Fatigue Self-Management Behaviors in Patients With Advanced Cancer: A Prospective Longitudinal Survey
Purpose/Objectives: To explore the fatigue self-management behaviors and factors associated with effectiveness of these behaviors in patients with advanced cancer.
Design: Prospective longitudinal interviewer-administered survey.
Setting: Royal Brisbane and Womenʼs Hospital in Queensland, Australia.
Sample: 152 outpatients with metastatic breast, lung, colorectal, and prostate cancer experiencing fatigue were recruited.
Methods: Patients were surveyed on three occasions: at baseline, four weeks, and eight weeks.
Main Research Variables: Fatigue self-management behavior (perceived effectiveness, self-efficacy, and frequency), medical and demographic characteristics (sites of primary cancer and metastasis, comorbidity, performance status), social support, depression, anxiety, and other symptoms were assessed.
Findings: The participants reported moderate levels of fatigue at baseline and maintained moderate levels at four and eight weeks. On average, participants consistently used about nine behaviors at each time point. Factors significantly associated with higher levels of perceived effectiveness of fatigue self-management behaviors were higher self-efficacy, higher education level, and lower levels of depressive symptoms.
Conclusions: The findings of this study demonstrate that patients with cancer, even those with advanced disease, still want and are able to use a number of behaviors to control their fatigue. Self-management interventions that aim to enhance self-efficacy and address any concurrent depressive symptoms have the potential to reduce fatigue severity.
Implications for Nursing: Nurses are well positioned to play a key role in supporting patients in their fatigue self-management.
Jump to a section
Cancer-related fatigue (CRF) is a distressing symptom and is reported in about 74% of patients with advanced cancer and 88% of those who are in the last weeks of life (Solano, Gomes, & Higginson, 2006; Teunissen et al., 2007). Fatigue experiences are debilitating and can reduce the quality of life of people with advanced cancer. The understanding of the etiology and pathophysiology, patient experience, and management of this symptom has improved (Bower, 2014). However, CRF is still not well managed in a notable proportion of patients with advanced cancer (Bruera et al., 2013; Yennurajalingam et al., 2013).
The management of CRF is complex and can involve a combination of pharmacologic and nonpharmacologic strategies (Minton, Richardson, Sharpe, Hotopf, & Stone, 2010; Payne, Wiffen, & Martin, 2012). For example, maintaining sleep hygiene, conserving energy, and exercising are commonly used strategies (Minton et al., 2010). The strategies required to manage CRF often involve a collaborative effort between patients and health professionals. Although patient self-management is likely to be an important component of CRF management (National Comprehensive Cancer Network, 2015), limited research exists to understand its role, particularly in patients with advanced disease.
During the past two decades, the literature has reported a number of behaviors used by patients in response to CRF. The authors’ literature review identified five studies that examined the use and effectiveness of these self-management behaviors from the perspective of patients with cancer (Borthwick, Knowles, McNamara, Dea, & Stroner, 2003; Chalise, Pandey, & Chalise, 2012; Lou, 2011; Richardson & Ream, 1997; So & Tai, 2005). However, none of these studies focused on advanced cancer, nor did they follow patients over time, which limits the capacity to predict the outcomes of these behaviors. Although some may think that patients with advanced cancer do not engage in self-management because they are too ill, empirical evidence indicates that patients with cancer, even at the advanced stage, still want and are able to use a number of behaviors to control their symptoms (Hopkinson, 2007; Hopkinson, Wright, McDonald, & Corner, 2006; Miaskowski et al., 2004; Sand, Harris, & Rosland, 2009).
Understanding patients’ rationales for fatigue self-management behaviors, how they use them, and how effective they perceive them to be is important. This understanding can guide a collaborative self-management care plan, wherein health professionals and patients discuss mutually defined goals, action plans, education, resources, and community support to optimize evidence-based self-management behaviors. However, of the studies described previously (Borthwick et al., 2003; Chalise et al., 2012; Lou, 2011; Richardson & Ream, 1997; So & Tai, 2005), only one prospective cross-sectional study on Chinese patients with cancer (Lou, 2011) explored the factors influencing the perceived effectiveness of some fatigue self-management behaviors. Lou (2011) reported that higher self-efficacy scores, more support from the neighborhood, and earlier stages of cancer were the potential influencing factors associated with fatigue self-management effectiveness. These findings suggest that targeting a combination of disease-related, individual, and contextual factors is needed to optimize self-management in this cohort.
However, robust research is required to answer the following questions that arise from gaps in the literature:
• What are the management strategies that patients choose to use (i.e., patient preferences)?
• How effective are these strategies from the perspective of the patient?
