Prevalence, Duration, Severity, and Distress of Chemotherapy-Related Gastrointestinal Symptoms in Patients With a Hematologic Malignancy
Purpose/Objectives: To describe prevalence, duration, severity, and distress of chemotherapy-related gastrointestinal (GI) symptoms, and evaluate inclusion of clinically relevant GI symptom items on cancer symptom questionnaires.
Design: Longitudinal descriptive design.
Setting: Inpatient and outpatient hematology settings.
Sample: 105 adults with a hematologic malignancy receiving their third or subsequent cycle of chemotherapy.
Methods: Participants completed weekly assessments of 19 GI symptoms during a three-week period of chemotherapy. Descriptive statistics were calculated to summarize GI symptom prevalence, duration, severity, and distress ratings at each week. Findings were compared to item content of 12 cancer multisymptom questionnaires identified in the literature.
Main Research Variables: GI symptom prevalence, duration, severity, and distress.
Findings: Participants reported an average of three to five GI symptoms at each time point that were typically experienced as mild to moderate in duration, severity, and distress. Only 3 of 11 clinically relevant GI symptoms were included on more than half of the cancer symptom questionnaires.
Conclusions: Patients receiving chemotherapy experience a moderate GI symptom burden across a wide range of potential GI symptoms.
Implications for Nursing: Future research should include measures of clinically relevant GI symptoms that may be emerging with new cancer therapies and toxicity prevention protocols.
Jump to a section
Chemotherapy is associated with side effects of varying prevalence, duration, severity, and distress, many of which are gastrointestinal (GI) symptoms. Evidence has shown that chemotherapy directly affects GI cell replacement within a few hours after administration (Mitchell, 2006). Perhaps the most well-known and well-studied GI symptoms in patients with cancer are nausea and vomiting. In the past five years, about 10,000 articles have been published concerning chemotherapy-related nausea and/or vomiting. However, chemotherapy is known to cause as many as 19 GI symptoms, including anticipatory nausea, anticipatory vomiting, dysphagia, eructation, xerostomia, oral mucositis, dysgeusia, anorexia, retching, nausea, vomiting, pyrosis, early satiety, bloating, diarrhea, constipation, rectal itching, rectal burning, and flatulence (Cherwin, 2012).
Patients with a hematologic malignancy are at particular risk for GI symptoms because of the high doses of chemotherapy needed to produce an effect on cancer cells in the blood, lymphatic tissue, and bone marrow (Camp-Sorrell, 2010). Few studies of chemotherapy-related symptoms focus exclusively on patients with a hematologic malignancy. Most report symptom data pooled from participants with solid tumors and hematologic malignancies (Bovio, Montagna, Bariani, & Baiardi, 2009; Spichiger et al., 2011). Bolukbas and Kutluturkan (2014) conducted one of the few studies reporting symptom prevalence, duration, severity, and distress exclusively among patients with a hematologic malignancy. Using the Memorial Symptom Assessment Scale (MSAS) to assess symptoms in patients with non-Hodgkin lymphoma receiving chemotherapy, they reported at least moderate symptom severity ratings on all 10 GI symptoms assessed.
A shortage of research exists on chemotherapy-related GI symptoms other than nausea and vomiting, perhaps related to the belief that other GI symptoms are not as prevalent or of sufficient duration, severity, or distress to be considered clinically relevant. Kazdin (1999) defined clinical relevance (or significance) of a symptom as the effect or meaning it has to the experiencing individual, including “difficulties in meeting role demands, interacting with others, and being restricted by what one can do in settings, situations, and activities in which one is involved” (p. 335). Although few studies have operationalized criteria for clinical relevance of symptoms, determinations appear to be based on multiple dimensions and consequences of the symptom experience. For example, Hwang et al. (2014) described clinically relevant fatigue as “a major factor causing significant decreases in cancer patients’ ability to perform usual activities and reduced quality of life” (p. 1454). Research has shown that symptoms rated as moderate to severe have a significant impact on levels of physical functioning and the ability to carry out daily tasks of living (Given et al., 2008) and interfere with quality of life (QOL) (Cleeland et al., 2014). This finding is consistent with symptom theories, such as the Symptom Experience Model (SEM), which highlights the importance of multiple symptom dimensions (including duration, severity, and distress), and the detrimental impact of symptoms on daily life and QOL (Armstrong, 2003). Therefore, the authors of the current article contend that, for a symptom to be clinically relevant, it should occur in more than a small subset of patients and be rated as greater than mild in at least one symptom dimension.
One of the major issues limiting GI symptom research is the general lack of representation of GI symptoms on symptom questionnaires. Cherwin (2012) reviewed GI symptom cluster literature and found no symptom questionnaires that included an item profile comprehensive of all GI symptoms. The symptom questionnaire used most frequently only assessed four GI symptoms: nausea, vomiting, anorexia, and xerostomia. In addition, few of the cancer symptom questionnaires allowed multidimensional evaluation of GI symptoms, with most evaluating only symptom severity. Research has shown that both symptom severity and symptom distress significantly correlate with symptom interference with daily life (McMillan, Tofthagen, & Morgan, 2008) and QOL (Akin, Can, Aydiner, Ozdilli, & Durna, 2010). By only describing symptoms based on one symptom dimension, a large piece of the patient symptom experience is overlooked and potentially unmanaged.
