I might as well risk getting an infection rather than stop living my life.” The desire of participants to reclaim their normal identities conflicted with the reminders of their cancer survivor status, such as going to follow-up appointments or wearing a medical alert bracelet or chain (in case an emergency blood transfusion was needed). Participants also struggled with feeling they were in an “aged” body because of fatigue and lost fitness, so increasing activity levels helped them to “feel part of the world again” (P6). Some maintained exercise programs during treatment, which seemed to lead to reduced fatigue and improved vitality in these participants. Fatigue took longer to resolve in others and meant returning to work was challenging, so participants sought to accept this new normal physical self.
Process 6: Re-establishing a sense of security and letting go of fears—Being prepared for the future and maintaining hope for parenthood: The transition from treatment to follow-up and the loss of the treatment “safety blanket” (P11) resulted in increased vulnerability as participants sought to re-establish a sense of security. A female participant at T1 (P6) explained that “nothing is certain anymore, [and] you’re suddenly faced with thinking about your life that you might not have.” The perceived abrupt ending of treatment led many participants to feel unprepared for life afterward, so some wanted more support between finishing treatment and going to their next follow-up appointment. A few participants expressed the need for an additional telephone call during this time to lessen their sense of abandonment from the often excellent care they felt they had received during treatment. A female participant at T1 (P24) described it in this way: “You were just kind of sent off back into the big wide world.” Some participants exhibited uncertainty about knowing what is normal in their physical recovery trajectory after treatment, such as the duration of ongoing symptoms; they felt that information from health professionals was lacking or vague, which led to greater anxiety. A male participant at T1 (P3) said he wanted “someone to say, ‘Well, this is what usually happens, [and] this is how long this will take to recover.’ . . . I haven’t got a clue how long it takes to recover.” Some discussed the need for the provision of more information before treatment completion (such as advice about when to stop medication, use of contraception, use of sun cream, when they could expect to resume work, socialization, and alcohol consumption). Participants also wanted information about where to seek psychosocial support. A male participant at T1 (P28) described his confusion.
We got a lot of conflicting information about whether me and my girlfriend have to use condoms. . . . Some doctors say, ‘Yes, you do,’ [and] some doctors [were] not so sure; we were left confused. Skincare as well because there’s very confusing information about whether you can go out in the sun or what you can do. Also working . . . knowing what you can and cannot do wasn’t made very clear at all.
In a few participants, fears of a recurrence resulted in frequent self-checking for “cancer lumps,” which could become obsessive and provoke anxiety. For those participants whose disease had not presented with palpable tumors, their fear of a recurrence being missed was magnified. A few desired greater information from health professionals about symptoms to lessen anxiety. Those who wanted children were aware of the threat of future disruption to their plans, so they sought to maintain hope for future parenthood. Female participants were given conflicting advice about when they could attempt conception; they were either told that a delay was unnecessary, or that they should wait one year or wait two years. Accepting this waiting game was a struggle, and some felt health professionals gave inadequate or vague information on fertility, particularly regarding the process of testing fertility, and wanted more written information. A lack of information heightened participants’ concerns and hindered their ability to feel they were getting on with life.
Discussion
These findings add to the very limited literature on the experiences and care needs of Hodgkin lymphoma survivors (Bober et al., 2007; Grinyer, 2010). Using the conceptual framework of the previously reported grounded theory (Matheson et al., 2016), the current authors have described the challenges these Hodgkin lymphoma survivors experienced and the processes that lead to a positive adjustment to cancer.
The study findings have several implications for nurses caring for young adult cancer survivors to enable positive adjustment. Providing greater information prior to cancer treatment completion would help better prepare young adults to re-establish a sense of normality and security soon after treatment completion. Oncology or hematology nurses need to share information about how long most young people with their type of cancer and stage of disease take to recover from side effects of treatment, particularly fatigue and alopecia. Young adults also need information about when to resume working, socializing in public, and consuming alcohol. As part of the treatment completion assessment and review, oncology or hematology nurses should also inform them about medication use, sun exposure and protection, and contraception and fertility interventions.