• What are the factors associated with the effectiveness of these strategies?
Although some research has explored fatigue self-management behaviors in patients with cancer undergoing active treatment with curative intent (Fitch, Mings, & Lee, 2008; Lou, 2011; Richardson & Ream, 1997), information is limited concerning such issues in patients with advanced disease. A deeper understanding of such behaviors will assist with the design of appropriate patient-centered interventions for this population.
The exploration of factors that influence self-management is an essential step to advance the development of self-management theories and theory-based interventions. Grey, Knafl, and McCorkle’s (2006) self- and family management framework (SFMF) was selected to guide this study of factors influencing fatigue self-management. The SFMF is consistent with Bandura’s (1977) self-efficacy theory and has been widely used to understand factors that influence individuals in their self-management of chronic illness (Grady, 2008; Lou, 2011). The main premise of the model is that risk and protective factors (e.g., severity of condition, age, gender, psychosocial characteristics, social support) can influence individuals’ ability to manage chronic illness and, in turn, health outcomes. In particular, this model guided the operationalization of the multivariable modeling in this study.
Methods
This prospective longitudinal, interviewer-administered survey study examined the fatigue self-management behaviors (levels of frequency, effectiveness, and self-efficacy) in patients with advanced cancer and assessed relationships between patients’ perceived effectiveness and a number of SMSF-informed factors, including sociodemographic characteristics, diagnosis, self-efficacy associated with fatigue self-management behaviors, physical symptoms, emotional state (depressive symptoms and anxiety), and level of social support. The study was approved by the Royal Brisbane and Women’s Hospital and the Queensland University of Technology Human Research Ethics Committee. Informed consent was obtained before patient participation.
Sample and Setting
In this study, the authors defined patients with advanced cancer as those with metastasis to distant organs or distant lymph nodes (National Cancer Institute, n.d.). Patients who fulfilled the following criteria were recruited from Royal Brisbane and Women's Hospital in Queensland, Australia, from December 2011 to May 2012:
• Diagnosed with breast, lung, colorectal, or prostate cancer with at least one distant metastasis
• Aged 18 years and older
• Completed first-line anticancer therapy
• Reported an average fatigue intensity score greater than 3 of 10 on a numeric rating scale (NRS) in the past seven days
• Had a life expectancy of greater than two months
Because the purpose of this study was to explore how patients managed fatigue, the limit of 3 of 10 on the NRS ensured that participants experienced moderate to severe fatigue. Patients were excluded if they were unable to speak or understand English, were deemed by treating clinicians to be too ill to participate, or were cognitively incapable of informed consent.
Data Collection
The first interview was conducted by the researcher at the outpatient clinic, with subsequent interviews being conducted via telephone or face to face. Sociodemographic and clinical characteristics (age, gender, ethnicity, education, living arrangement, income, marital status, anticancer therapy, primary cancer site, and metastasis), Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987), Australia-modified Karnofsky Performance Status (Abernethy, Shelby-James, Fazekas, Woods, & Currow, 2005), Hospital Anxiety and Depression Scale, and Short Medical Outcomes Study Social Support Survey (Sherbourne & Stewart, 1991) were collected at baseline. Other measures, including the Brief Fatigue Inventory (BFI) (Mendoza et al., 1999), Edmonton Symptom Assessment System (Bruera, Kuehn, Miller, Selmser, & Macmillan, 1991), Self-Efficacy in Managing Symptoms Scale—Fatigue Subscale for Patients With Advanced Cancer (SMSFS-A) (Chan, Yates, & McCarthy, 2016) were administered at baseline, four weeks, and eight weeks.
The SMSFS-A was developed by the researchers for the purpose of examining the frequency, perceived effectiveness, and self-efficacy of using fatigue self-management behaviors in patients with advanced cancer (Chan, 2014). The SMSFS-A includes 16 distinct behaviors, which are grouped into five categories: activities, complementary or alternative therapies, cognitive, psychological, and nutrition (see Figure 1). The development of this instrument involved a comprehensive literature review (Chan, Yates, & McCarthy, 2011), semistructured interviews, expert panel reviews, and pilot testing (Chan et al., 2016). Preliminary testing of the tool indicated content validity, face validity, and acceptable test-retest reliability (Chan et al., 2016).
All final items of the tool achieved a content validity index of 1 for relevance. Bland–Altman plots demonstrated agreement between test-retest for self-perceived overall effectiveness and confidence levels for fatigue self-management.