By focusing on patients with hematologic disorders receiving chemotherapy and by assessing symptoms across the dimensions of presence, duration, severity, and distress, the current work advances science in the understudied area of chemotherapy-related GI symptoms, beyond nausea and vomiting. The primary aim of this study was to describe prevalence, duration, severity, and distress of GI symptoms at weekly intervals during a three-week cycle of chemotherapy in a sample of patients with a hematologic malignancy. The second aim of this study was to determine if those GI symptoms identified as clinically relevant were included as items on common cancer symptom questionnaires.
Methods
Design and Sample
This longitudinal descriptive study recruited a convenience sample of patients (N = 105) with a hematologic malignancy receiving inpatient or outpatient chemotherapy at a large comprehensive cancer center from December 2013 to December 2014. Participants were adults, aged 18 years or older, who had a diagnosis of leukemia, lymphoma, or myelodysplastic syndrome, were able to read and write in English, were beginning at least a third cycle of chemotherapy, and were receiving chemotherapy in three-week (21-day) or four-week (28-day) cycles. Patients were excluded if they had cognitive impairment or confusion that would prevent completing questionnaires. To ensure that GI symptoms were primarily chemotherapy-related, patients were excluded if they were receiving radiation therapy currently or within the past six months, or if they had a GI comorbidity, such as irritable bowel syndrome or gastroesophageal reflux disease, that preceded the cancer diagnosis. Participants were recruited at or after the third administration of chemotherapy to allow time for conditioned responses, such as anticipatory nausea and vomiting, to occur (Hickok, Roscoe, & Morrow, 2001). Cycle lengths of three and four weeks were chosen because these are the most common schedules for hematology chemotherapy regimens (Chu, 2010). The current study combines symptom questionnaire data from a smaller feasibility study with equivalent procedures (N = 17) and the larger study that followed (N = 88). Participants in the larger study were screened for preexisting GI comorbidities through self-report prior to enrollment. Participants in the smaller feasibility study did not complete this screening item; however, medical record reviews revealed that none of these participants had a documented preexisting GI comorbidity. Although medical record review does not ensure that these 17 patients did not have preexisting GI comorbidities, it provided reasonable evidence to combine the two samples.
Instruments
A demographic questionnaire was used to collect the participants’ age, gender, ethnicity, race, education, partner status, and income. Clinical characteristics were collected from the medical record, including diagnosis, chemotherapy regimen dose (e.g., standard or reduced dose), emetogenicity rating, cycle number, and any GI supportive medications. A GI supportive medication was defined as any medication with a mechanism to influence GI symptoms and included antiemetics, antidiarrheals, antacids, stool softeners, anti-gas agents, appetite stimulants, GI motility stimulants, topical or systemic analgesics, and oral care products (i.e., medicated mouthwash or saliva substitute). GI supportive medications were collected from the medical record to describe participants’ management of cancer-related GI symptoms.
GI symptoms were measured using a modified version of the MSAS (Portenoy et al., 1994). The modified MSAS included 41 symptoms, 32 from the original MSAS and 9 additional GI symptoms (total of 19 GI and 22 other symptoms). The additional chemotherapy-related GI symptoms were identified through a review of literature and consultation with cancer symptom experts. Participants were asked to review the list of symptoms and indicate if a symptom was present in the past week. For each symptom present, duration was rated on a 1–4 scale, from “rarely” to “almost constantly”; severity was rated on a 1–4 scale, from “slight” to “very severe”; and distress was rated on a 0–4 scale, from “not at all” to “very much.” Three GI symptoms were not appropriate for duration ratings (e.g., oral mucositis, dysgeusia, constipation) because they are symptoms that, by nature, do not come and go on a day-by-day basis but are typically noted as being either present or absent. Two items assessing anticipatory nausea and anticipatory vomiting asked participants to rate these symptoms as they occurred from the evening prior up to the day of chemotherapy because, by definition, anticipatory nausea and vomiting only occur within 24 hours prior to chemotherapy administration (Hickok et al., 2001). The original MSAS has well-documented reliability and validity among the oncology population (a = 0.83–0.88) (Chang, Hwang, Feuerman, & Kasimis, 2000; Kirkova et al., 2006; Portenoy et al., 1994). The modified MSAS, with the additional GI symptoms, was found to have good content validity among practicing cancer clinician experts (content validity index = 0.83) (Cherwin & Kwekkeboom, 2013) and good internal consistency (alpha = 0.87–0.9) in the current sample. This article reports data from the 19 GI symptoms only.
Procedure
Hematology practitioners (RNs, NPs, or MDs) identified eligible patients and referred them to the nurse researcher, who met with potential participants to explain the study and obtain consent. After obtaining consent, the nurse researcher arranged to meet the participant on the first day of a new cycle of chemotherapy (day 1). On day 1, participants completed the demographic questionnaire and the modified MSAS. Participants were asked to provide weekly symptom assessments during the three weeks of ongoing treatment. On days 7, 14, and 21, participants completed the modified MSAS again at home and returned the completed questionnaire by mail.
Data Analyses
Descriptive statistics were used to summarize demographic and clinical characteristics of this sample, and to describe the prevalence, duration, severity, and distress of GI symptoms at each of the four time points (days 1, 7, 14, and 21). Symptom duration, severity, and distress ratings were summarized only from those who reported the symptom as “present.”