Female participants were given conflicting information by clinicians on when they could safely attempt conception. This finding reflects previous literature that fertility information is perceived as inadequate (Absolom et al., 2009; Wright, Coad, Morgan, Stark, & Cable, 2014). A need exists for a robust body of current evidence to be able to categorize any risks regarding pregnancy following treatment. In the United Kingdom, the Lymphoma Association (n.d.) recommends a two-year delay between receiving treatment and attempting conception, yet acknowledges the variability in advice given. Oncology or hematology nurses can help young adults who have fertility concerns by working with clinicians to provide consistent information and psychological support.
A key role of oncology or hematology nurses is providing patient education (Oncology Nursing Society, 2011). Participants in this study particularly wanted education about monitoring signs and symptoms of disease recurrence. Providing this education may help to increase self-efficacy (confidence to perform a given behavior), which was lacking in some participants. Survivors who presented with nonpalpable disease would need information about how their remission will be monitored to reduce fears of recurrence. Some participants expressed the need for at least one additional telephone call after treatment completion, preferably by an oncology or hematology nurse, to bridge the gap in support between treatment and follow-up appointments. The current authors’ findings suggest a need for greater survivorship care planning for young adults who have been treated for Hodgkin lymphoma. In the United Kingdom, the National Cancer Survivorship Initiative recommends that oncology or hematology nurses conduct a holistic needs assessment with an individual prior to his or her completion of cancer treatment (Department of Health et al., 2013). Overall, the current authors’ findings suggest that young Hodgkin lymphoma survivors need greater preparation for the end of treatment because the first few months on a follow-up regimen are particularly challenging. Information and support to facilitate self-management should, therefore, be given prior to treatment completion to help young adults prepare for this transition to follow-up (see Figure 2).
Young adult cancer survivors place high importance on informal peer support, and some also wish to have professional psychological therapy (Kent et al., 2013; Matheson et al., 2016; Taylor, Pearce, Gibson, Fern, & Whelan, 2013; Tsangaris et al., 2014). Methods for facilitating such support warrant additional investigation. Oncology or hematology nurses who guide individuals to support services should use a person-centered approach that takes into consideration biopsychosocial aspects of their care needs. Participants who were guided (often by the oncology or hematology nurse) to support services found this highly valuable because of their physical, psychological, and social needs regarding child care, income, and peer support. Because of time constraints on oncology or hematology nurses, the role of navigators for patients with cancer (Freeman & Rodriguez, 2011) or trained support volunteers could be explored further; they have been shown to provide emotional support, information, and guidance to services (Gabitova & Burke, 2014; Robinson-White, Conroy, Slavish, & Rosenzweig, 2010). The Lymphoma Association (n.d.) offers a patient buddy system in the United Kingdom, which is a useful resource.
The authors’ findings show that the active acceptance of the impact of cancer, shown previously as a useful coping strategy (Allart, Soubeyran, & Cousson-Gélie, 2013; Barroilhet Díez, Forjaz, & Garrido Landívar, 2005), helped to facilitate positive adjustment, particularly toward appearance and fertility concerns. This suggests that interventions that promote active acceptance may be useful, such as mindfulness-based interventions, including Acceptance and Commitment Therapy (Chambers, Foley, Galt, Ferguson, & Clutton, 2012; Hulbert-Williams, Storey, & Wilson, 2015; Jones et al., 2013; Piet, Würtzen, & Zachariae, 2012; Shennan, Payne, & Fenlon, 2011). Additional research is needed to test such interventions in young adults with cancer.
Some participants maintained exercise routines during treatment and found this to be a helpful strategy for reducing fatigue. Exercise or activity-based interventions have been shown to be effective in reducing fatigue in Hodgkin lymphoma survivors (Oldervoll, Kaasa, Knobel, & Loge, 2003), as well as other young adult survivors (Hauken, Holsen, Fismen, & Larsen, 2015; Rosenberg, Lange, Zebrack, Moulton, & Kosslyn, 2014). Systematic review evidence also indicates that exercise has many benefits for people with cancer during and after treatment completion (Cramp & Byron-Daniel, 2012; Mishra et al., 2012).