Statistical Analysis
All analyses were conducted using SPSS®, version 17.0. Fatigue severity and perceived effectiveness and self-efficacy levels of fatigue self-management behaviors were summarized with mean scores and standard deviations. Percentages described the use of behaviors. Bivariate analyses, such as the Pearson correlation coefficient and analysis of variance, examined relationships between fatigue self-management effectiveness outcomes (total and global) and selected independent variables (gender, age, anxiety and depression, self-efficacy, primary tumor type, comorbidities, fatigue severity, other concurrent symptom severity, living arrangement, and level of social support). Generalized estimating equation modeling examined the factors that influenced the perceived effectiveness of self-management behaviors. Independent variables associated with the outcome at the bivariate level at a p value of less than 0.25 were entered into the multivariable analysis (Katz, 2011). In the current study, two separate multivariable models were tested using two effectiveness scores (global and total summary), on the premise that these scores provided different information and, therefore, could yield different predictive factors. That is, the summary score captured the effectiveness of each behavior used, where the weight of each behavior was equal. The global score, on the other hand, provided an overall rating of the effectiveness of the patient’s fatigue self-management strategies, where the weight of each behavior may not be equal (Coens, Bottomley, Efficace, Flechtner, & Aaronson, 2005). Because of the exploratory nature of the study, the authors were interested in both outcomes.
Results
One hundred fifty-two patients with advanced cancer participated in the current study. Table 1 summarizes the sociodemographic and clinical characteristics of participants at baseline. The majority had breast cancer and were relatively young. Participants were mainly partnered, did not complete high school, were low-income earners, reported good social support, and had a relatively high functional status. During the duration of the study, 21 patients were lost to follow-up.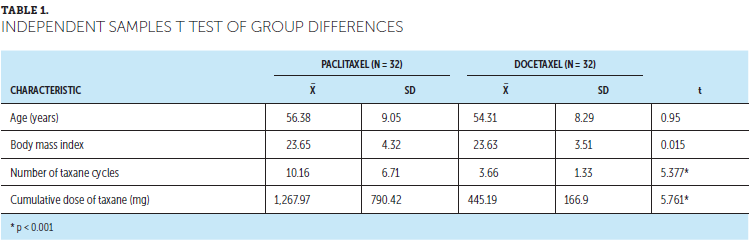
Fatigue Severity
Overall, fatigue severity scores were normally distributed at each time point (see Table 2). Fatigue severity was consistently moderate (4–6 of 10) from baseline to eight weeks. The descriptive data indicate that changes in fatigue severity and distress over time were not clinically meaningful (Revicki et al., 2006; Siu et al., 2013), with mean differences less than 1 between all time points. Therefore, no additional testing was undertaken to determine statistically significant differences between time points. Data were examined to determine any differences in fatigue severity between the group that dropped out and the group that did not in terms of age, gender, ethnicity, education, living arrangements, income level, marital status, functional status, and other symptom severity scores. At baseline, the group lost to follow-up had worse functional status (t = 2.36, p < 0.05, df = 151), had higher scores of fatigue at the moment (t = –0.238, p < 0.05, df = 151), and demonstrated a trend toward a higher level of usual fatigue over the past 24 hours (t = –2.09, p < 0.05, df = 151) compared to those who remained in the study. No other differences were seen between these two groups.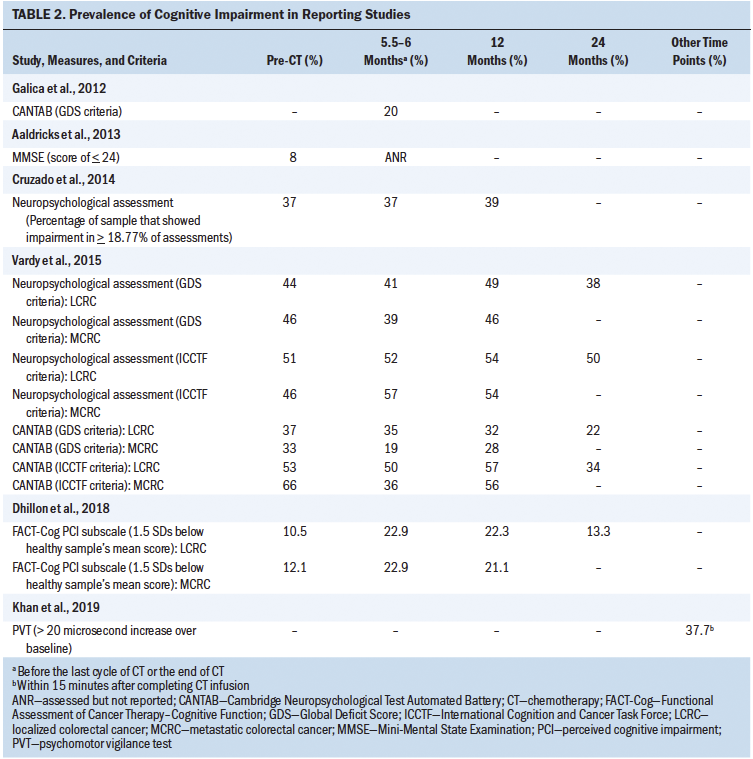