To determine if clinically relevant GI symptoms were represented on cancer symptom questionnaires, the authors first developed an operational definition of a clinically relevant symptom. For the purposes of this study, a symptom was deemed clinically relevant if it (a) was present in 15% or more of the sample and (b) had duration, severity, or distress rated moderate or severe at some point during the chemotherapy cycle. In identifying a core set of treatment-related symptoms for assessment, Cleeland et al. (2013) deemed symptoms to be highly prevalent and worthy of assessment if the prevalence was at least 15%. Several investigators have described increases in interference with daily life when cancer symptoms progress from mild to moderate or severe (Given et al., 2008; Jeon, Given, Sikorskii, & Given, 2009; Serlin, Mendoza, Nakamura, Edwards, & Cleeland, 1995). Using the verbal descriptors from the MSAS, a symptom rating of 2 or greater differentiated between mild and moderate symptom duration, severity, or distress. Symptoms with clinically relevant ratings were compared to those on 12 multisymptom questionnaires identified in a systematic review of cancer symptom assessment instruments (Kirkova et al., 2006).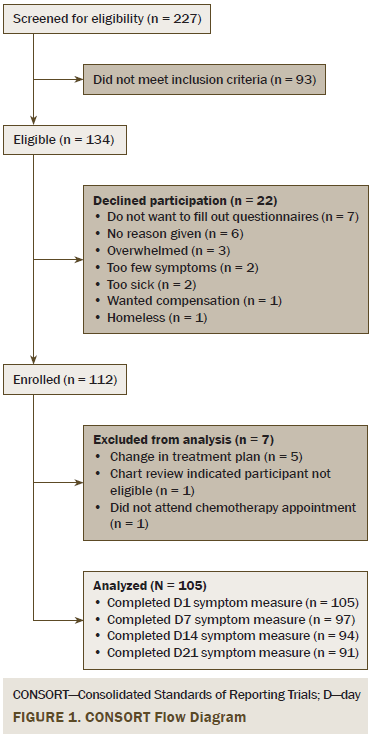
Results
Sample Characteristics
In total, 227 patients were screened. Eighty-four percent of eligible patients agreed to participate, and 94% of those (N = 105) provided data for analysis. Flow of participants through the study is reported in Figure 1. Demographic characteristics of the sample are reported in Table 1.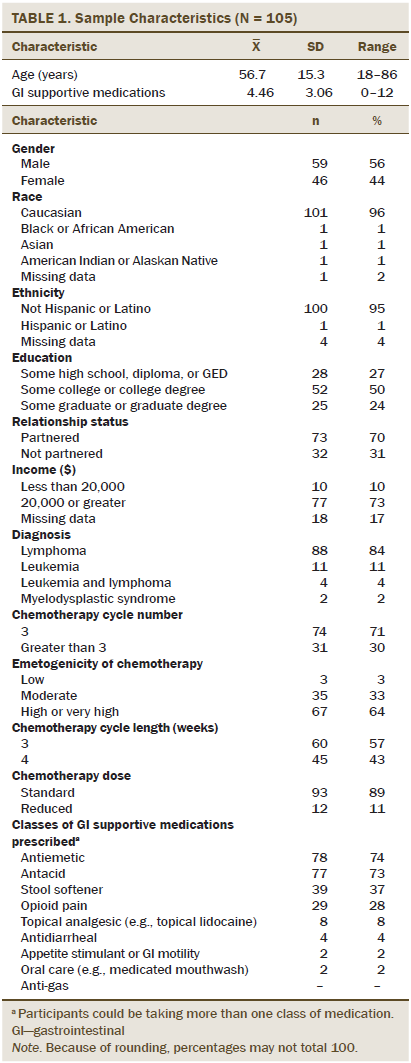
Gastrointestinal Symptom Burden and Symptom Prevalence
Patients reported a mean of 3.46 (SD = 3.2) GI symptoms at day 1, 4.43 (SD = 3.08) GI symptoms at day 7, 3.43 (SD = 3.09) GI symptoms at day 14, and 3.21 (SD = 3.27) GI symptoms at day 21. Prevalence rates of individual GI symptoms ranged from 3% (retching, days 7 and 21) to 51% (nausea, day 7; dysgeusia, day 7). Twelve GI symptoms were reported by at least 15% of participants at one or more assessment points, including xerostomia (35%–47%), anorexia (25%–38%), pyrosis (14%–24%), eructation (10%–22%), bloating (23%–33%), nausea (25%–51%), early satiety (11%–15%), flatulence (40%–44%), diarrhea (13%–25%), dysgeusia (35%–51%), oral mucositis (13%–23%), and constipation (18%–35%). Seven of these GI symptoms (xerostomia, anorexia, bloating, nausea, flatulence, dysgeusia, and constipation) were reported by 15% or more of the participants at all four points in time (see Tables 2–5).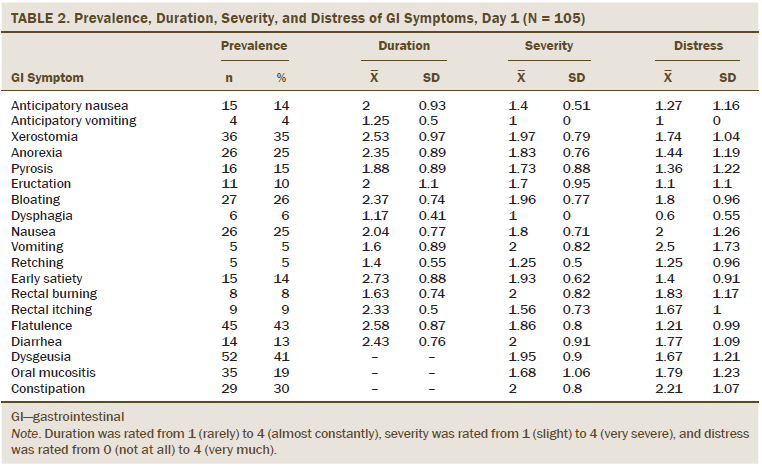