Limitations
Survival outcomes following treatment for Hodgkin lymphoma are good, and most patients with these diagnoses have a good prognosis. However, the authors’ theory may not be relevant to young adults who have tumors with lower survival rates and overall poorer prognoses. Additional research is required to test the value of the grounded theory for young adults with other tumor types, as well as for older cancer survivors. Other limitations include a small sample size and probable self-selected sample bias because of the authors’ relatively low response rate. Patients who had not made a positive adjustment may have been reluctant to participate. Most participants had a partner, so the authors recognize that the experience of single participants is not captured thoroughly. Future research is needed specifically to examine the effect of treatment for, and survivorship from, cancer in young adults who are single or childless.
Conclusion
Using a grounded theory of positive psychosocial adjustment to cancer, this article describes how young adults treated for Hodgkin lymphoma can successfully transition into survivorship. Positive psychosocial adjustment to cancer survivorship is facilitated by having informal peer support; being able to positively reframe, accept, and normalize their experience; and being prepared for the future. Findings from the current study have implications for oncology or hematology nurses regarding survivorship care planning with young adult Hodgkin lymphoma survivors. This requires provision of accurate and effective information and support. All patients should receive a holistic assessment of their needs prior to treatment completion and transition into survivorship. This would enable nurses to provide individually tailored education about the expected duration of recovery, signs of concern that may suggest a relapse, resumption of employment and social activities, and conception and fertility. Additional investigation is warranted regarding the use of informal support mechanisms to equip young adults to positively adapt to cancer survivorship (e.g., peer support). The current authors’ findings suggest that this additional support may help young people positively adjust to life beyond their cancer treatment.
References
Absolom, K., Eiser, C., Michel, G., Walters, S.J., Hancock, B.W., Coleman, R.E., . . . Greenfield, M. (2009). Follow-up care for cancer survivors: Views of the younger adult. British Journal of Cancer, 101, 561–567. doi:10.1038/sj.bjc.6605213
Adams, E., McCann, L., Armes, J., Richardson, A., Stark, D., Watson, E., & Hubbard, G. (2011). The experiences, needs and concerns of younger women with breast cancer: A meta-ethnography. Psycho-Oncology, 20, 851–861. doi:10.1002/pon.1792
Adler, N.E., & Page, A.E.K. (Eds.). (2008). Cancer care for the whole patient: Meeting psychosocial health needs. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK4015
Allart, P., Soubeyran, P., & Cousson-Gélie, F. (2013). Are psychosocial factors associated with quality of life in patients with haematological cancer? A critical review of the literature. Psycho-Oncology, 22, 241–249. doi:10.1002/pon.3026
Barroilhet Díez, S., Forjaz, M.J., & Garrido Landívar, E. (2005). Concepts, theories and psychosocial factors in cancer adaptation. Actas Españolas de Psiquiatría, 33, 390–397.
Bellizzi, K.M., Smith, A., Schmidt, S., Keegan, T.H., Zebrack, B., Lynch, C.F., . . . Simon, M. (2012). Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer, 118, 5155–5162. doi:10.1002/cncr.27512
Bleyer, A., Viny, A., & Barr, R. (2006). Cancer in 15- to 29-year-olds by primary site. Oncologist, 11, 590–601. doi:10.1634/theoncologist.11-6-590
Bober, S.L., Park, E.R., Schmookler, T., Medeiros Nancarrow, C., & Diller, L. (2007). Perceptions of breast cancer risk and cancer screening: A qualitative study of young, female Hodgkin’s disease survivors. Journal of Cancer Education, 22, 42–46.