Frequency, Effectiveness, and Self-Efficacy of Self-Management Behaviors
The levels of perceived effectiveness of self-management behaviors and the respective frequency and perceived self-efficacy scores at each time point are summarized in Tables 3, 4, and 5. On average, the participants used about nine behaviors in total during the preceding seven days at all three time points. From the perspective of the participants, the five most effective behaviors for relieving fatigue were pacing activity, taking a short sleep during the day, planning activities to make the most of energy levels throughout the day, doing things that distract from fatigue, and doing things to improve sleep at night. Participants were generally confident in undertaking all of the behaviors, with mean self-efficacy scores greater than 7 of 10 (higher scores represent greater levels of self-efficacy). Regarding dropout, post-hoc analysis examined if any differences existed at baseline between those who dropped out and those who remained in the study for a number of outcomes associated with fatigue self-management (the total summary and global effectiveness scores, total summary and global self-efficacy scores, and total level of frequency of fatigue self-management). Using independent sample t tests, no differences were seen between groups in any outcome, except for total frequency of fatigue self-management (t = –2.82, p < 0.01). That is, participants who dropped out at either four or eight weeks used self-management strategies less frequently than those who remained in the study.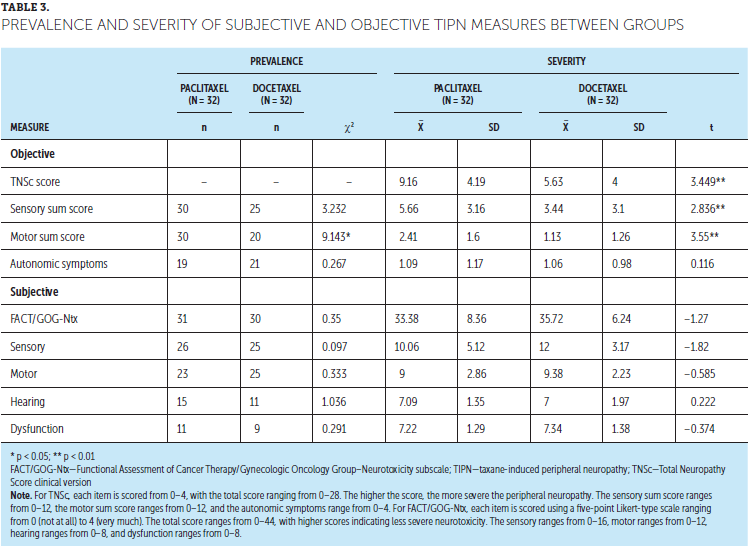
Predictive Factors of Perceived Effectiveness of Self-Management Behaviors
Higher levels of education (p = 0.02) and higher total (p = 0.001) and global self-efficacy (p < 0.001) scores were significant independent predictors of the total perceived effectiveness levels of self-management behaviors during a two-month period. Lower levels of depressive symptoms (p = 0.04) and higher levels of global self-efficacy (p < 0.001) were also significant independent predictors of the global perceived effectiveness levels of self-management behaviors during a two-month period. Although ethnicity was a significant predictor (p = 0.02), the small sample size of individuals who were non-Caucasian (n = 6, 4%) did not allow a robust evaluation for this question.
[[{"type":"media","view_mode":"media_original","fid":"26651","attributes":{"alt":"","class":"media-image","height":"586","typeof":"foaf:Image","width":"754"}}]]
Discussion
The purpose of the current study was to explore fatigue self-management behaviors in patients with distant metastatic disease with moderate to severe fatigue at baseline. Participants employed a range of behaviors to manage their fatigue. The number of behaviors used by patients in the current study is greater than that reported by two previous studies that measured this outcome in patients undergoing active anticancer therapy (Lou, 2011; Yates et al., 2001), with participants reporting five behaviors at a single time point. The difference could be because of the varied populations studied or the different research instruments used in the studies (Lou, 2011; Yates et al., 2001).