Gastrointestinal Symptom Duration
Among those reporting the GI symptoms as present, mean duration ratings ranged from 1.17 (dysphagia, day 1) to 2.73 (early satiety, day 1) on the 1 (rarely) to 4 (almost constantly) scale. Twelve GI symptoms had duration ratings of 2 or greater at one or more time points during the chemotherapy cycle, including anticipatory nausea (2), xerostomia (2.22–2.7), anorexia (2.35–2.68), pyrosis (1.83–2.15), eructation (2–2.43), bloating (2.1–2.69), nausea (1.96–2.18), early satiety (2.4–2.73), rectal burning (1.58–2.2), rectal itching (1.83–2.33), flatulence (2.25–2.58), and diarrhea (1.94–2.43). Six GI symptoms (xerostomia, anorexia, pyrosis, bloating, early satiety, and flatulence) were rated 2 or greater for all four points in time.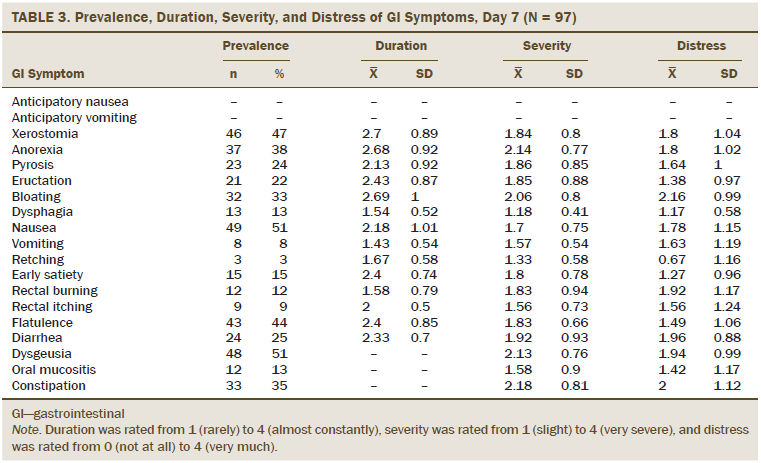
Gastrointestinal Symptom Severity
Among those reporting the GI symptoms as present, mean severity ranged from 1 (anticipatory vomiting and dysphagia, day 1) to 2.18 (constipation, day 7) on the 1 (slight) to 4 (very severe) scale. Nine GI symptoms had severity ratings of 2 or greater at some point during the chemotherapy cycle, including anorexia (1.7–2.14), pyrosis (1.73–2.15), bloating (1.81–2.06), vomiting (1.5–2), early satiety (1.8–2.09), rectal burning (1.6–2), diarrhea (1.67–2), dysgeusia (1.84–2.13), and constipation (1.79–2.18). None of the GI symptoms had severity ratings of 2 or greater for all four points in time.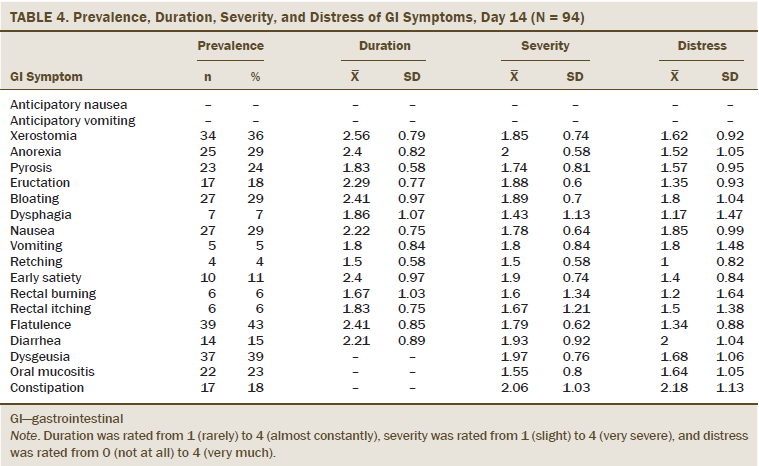
Gastrointestinal Symptom Distress
Among those reporting the GI symptoms as present, mean distress ratings ranged from 0.5 (dysphagia, day 1) to 2.5 (vomiting, day 1) on the 0 (not at all) to 4 (very much) scale. Five GI symptoms had a distress rating of 2 or greater at some point during the chemotherapy cycle, including bloating (1.55–2.16), nausea (1.64–2), vomiting (1.25–2.5), diarrhea (1.39–2), and constipation (1.94–2.21). None of the GI symptoms had distress ratings of 2 or greater for all four points in time.