Brennan, J. (2001). Adjustment to cancer—Coping or personal transition? Psycho-Oncology, 10, 1–18. doi:10.1002/1099-1611(200101/02)10:1<1::AID-PON484>3.0.CO;2-T
Cancer Research UK. (n.d.). Hodgkin lymphoma statistics. Retrieved from http://www.cancerresearchuk.org/health-professional/cancer-statistics/s…
Chambers, S.K., Foley, E., Galt, E., Ferguson, M., & Clutton, S. (2012). Mindfulness groups for men with advanced prostate cancer: A pilot study to assess feasibility and effectiveness and the role of peer support. Supportive Care in Cancer, 20, 1183–1192. doi:10.1007/s00520-011-1195-8
Charmaz, K. (2006). Constructing grounded theory: A practical guide through qualitative analysis. London, UK: Sage.
Costanzo, E.S., Lutgendorf, S.K., Mattes, M.L., Trehan, S., Robinson, C.B., Tewfik, F., & Roman, S.L. (2007). Adjusting to life after treatment: Distress and quality of life following treatment for breast cancer. British Journal of Cancer, 97, 1625–1631. doi:10.1038/sj.bjc.6604091
Costanzo, E.S., Ryff, C.D., & Singer, B.H. (2009). Psychosocial adjustment among cancer survivors: Findings from a national survey of health and well-being. Health Psychology, 28, 147–156. doi:10.1037/a0013221
Cramp, F., & Byron-Daniel, J. (2012). Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews, 2012, CD006145. doi:10.1002/14651858.CD006145.pub3
Daniëls, L.A., Oerlemans, S., Krol, A.D., van de Poll-Franse, L.V., & Creutzberg, C.L. (2013). Persisting fatigue in Hodgkin lymphoma survivors: A systematic review. Annals of Hematology, 92, 1023–1032. doi:10.1007/s00277-013-1793-2
Department of Health, Macmillan Cancer Support, & NHS Improvement. (2010). National Cancer Survivorship Initiative (NCSI) vision. Retrieved from http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh…
Department of Health, Macmillan Cancer Support, & NHS Improvement. (2013). Living with and beyond cancer: Taking action to improve outcomes. Retrieved from https://www.gov.uk/government/uploads/system/uploads/attachment_data/fi…
Foster, C., & Fenlon, D. (2011). Recovery and self-management support following primary cancer treatment. British Journal of Cancer, 105(Suppl. 1), S21–S28. doi:10.1038/bjc.2011.419
Freeman, H.P., & Rodriguez, R.L. (2011). History and principles of patient navigation. Cancer, 117(Suppl. 15), 3537–3540. doi:10.1002/cncr.26262
Gabitova, G., & Burke, N.J. (2014). Improving healthcare empowerment through breast cancer patient navigation: A mixed methods evaluation in a safety-net setting. BMC Health Services Research, 14, 407. doi:10.1186/1472-6963-14-407
Grinyer, A. (2009). Life after cancer in adolescence and young adulthood: The experience of survivorship. London, UK: Routledge.