[[{"type":"media","view_mode":"media_original","fid":"26656","attributes":{"alt":"","class":"media-image","height":"586","typeof":"foaf:Image","width":"757"}}]]
In the current study, despite the relatively high number of fatigue strategies used, self-reported fatigue severity did not significantly change during the eight-week study period. In addition, the global effectiveness score decreased (although not significantly) over time, ranging from 5–6 of 10 (10 indicating the most effective). Two potential reasons exist for this finding. Some behaviors, such as doing relaxing things or using distraction, may only provide relief for a limited time. The BFI does not measure how long fatigue relief lasts. In addition, the sample for the current study had progressive advanced disease. Participants’ fatigue severity possibly could increase over time. Their engagement in fatigue self-management could be effective to the extent of keeping their fatigue severity stable, rather than reducing their fatigue severity. In this context, of note is that this observational study was exploratory in nature. It was not designed to establish cause and effect, nor measure how each behavior contributed to the magnitude of fatigue relief.
However, the authors’ findings highlight that perceived self-efficacy, education level, and depressive symptoms are important factors associated with perceived effectiveness of fatigue self-management behaviors. The finding that self-efficacy is a significant factor is consistent with findings of a previous study of Chinese patients with cancer undergoing chemotherapy (Lou, 2011). The relationship between self-efficacy and perceived effectiveness of fatigue self-management behaviors can be understood with reference to Bandura’s self-efficacy theory (Bandura, 1977, 1985, 1997; Bandura, Adams, & Beyer, 1977). Those with greater self-efficacy could perceive fatigue as modifiable and, therefore, invest more effort in self-management behaviors to alleviate fatigue. Individuals with greater self-efficacy also could be more persistent when confronting difficulties, obstacles, or adverse outcomes in the process of achieving goals (Bandura, 1977).
Regarding education level, compared to participants who did not complete high school, participants who completed high school reported that self-management behaviors were more effective. This finding is congruent with a number of studies of patients with chronic disease (Elsie et al., 2012; Fu et al., 2003; Lorig et al., 1999). Specifically, two studies of patients with various types of chronic disease reported that patients with a higher education level not only had better self-efficacy outcomes but also lower levels of fatigue (Elsie et al., 2012; Fu et al., 2003). People with higher education may have higher self-efficacy and health literacy to make use of self-management support and, in turn, may have better self-management outcomes.
Fewer depressive symptoms were predictive of greater levels of fatigue self-management effectiveness. This finding is consistent with those reported by self-management studies of other chronic illnesses (Egede & Ellis, 2008; Jerant, Kravitz, Moore-Hill, & Franks, 2008). Depressed individuals may lack the energy and motivation to self-manage their fatigue (Lustman et al., 2000). The co-occurrence of depressive symptoms and fatigue often is reported in patients with advanced cancer (Hagelin, Wengström, & Fürst, 2009; Yennurajalingam, Palmer, Zhang, Poulter, & Bruera, 2008). The results of the current study suggest a potential role of self-management outcomes serving as mediators between depression and fatigue in this population.
Limitations
The authors acknowledge several limitations. The population of interest in the current study is comprised of patients with metastatic disease, requiring treatments or follow-up appointments at a tertiary cancer center. Given that the majority of the sample was receiving anticancer therapy at the time of enrollment and had a relatively high performance status, the participants likely were at an earlier stage of their advanced disease. Twenty-one participants were lost to follow-up mainly because of being too sick.
The dropout analysis showed that the fatigue severity scores and other outcomes reported at four and eight months likely could be underestimated in this subgroup. Known risk factors for CRF, such as anemia, cachexia, weight loss, and administration of certain anticancer therapies known to increase fatigue, were not measured because of consideration of patient burden and the exploratory nature of the study; therefore, cause and effect were not established. Lastly, the SMSFS-A tool was developed for the purpose of the current study. Although the tool was developed carefully and preliminary testing was undertaken, this tool requires additional testing in other populations to establish its reliability and validity.
Implications for Nursing Practice
The results of the current study have several implications for nursing practice. Oncology nurses need to be aware that patients with advanced cancer can and do engage in fatigue self-management behaviors, at least in the earlier stages of the advanced disease trajectory. Therefore, self-management support should not be limited to those with early-stage cancer or those receiving active treatment. Many patients autonomously adopt self-management strategies (e.g., doing relaxing things, resting during the day without falling asleep). Oncology nurses can be involved in planning these with patients and in ensuring that patients have the confidence, right techniques, and skills to use these behaviors effectively.
Some evidence-based strategies, such as carefully planned exercise, are not commonly used by patients, despite their benefits. Oncology nurses can partner with patients, families, and caregivers to identify reasons for not using these behaviors or barriers to using them. Nurses and patients can work collaboratively to address these barriers. For example, stretching exercises tailored to the individual’s capacity can be safely incorporated into a self-management plan. Additional barriers to self-management, such as lack of motivation, time, and partner or professional guidance, could be identified and further addressed. Other important clinical factors for consideration during care planning include bone metastases and pain, thrombocytopenia, anemia, fever or active infection, and assessment of safety issues, such as risk of falls and stability (National Comprehensive Cancer Network, 2015).