Presence of Clinically Relevant Gastrointestinal Symptoms on Cancer Symptom Questionnaires
Based on the authors’ conceptualization of a clinically relevant symptom, 11 GI symptoms were found to meet the threshold for meaningful prevalence ratings and a meaningful duration, severity, or distress rating. These symptoms included xerostomia, anorexia, pyrosis, eructation, bloating, nausea, early satiety, flatulence, diarrhea, dysgeusia, and constipation. The authors compared the GI symptoms deemed clinically relevant to the GI symptoms present on 12 cancer multisymptom questionnaires identified in the review by Kirkova et al. (2006). Of the 11 clinically relevant GI symptoms, 8 were included on at least one cancer multisymptom questionnaire. Only 3 of the 11 clinically relevant GI symptoms were included on more than half of the cancer symptom questionnaires. Nausea was assessed on all instruments (n = 12, anorexia on 10, constipation on 9, diarrhea and xerostomia on 5, dysgeusia and pyrosis on 2, and bloating on 1. Eructation, early satiety, and flatulence were not assessed by any of the cancer symptom questionnaires (see Table 6).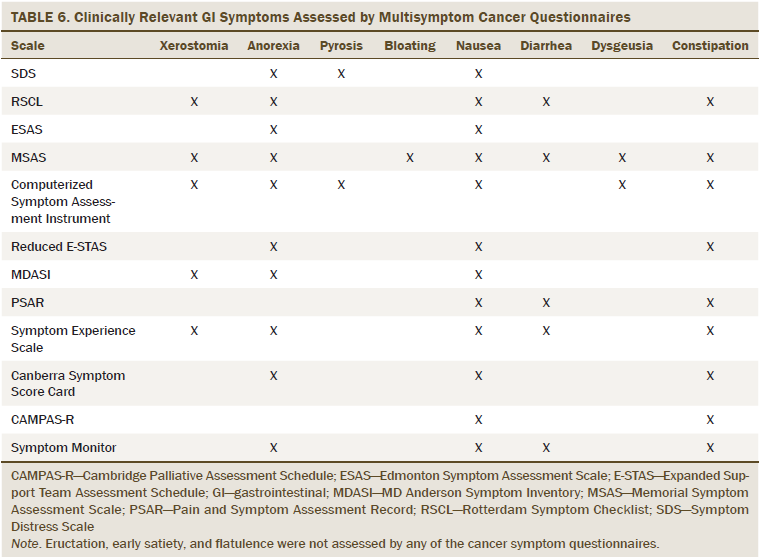
Discussion
The authors found that most patients experienced several concurrent GI symptoms at any given assessment point; however, all 19 symptoms were reported by some participants. This would indicate that a wide variety in GI symptoms are experienced across participants, with many individual differences in GI symptoms between participants.
The variety in GI symptoms experienced by participants is perhaps related, in large part, to the chemotherapy regimens used. Indeed, although the authors aimed for a more homogenous sample with regard to disease characteristics (e.g., patients with a hematologic malignancy not receiving a stem cell transplantation), participants could be receiving 1 of 22 chemotherapy regimens. The most commonly used chemotherapy regimens in this sample included RCHOP/CHOP (cyclophosphamide [Cytoxan®], doxorubicin [Adriamycin®], vincristine [Oncovin®], and prednisone [Deltasone®] with or without rituximab [Rituxan®]) (n = 32, 31%), rituximab and bendamustine (Treanda®) (n = 23, 22%), ABVD (doxorubicin, bleomycin [Blenoxane®], vinblastine [Velbe®], and dacarbazine [DTIC®]) (n = 13, 12%), and R-EPOCH/EPOCH (prednisone, etoposide [Toposar®], vincristine, cyclophosphamide, and doxorubicin with or without rituximab) (n = 12, 11%). Each of the regimens has a unique combination of drugs, and the individual chemotherapies that comprise these regimens have differing GI toxicities. For example, vincristine is associated with constipation (Yarbro, Frogge, & Goodman, 2005); doxorubicin and etoposide are associated with mucositis (Yarbro et al., 2005); bendamustine is associated with diarrhea and constipation (Kahl et al., 2010); and cyclophosphamide, dacarbazine, and doxorubicin are associated with dysgeusia (Yarbro et al., 2005). The GI symptom duration, severity, and distress experienced by any given patient depends on the dose of the chemotherapy used, as well as how each individual drug interacts with others in the multidrug regimen.
The authors also found that, although a large number of GI symptoms had meaningful prevalence and duration ratings, severity and distress ratings were relatively mild in the current sample. The findings conflict with those of Bolukbas and Kutluturkan (2014), who reported mean severity of 2 or greater on 10 MSAS GI symptom items, and distress ratings of 2 or greater on 5 GI symptoms among patients with non-Hodgkin lymphoma. GI symptom reports in the solid tumor population are mixed, with 5 of 10 MSAS GI symptoms rated 2 or greater in severity and none of the GI symptoms rated 2 or greater in distress among women with breast cancer (Hofsø, Miaskowski, Bjordal, Cooper, & Rustøen, 2012). Dapueto, Abreu, Francolino, and Levin (2014) reported that all 10 GI symptoms had a mean distress rating of 2 or greater in patients with various solid tumor diagnoses.