Grinyer, A. (2010). The late effects of mantle field radiotherapy: The information and support needs of women survivors of Hodgkin’s disease. European Journal of Oncology Nursing, 14, 183–189. doi:10.1016/j.ejon.2009.12.006
Hall, A.E., Boyes, A.W., Bowman, J., Walsh, R.A., James, E.L., & Girgis, A. (2012). Young adult cancer survivors’ psychosocial well-being: A cross-sectional study assessing quality of life, unmet needs, and health behaviors. Supportive Care in Cancer, 20, 1333–1341. doi:10.1007/s00520-011-1221-x
Hauken, M.A., Holsen, I., Fismen, E., & Larsen, T.M. (2015). Working toward a good life as a cancer survivor: A longitudinal study on positive health outcomes of a rehabilitation program for young adult cancer survivors. Cancer Nursing, 38, 3–15. doi:10.1097/ncc.0000000000000138
Heutte, N., Flechtner, H.H., Mounier, N., Mellink, W.A., Meerwaldt, J.H., Eghbali, H., . . . Henry-Amar, M. (2009). Quality of life after successful treatment of early-stage Hodgkin’s lymphoma: 10-year follow-up of the EORTC–GELA H8 randomised controlled trial. Lancet. Oncology, 10, 1160–1170. doi:10.1016/S1470-2045(09)70258-X
Hulbert-Williams, N.J., Storey, L., & Wilson, K.G. (2015). Psychological interventions for patients with cancer: Psychological flexibility and the potential utility of Acceptance and Commitment Therapy. European Journal of Cancer Care, 24, 15–27. doi:10.1111/ecc.12223
Jones, P., Blunda, M., Biegel, G., Carlson, L.E., Biel, M., & Wiener, L. (2013). Can mindfulness-based interventions help adolescents with cancer? Psycho-Oncology, 22, 2148–2151. doi:10.1002/pon.3251
Kelly, D., & Gibson, F. (Eds.). (2008). Cancer care for adolescents and young adults. Oxford, UK: Blackwell.
Kent, E.E., Smith, A.W., Keegan, T.H., Lynch, C.F., Wu, X.C., Hamilton, A.S., . . . Harlan, L.C. (2013). Talking about cancer and meeting peer survivors: Social information needs of adolescents and young adults diagnosed with cancer. Journal of Adolescent and Young Adult Oncology, 2, 44–52. doi:10.1089/jayao.2012.0029
Kwak, M., Zebrack, B.J., Meeske, K.A., Embry, L., Aguilar, C., Block, R., . . . Cole, S. (2013). Trajectories of psychological distress in adolescent and young adult patients with cancer: A 1-year longitudinal study. Journal of Clinical Oncology, 31, 2160–2166. doi:10.1200/jco.2012.45.9222
Lymphoma Association. (n.d.). Buddy scheme. Retrieved from http://www.lymphomas.org.uk/how-we-can-support-you/buddy-scheme
Matheson, L., Boulton, M., Lavender, V., Protheroe, A., Brand, S., Wanat, M., & Watson, E. (2016). Dismantling the present and future threats of testicular cancer: A grounded theory of positive and negative adjustment trajectories. Journal of Cancer Survivorship, 10, 194–205. doi:10.1007/s11764-015-0466-7
Mishra, S.I., Scherer, R.W., Geigle, P.M., Berlanstein, D.R., Topaloglu, O., Gotay, C.C., & Snyderm C. (2012). Exercise interventions on health-related quality of life for cancer survivors Cochrane Database of Systematic Reviews, 2012, CD007566. doi:10.1002/14651858.CD007566.pub2
National Cancer Institute. (2014). A snapshot of adolescent and young adult cancers. Retrieved from http://www.cancer.gov/researchandfunding/snapshots/adolescent-young-adu…
National Cancer Institute. (2015). Adjustment to cancer: Anxiety and distress (PDQ®)—Health professional version. Retrieved from http://www.cancer.gov/cancertopics/pdq/supportivecare/adjustment/Health…
National Cancer Institute. (2016). SEER stat fact sheets: Hodgkin lymphoma. Retrieved from http://seer.cancer.gov/statfacts/html/hodg.html
National Coalition for Cancer Survivorship. (n.d.). Policy priorities. Retrieved from http://www.canceradvocacy.org/cancer-policy/policy-priorities
Oldervoll, L.M., Kaasa, S., Knobel, H., & Loge, J.H. (2003). Exercise reduces fatigue in chronic fatigued Hodgkins disease survivors—Results from a pilot study. European Journal of Cancer, 39, 57–63. doi:10.1016/S0959-8049(02)00483-5