Patients should be informed when they report self-initiated behaviors that are not supported by evidence or which have the potential for adverse effects. A good example is drinking beverages with caffeine, which has a rebound effect on sleep. The results of the current study suggest that drinking beverages with caffeine is a popular behavior used by 91% of participants, so the reasons for discouraging it should be clearly explained. That said, nurses should understand when and how patient-initiated actions can produce personal benefit, even when no clinical benefit exists, and accommodate this in the care plan. Patients’ decisions about how to respond to various symptoms are complex and likely account for a range of factors that are not always apparent to the clinician. For example, the hypothesized action is the adenosine pathway caused by caffeine’s antagonism (Rétey et al., 2005). However, the differential sensitivity to caffeine could explain individual differences in caffeine-related sleep disturbances. Evidence suggests that caffeine-related sleep disturbance is closely associated with several genes in the general population (Byrne et al., 2012). These findings indicate that individuals respond to caffeine differently and that advising all patients to reduce caffeine intake may not be necessary.
Implications for Nursing Research
Findings from the current study suggest the potential role of self-efficacy enhancement and depressive symptom management in fatigue self-management. Additional studies in this field could investigate interventions to enhance patients’ self-efficacy and the additive role of depressive symptoms in this population. The intervention should be carefully designed and tested using a three-arm pragmatic randomized, controlled trial (arm 1: self-management intervention with self-efficacy enhancement, arm 2: the intervention in arm 1 incorporating evidence-based depressive symptom management, arm 3: control). Outcomes should include behavioral uptake, as well as fatigue severity.
[[{"type":"media","view_mode":"media_original","fid":"26661","attributes":{"alt":"","class":"media-image","height":"189","typeof":"foaf:Image","width":"365"}}]]
Conclusion
The current study focused on the perspectives of patients, highlighting a number of issues requiring further attention in clinical practice. Self-management is one patient response to the symptom experience. Because patients appear to often engage in self-management of fatigue, they are likely to obtain relief from these behaviors, as well as some sense of control. This process is extremely complex, requiring competent oncology nurses to provide high-quality, evidence-based self-management support.
References
Abernethy, A.P., Shelby-James, T., Fazekas, B.S., Woods, D., & Currow, D.C. (2005). The Australia-modified Karnofsky Performance Status (AKPS) scale: A revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliative Care, 4, 7. doi:10.1186/1472-684X-4-7
Bandura, A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84, 191–215.
Bandura, A. (1985). Social foundations of thought and action: A social cognitive theory. Upper Saddle River, NJ: Prentice Hall.
Bandura, A. (1997). Self-efficacy: The exercise of control. New York, NY: Freeman.
Bandura, A., Adams, N.E., & Beyer, J. (1977). Cognitive processes mediating behavioral change. Journal of Personality and Social Psychology, 35, 125–139. doi:10.1037/0022-3514.35.3.125
Borthwick, D., Knowles, G., McNamara, S., Dea, R.O., & Stroner, P. (2003). Assessing fatigue and self-care strategies in patients receiving radiotherapy for non-small cell lung cancer. European Journal of Oncology Nursing, 7, 231–241. doi:10.1016/S1462-3889(03)00046-2
Bower, J.E. (2014). Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology, 11, 597–609. doi:10.1038/nrclinonc.2014.127
Bruera, E., Kuehn, N., Miller, M., Selmser, P., & Macmillan, K. (1991). The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. Journal of Palliative Care, 7(2), 6–9.
Bruera, E., Yennurajalingam, S., Palmer, J.L., Perez-Cruz, P.E., Frisbee-Hume, S., Allo, J.A., . . . Cohen, M.Z. (2013). Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: A randomized, placebo-controlled, phase II trial. Journal of Clinical Oncology, 31, 2421–2427. doi:10.1200/JCO.2012.45.3696
Byrne, E.M., Johnson, J., McRae, A.F., Nyholt, D.R., Medland, S.E., Gehrman, P.R., . . . Martin, N.G. (2012). A genome-wide association study of caffeine-related sleep disturbance: Confirmation of a role for a common variant in the adenosine receptor. Sleep, 35, 967–975. doi:10.5665/sleep.1962
Chalise, P., Pandey, R.A., & Chalise, H.N. (2012). Self-care practices and their perceived effectiveness among fatigued cancer patients in Nepal. Asia-Pacific E-Journal of Health Social Science, 1, 1–4.