One possible explanation for the low symptom severity and distress reports in the sample could be related to proactive symptom prevention efforts. The research done by Hofsø et al. (2012), Dapueto et al. (2014), and Bolukbas and Kutluturkan (2014) all took place outside of the United States, where supportive medication regimens may be different than those offered in U.S. hospitals and clinics. None of these studies reported the symptom management medications their participants may have received. Review of the current participants’ medical records demonstrated that most had nearly five (mean = 4.46) GI supportive medications prescribed. In the cancer center where this study took place, all chemotherapy treatment protocols include at least a preventive antiemetic regimen. It is reasonable to conclude that guideline-based aggressive prevention and management of chemotherapy-related symptoms may have resulted in well-controlled symptoms during this active period of chemotherapy treatment.
The authors chose to exclude participants with preexisting GI comorbidities to ensure a high probability that the GI symptoms reported were from chemotherapy and not related to other reasons. However, GI symptoms may potentially be worse in patients who are already experiencing GI comorbidities, such as irritable bowel syndrome or gastric reflux. Future studies should explore the GI symptom experiences of these patients because their symptoms may be more difficult to manage and need more intensive intervention.
Through examining symptom reports, the authors found that 11 of 19 GI symptoms assessed met the criteria to be considered clinically relevant. Despite the large number of clinically relevant GI symptoms, many cancer symptom questionnaires only assess a handful of these symptoms, and none of the cancer symptom questionnaires identified by Kirkova et al. (2006) assess all of the clinically relevant symptoms identified by the current authors. Studies that review cancer symptom questionnaires repeatedly demonstrate that the most commonly used cancer multisymptom questionnaire is the MD Anderson Symptom Inventory (MDASI) (Cherwin, 2012; Kim, Dodd, Aouizerat, Jahan, & Miaskowski, 2009; Reilly et al., 2013). Although the MDASI has a number of benefits that include brevity and established reliability and validity, it includes only four GI symptoms assessed solely on the dimension of symptom severity. Treatment-related symptoms in people with cancer can be highly varied and span multiple symptom dimensions. A symptom questionnaire more comprehensive than the MDASI may be necessary to more completely capture the GI symptom experience. The MSAS has been recommended as superior for GI symptom assessment (Cherwin, 2012; Kirkova et al., 2006).
In addition to advocating for the need to use more comprehensive symptom questionnaires, the authors also argue that a need exists to refine symptom management efforts to keep pace with advances in cancer treatment and toxicity prophylaxis. Nausea and vomiting are among the symptoms most commonly included on cancer symptom questionnaires (12 and 10, respectively) (Kirkova et al., 2006), however, the current data demonstrate that vomiting was not clinically relevant in a sample where antiemetic prophylaxis was routine. Indeed, a majority of the participants in this study received at least one antiemetic. Without antiemetics, vomiting prevalence, duration, severity, and distress may be substantially higher. The authors argue that assessing less well-known but clinically relevant GI symptoms may raise attention to their management, and could ultimately lead to better guideline-based prevention and management, as has been the case for chemotherapy-induced vomiting.
Educating patients about the need to report symptoms to their healthcare providers, regardless of whether or not they are directly queried, is also important. As patient advocates, nurses can empower patients to report symptoms that interfere with their lives and provide recommendations for intervention. By encouraging patients to self-monitor and report bothersome symptoms, symptom management efforts can be greatly improved.
The results from this study show that a variety of GI symptoms contributed to patients’ symptom burden. Other clinically relevant GI symptoms included pyrosis, xerostomia, anorexia, early satiety, bloating, eructation, flatulence, constipation, and diarrhea, and each of these symptoms has pharmacologic management options available. Although many patients did receive medication to address pyrosis (73%) and constipation (37%), only a small number of participants received medications to address diarrhea (4%), appetite or GI motility issues (2%), or xerostomia (2%). No patients received medications to prevent or control issues with bloating, eructation, or flatulence. Of note, bloating and diarrhea were prevalent symptoms and met the threshold to be considered clinically relevant for duration, severity, and distress, but were poorly managed pharmacologically in this sample.
One explanation for the underuse of medications for some of the GI symptoms could be that medications for many GI symptoms are not built into existing treatment protocols. Another possible explanation is that the GI symptoms traditionally associated with cytotoxic chemotherapies are changing as new targeted therapeutic agents, immunotherapies, and cancer vaccines are introduced. Symptoms that were once prevalent in the earlier days of chemotherapy treatment, such as vomiting, are better managed through improved antiemetic medications. Simultaneously, previously unrecognized or less prevalent GI symptoms, such as early satiety, eructation, bloating, flatulence, and diarrhea, may be emerging as problematic symptoms with higher occurrence rates. Symptom assessment and management efforts should evolve as therapies and protocols advance. Comprehensive assessment is the first step toward understanding which symptoms are emerging as relevant for a patient population.