Oncology Nursing Society. (2011, April 28). Oncology Nursing Society Joins Journey Forward Collaborative to benefit cancer survivors [Press release]. Retrieved from http://www.prnewswire.com/news-releases/oncology-nursing-society-joins-…
Parker, P.A., Baile, W.F., de Moor, C., & Cohen, L. (2003). Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psycho-Oncology, 12, 183–193. doi:10.1002/pon.635
Piet, J., Würtzen, H., & Zachariae, R. (2012). The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 80, 1007–1020. doi:10.1037/a0028329
Robinson-White, S., Conroy, B., Slavish, K.H., & Rosenzweig, M. (2010). Patient navigation in breast cancer: A systematic review. Cancer Nursing, 33, 127–140. doi:10.1097/NCC.0b013e3181c40401
Roper, K., McDermott, K., Cooley, M.E., Daley, K., & Fawcett, J. (2009). Health-related quality of life in adults with Hodgkin’s disease: The state of the science. Cancer Nursing, 32, E1–E17. doi:10.1097/NCC.0b013e3181aa4a33
Rosenberg, R.S., Lange, W., Zebrack, B., Moulton, S., & Kosslyn, S.M. (2014). An outdoor adventure program for young adults with cancer: Positive effects on body image and psychosocial functioning. Journal of Psychosocial Oncology, 32, 622–636. doi:10.1080/07347332.2014.936652
Shennan, C., Payne, S., & Fenlon, D. (2011). What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psycho-Oncology, 20, 681–697. doi:10.1002/pon.1819
Taylor, R.M., Pearce, S., Gibson, F., Fern, L., & Whelan, J. (2013). Developing a conceptual model of teenage and young adult experiences of cancer through meta-synthesis. International Journal of Nursing Studies, 50, 832–846. doi:10.1016/j.ijnurstu.2012.09.011
Thompson, K., Palmer, S., & Dyson, G. (2009). Adolescents and young adults: Issues in transition from active therapy into follow-up care. European Journal of Oncology Nursing, 13, 207–212. doi:10.1016/j.ejon.2009.05.001
Tsangaris, E., Johnson, J., Taylor, R., Fern, L., Bryant-Lukosius, D., Barr, R., . . . Klassen, A. (2014). Identifying the supportive care needs of adolescent and young adult survivors of cancer: A qualitative analysis and systematic literature review. Supportive Care in Cancer, 22, 947–959. doi:10.1007/s00520-013-2053-7
Wright, C.I., Coad, J., Morgan, S., Stark, D., & Cable, M. (2014). ‘Just in case’: The fertility information needs of teenagers and young adults with cancer. European Journal of Cancer Care, 23, 189–198. doi:10.1111/ecc.12137
Zebrack, B. (2009). Information and service needs for young adult cancer survivors. Supportive Care in Cancer, 17, 349–357. doi:10.1007/s00520-008-0469-2
Zebrack, B., & Isaacson, S. (2012). Psychosocial care of adolescent and young adult patients with cancer and survivors. Journal of Clinical Oncology, 30, 1221–1226. doi:10.1200/JCO.2011.39.5467
Zebrack, B.J. (2011). Psychological, social, and behavioral issues for young adults with cancer. Cancer, 117(Suppl. 10), 2289–2294. doi:10.1002/cncr.26056
About the Author(s)
Matheson is a postdoctoral research assistant, Boulton is a professor of health sociology, and Lavender is a senior lecturer in cancer care, all in the Faculty of Health and Life Sciences at Oxford Brookes University; Collins is a consultant hematologist and Mitchell-Floyd is a lymphoma specialist nurse practitioner, both in the Department of Clinical Haematology at the Oxford Cancer and Haematology Centre of the Oxford University Hospitals NHS Foundation Trust; and Watson is a professor of supportive cancer care in the Faculty of Health and Life Sciences at Oxford Brookes University, all in Oxford, England. Matheson, Boulton, Lavender, and Watson contributed to the conceptualization and design and provided the analysis. Matheson completed the data collection, and Collins and Mitchell-Floyd assisted with recruitment. All authors contributed to the manuscript preparation. Matheson can be reached at l.matheson@brookes.ac.uk, with copy to editor at ONFEditor@ons.org. Submitted November 2015. Accepted for publication January 21, 2016.