Chan, R. (2014). Self-management associated with fatigue in patients with advanced cancer: A prospective longitudinal study. Retrieved from http://eprints.qut.edu.au/69858/2/Raymond_Chan_Thesis.pdf
Chan, R.J., Yates, P., & McCarthy, A.L. (2011). The aetiology, impact and management of cancer-related fatigue in patients with advanced cancer. Australian Journal of Cancer Nursing, 12(2), 4–11.
Chan, R.J., Yates, P., & McCarthy, A.L. (2016). The development and preliminary testing of an instrument for assessing fatigue self-management outcomes in patients with advanced cancer. Cancer Nursing. Advance online publication.
Charlson, M.E., Pompei, P., Ales, K.L., & MacKenzie, C.R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373–383. doi:10.1016/0021-9681(87)90171-8
Coens, C., Bottomley, A., Efficace, F., Flechtner, H., & Aaronson, N. (2005). Variability and sample size requirements for health-related quality-of-life measures: Understanding the challenges facing investigators. Journal of Clinical Oncology, 23, 8541–8542.
Egede, L.E., & Ellis, C. (2008). The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technology and Therapeutics, 10, 213–219. doi:10.1089/dia.2007.0278
Elsie, H., Chan, W., Wong, S., Wong, R., Li, S., & Woo, J. (2012). Chronic disease self-management: Do patient demographics and leader characteristics affect outcomes? Primary Health Care, 2(2), 1–7. doi:10.4172/2167-1079.1000112
Fitch, M.I., Mings, D., & Lee, A. (2008). Exploring patient experiences and self-initiated strategies for living with cancer-related fatigue. Canadian Oncology Nursing Journal, 18, 124–140. doi:10.5737/1181912x184124131
Fu, D., Fu, H., McGowan, P., Shen, Y.E., Zhu, L., Yang, H., . . . Wei, Z. (2003). Implementation and quantitative evaluation of chronic disease self-management programme in Shanghai, China: Randomized controlled trial. Bulletin of the World Health Organization, 81, 174–182.
Grady, K.L. (2008). Self-care and quality of life outcomes in heart failure patients. Journal of Cardiovascular Nursing, 23, 285–292. doi:10.1097/01.JCN.0000305092.42882.ad
Grey, M., Knafl, K., & McCorkle, R. (2006). A framework for the study of self- and family management of chronic conditions. Nursing Outlook, 54, 278–286. doi:10.1016/j.outlook.2006.06.004
Hagelin, C.L., Wengström, Y., & Fürst, C.J. (2009). Patterns of fatigue related to advanced disease and radiotherapy in patients with cancer—A comparative cross-sectional study of fatigue intensity and characteristics. Supportive Care in Cancer, 17, 519–526. doi:10.1007/s00520-008-0502-5
Hopkinson, J.B. (2007). How people with advanced cancer manage changing eating habits. Journal of Advanced Nursing, 59, 454–462. doi:10.1111/j.1365-2648.2007.04283.x
Hopkinson, J.B., Wright, D.N., McDonald, J.W., & Corner, J.L. (2006). The prevalence of concern about weight loss and change in eating habits in people with advanced cancer. Journal of Pain and Symptom Management, 32, 322–331. doi:10.1016/j.jpainsymman.2006.05.012
Jerant, A., Kravitz, R., Moore-Hill, M., & Franks, P. (2008). Depressive symptoms moderated the effect of chronic illness self-management training on self-efficacy. Medical Care, 46, 523–531. doi:10.1097/MLR.0b013e31815f53a4
Katz, M.H. (2011). Multivariable analysis: A practical guide for clinicians and public health researchers (3rd ed.). New York, NY: Cambridge University Press.