Limitations
The study used a relatively small convenience sample (N = 105) of exclusively patients with hematologic disorders recruited from a National Cancer Institute–designated comprehensive cancer center. Therefore, results are not representative of patients with other cancer diagnoses or those receiving care in community cancer settings and cannot be generalized to the cancer population as a whole. Limitations in the sampling design may have influenced how symptoms were reported. Although the symptom reports used in this study came entirely from patients with hematologic malignancies, the chemotherapy regimens differed. The authors also included participants with three- and four-week treatment cycles but only collected symptom data for three weeks of treatment. In addition, the authors did not note whether a patient’s disease was recurrent or not. All three of these factors can be linked to patient symptom experience. Finally, the GI symptom duration, severity, and distress ratings do not reflect the entire sample, but rather only those who experienced the symptom. Therefore, the means, as reported, may be biased by the experiences of a small number of outliers. The authors attempted to account for this by operationalizing the definition of clinically relevant symptoms to include a meaningful threshold of prevalence combined with a relevant duration, severity, or distress rating, therefore excluding any symptoms with consistently low prevalence ratings.
The limitations of this work are countered by a number of strengths. First, the authors attempted to ensure that any GI symptoms reported could be attributed to treatment and not to other causes by excluding patients with noncancer causes of GI symptoms. Second, the authors assessed GI symptoms across four relevant symptom dimensions and over time. A vast majority of symptom research is cross-sectional and focuses on a single symptom dimension. By including multiple symptom dimensions and multiple time points, this work provides a more complete picture of the week-to-week symptom experience of a patient with a hematologic malignancy receiving chemotherapy.
Implications for Practice and Conclusion
The results of the study indicate that patients experience a wide variety of GI symptoms and that current cancer symptom questionnaires are missing a large number of clinically relevant GI symptoms. Cancer symptom research has shifted toward a personalized medicine approach: treating each patient’s experience as unique and acknowledging that one-size-fits-all assessment and intervention does not serve patients well. By using cancer symptom questionnaires with narrow assessments, the authors cannot hope to properly document and intervene for symptoms relevant to each person. The results of this study should influence researchers to use more comprehensive symptom assessments and update their knowledge of what symptoms are most meaningful to patients; however, further research in larger samples is needed to confirm findings and clarify directions for changes in practice. A revision of commonly used symptom measures is needed to add symptoms that may be common toxicities of new therapies and include symptom dimensions beyond prevalence and severity. With new symptom data and new symptom measures, researchers can work with clinicians for better symptom management in patients with cancer.
References
Akin, S., Can, G., Aydiner, A., Ozdilli, K., & Durna, Z. (2010). Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. European Journal of Oncology Nursing, 14, 400–409. doi:10.1016/j.ejon.2010.01.003
Armstrong, T.S. (2003). Symptoms experience: A concept analysis. Oncology Nursing Forum, 30, 601–606. doi:10.1188/03.ONF.601-606
Bolukbas, F., & Kutluturkan, S. (2014). Symptoms and symptom clusters in non-Hodgkin’s lymphoma patients in Turkey. Asian Pacific Journal of Cancer Prevention, 15, 7153–7158. doi:10.7314/APJCP.2014.15.17.7153
Bovio, G., Montagna, G., Bariani, C., & Baiardi P. (2009). Upper gastrointestinal symptoms in patients with advanced cancer: Relationship to nutritional and performance status. Supportive Care in Cancer, 17, 1317–1324. doi:10.1007/s00520-009-0590-x
Camp-Sorrell, D. (2010). Chemotherapy toxicities and management. In C.H. Yarbro, D. Wujcik, & B.H. Gobel (Eds.), Cancer nursing: Principles and practice (pp. 458–503). Sudbury, MA: Jones and Bartlett.
Chang, V.T., Hwang, S.S., Feuerman, M., & Kasimis, B.S. (2000). Symptom and quality of life survey of medical oncology patients at a Veterans Affairs medical center: A role for symptom assessment. Cancer, 88, 1175–1183. doi:10.1002/(SICI)1097-0142 (20000301)88:5<1175::AID-CNCR30>3.0.CO;2-N
Cherwin, C., & Kwekkeboom, K. (2013, March). Content validity of the GI module of the modified Memorial Symptom Assessment Scale (MSAS) in hematologic cancer patients receiving chemotherapy. Poster presented at the 37th annual Midwest Nursing Research Society Conference in Chicago, IL.
Cherwin, C.H. (2012). Gastrointestinal symptoms in patients with cancer: A synthesis of the literature. Oncology Nursing Forum, 39, 157–165. doi:10.1188/12.ONF.157-165
Chu, E. (2010). Pocket guide to chemotherapy protocols. Sudbury, MA: Jones and Bartlett.
Cleeland, C.S., Mayer, M., Dreyer, N.A., Yim, Y.M., Yu, E., & Kaufman, P.A. (2014). Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: Results from a substudy of the VIRGO observational cohort study. Breast, 23, 763–769. doi:10.1016/j.breast.2014.08.004
Cleeland, C.S., Zhao, F., Chang, V.T., Sloan, J.A., O’Mara, A.M., & Fisch, M.J. (2013). The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer, 119, 4333–4340. doi:10.1002/cncr.28376
Dapueto, J.J., Abreu, M.D.C., Francolino, C., & Levin, R. (2014). Psychometric assessment of the MSAS-SF and the FACIT-Fatigue Scale in Spanish-speaking patients with cancer in Uruguay. Journal of Pain Symptom Management, 47, 936–945. doi:10.1016/j.jpainsymman.2013.06.020
Given, B., Given, C.W., Sikorskii, A., Jeon, S., McCorkle, R., & Decker D. (2008). Establishing mild, moderate, and severe scores for cancer-related symptoms: How consistent and clinically meaningful are interference-based severity cut-points? Journal of Pain and Symptom Management, 35, 126–135. doi:10.1016/j.jpainsym man.2007.03.012
Hickok, J.T., Roscoe, J.A., & Morrow, G.R. (2001). The role of patients’ expectations in the development of anticipatory nausea related to chemotherapy for cancer. Journal of Pain and Symptom Management, 22, 843–850. doi:10.1016/S0885-3924(01)00317-7
Hofsø, K., Miaskowski, C., Bjordal, K., Cooper, B.A., & Rustøen, T. (2012). Previous chemotherapy influences the symptom experience and quality of life of women with breast cancer prior to radiation therapy. Cancer Nursing, 35, 167–177. doi:10.1097/NCC.0b013e31821f5eb5
Hwang, I.C., Yun, Y.H., Kim, Y.W., Ryu, K.W., Kim, Y.A., & Sohn, T.S. (2014). Factors related to clinically relevant fatigue in disease-free stomach cancer survivors and expectation–outcome consistency. Supportive Care in Cancer, 22, 1453–1460. doi:10.1007/s00520-013-2110-2.
Jeon, S., Given, C.W., Sikorskii, A., & Given, B. (2009). Do interference-based cut-points differentiate mild, moderate, and severe levels of 16 cancer-related symptoms over time? Journal of Pain and Symptom Management, 37, 220–232. doi:10.1016/j.jpainsymman.2008.01.010
Kahl, B.S., Bartlett, N.L., Leonard, J.P., Chen, L., Ganjoo, K., & Cheson, B.D. (2010). Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: Results from a multicenter study. Cancer, 116, 106–114. doi:10.1002/cncr.24714
Kazdin, A.E. (1999). The meanings and measurement of clinical significance. Journal of Consulting and Clinical Psychology, 67, 332–339.
Kim, J.E., Dodd, M.J., Aouizerat, B.E., Jahan, T., & Miaskowski, C. (2009). A review of the prevalence and impact of multiple symptoms in oncology patients. Journal of Pain and Symptom Management, 37, 715–736. doi:10.1016/j.jpainsymman.2008.04.018
Kirkova, J., Davis, M.P., Walsh, D., Tiernan, E., O’Leary, N., & Russell, K.M. (2006). Cancer symptom assessment instruments: A systematic review. Journal of Clinical Oncology, 24, 1459–1473. doi:10.1200/JCO.2005.02.8332
McMillan, S.C., Tofthagen, C., & Morgan, M.A. (2008). Relationships among pain, sleep disturbances, and depressive symptoms in outpatients from a comprehensive cancer center. Oncology Nursing Forum, 35, 603–611. doi:10.1188/08.ONF.603-611
Mitchell, E.P. (2006). Gastrointestinal toxicity of chemotherapeutic agents. Seminars in Oncology, 33, 106–120.
Portenoy, R.K., Thaler, H.T., Kornblith, A.B., Lepore, J.M., Friedlander-Klar, H., Kiyasu, E., . . . Norton, L. (1994). The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer, 30A, 1326–1336.
Reilly, C.M., Bruner, D.W., Mitchell, S.A., Minasian, L.M., Basch, E., Dueck, A.C., & Reeve, B.B. (2013). A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Supportive Care in Cancer, 21, 1525–1550. doi:10.1007/s00520-012-1688-0
Serlin, R.C., Mendoza, T.R., Nakamura, Y., Edwards, K.R., & Cleeland, C.S. (1995). When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain, 61, 277–284.
Spichiger, E., Müller-Fröhlich, C., Denhaerynck, K., Stoll, H., Hantikainen, V., & Dodd, M. (2011). Prevalence of symptoms, with a focus on fatigue, and changes of symptoms over three months in outpatients receiving cancer chemotherapy. Swiss Medical Weekly, 141, w13303. doi:10.4414/smw.2011.13303
Yarbro, C.H., Frogge, M.H., & Goodman, M. (2005). Chemotherapy toxicities and management. Cancer nursing: Principles and practice (6th ed., pp. 419–437). Sudbury, MA: Jones and Bartlett.
About the Author(s)
Cherwin is a faculty associate in the College of Nursing at the University of Iowa in Iowa City, and Kwekkeboom is a professor in the School of Nursing at the University of Wisconsin in Madison. This research was funded by grants (F31NR014062 and R01NR013468) from the National Institute of Nursing Research of the National Institutes of Health, a grant (121310-DSCH-11-278-01-SCN) from the American Cancer Society Doctoral Degree Scholarship in Cancer Nursing, and an Eckburg Research Award from the School of Nursing at the University of Wisconsin in Madison. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Both authors contributed to the conceptualization and design, analysis, and manuscript preparation. Cherwin completed the data collection and provided statistical support. Cherwin can be reached at catherine-cherwin@uiowa.edu, with copy to editor at ONFEditor@ons.org. Submitted October 2015. Accepted for publication December 17, 2015.