Lorig, K.R., Sobel, D.S., Stewart, A.L., Brown, B.W., Jr., Bandura, A., Ritter, P., . . . Holman, H.R. (1999). Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Medical Care, 37, 5–14. doi:10.1097/00005650-199901000-00003
Lou, Y. (2011). Self-management of cancer treatment-related fatigue, nausea, vomiting and oral mucositis in Chinese cancer patients. Retrieved from http://eprints.qut.edu.au/44127/1/Yan_Lou_Thesis.pdf
Lustman, P.J., Anderson, R.J., Freedland, K.E., de Groot, M., Carney, R.M., & Clouse, R.E. (2000). Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care, 23, 934–942. doi:10.2337/diacare.23.7.934
Mendoza, T.R., Wang, X.S., Cleeland, C.S., Morrissey, M., Johnson, B.A., Wendt, J.K., & Huber, S.L. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85, 1186–1196. doi:10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N
Miaskowski, C., Dodd, M., West, C., Schumacher, K., Paul, S.M., Tripathy, D., & Koo, P. (2004). Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. Journal of Clinical Oncology, 22, 1713–1720. doi:10.1200/JCO.2004.06.140
Minton, O., Richardson, A., Sharpe, M., Hotopf, M., & Stone, P. (2010). Drug therapy for the management of cancer-related fatigue. Cochrane Database of Systematic Reviews, 7, CD006704. doi:10.1002/14651858.CD006704.pub3
National Cancer Institute. (n.d.). NCI dictionary of cancer terms. Retrieved from https://www.cancer.gov/publications/dictionaries/cancer-terms
National Comprehensive Cancer Network. (2015). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Cancer-related fatigue [v.1.2016]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
Payne, C., Wiffen, P.J., & Martin, S. (2012). Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database of Systematic Reviews, 1, CD008427. doi:10.1002/14651858.CD008427.pub2
Rétey, J.V., Adam, M., Honegger, E., Khatami, R., Luhmann, U.F.O., Jung, H.H., . . . Landolt, H.P. (2005). A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proceedings of the National Academy of Sciences of the United States of America, 102, 15676–15681. doi:10.1073/pnas.0505414102
Revicki, D.A., Cella, D., Hays, R.D., Sloan, J.A., Lenderking, W.R., & Aaronson, N.K. (2006). Responsiveness and minimal important differences for patient reported outcomes. Health and Quality of Life Outcomes, 4, 70. doi:10.1186/1477-7525-4-70
Richardson, A., & Ream, E. (1997). Self-care behaviours initiated by chemotherapy patients in response to fatigue. International Journal of Nursing Studies, 34, 35–43. doi:10.1016/S0020-7489(96)00031-4
Sand, A.M., Harris, J., & Rosland, J.H. (2009). Living with advanced cancer and short life expectancy: Patients’ experiences with managing medication. Journal of Palliative Care, 25, 85–91.
Sherbourne, C.D., & Stewart, A.L. (1991). The MOS social support survey. Social Science and Medicine, 32, 705–714. doi:10.1016/0277-9536(91)90150-B
Siu, S.W., Law, M., Liu, R.K., Wong, K.H., Soong, I.S., Kwok, A.O., . . . Leung, T.W. (2013). Use of methylphenidate for the management of fatigue in Chinese patients with cancer. American Journal of Hospice and Palliative Care, 31, 281–286. doi:10.1177/1049909113487022
So, W.K., & Tai, J.W. (2005). Fatigue and fatigue-relieving strategies used by Hong Kong Chinese patients after hemopoietic stem cell transplantation. Nursing Research, 54, 48–55. doi:10.1097/00006199-200501000-00007
Solano, J., Gomes, B., & Higginson, I.J. (2006). A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of Pain and Symptom Management, 31, 58–69. doi:10.1016/j.jpainsymman.2005.06.007
Teunissen, S.C., Wesker, W., Kruitwagen, C., de Haes, H.C., Voest, E.E., & de Graeff, A. (2007). Symptom prevalence in patients with incurable cancer: A systematic review. Journal of Pain and Symptom Management, 34, 94–104. doi:10.1016/j.jpainsymman.2006.10.015
Yates, P., Hargraves, M., Campbell, J., Mirolo, B., Baker, D., & Clinton, M. (2001). Factors influencing patient’s self-efficacy with managing cancer-related symptoms. Oncology Nursing Forum, 28, 339–340.
Yennurajalingam, S., Frisbee-Hume, S., Palmer, J.L., Delgado-Guay, M.O., Bull, J., Phan, A.T., . . . Bruera, E. (2013). Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. Journal of Clinical Oncology, 31, 3076–3082. doi:10.1200/JCO.2012.44.4661
Yennurajalingam, S., Palmer, J.L., Zhang, T., Poulter, V., & Bruera, E. (2008). Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Supportive Care in Cancer, 16, 1125–1130. doi:10.1007/s00520-008-0466-5
About the Author(s)
Chan is an associate professor in the Cancer Nursing Professional Precinct at the Queensland University of Technology and Royal Brisbane and Women's Hospital in Australia; and Yates is the head of the School of Nursing and McCarthy is a professor and chair of Cancer Nursing, both in the Institute of Health and Biomedical Innovation at the Queensland University of Technology in Australia. This research was funded, in part, by the Royal Brisbane and Women’s Hospital Foundation PhD Scholarship. Chan is supported by a National Health and Medical Research Council Health Professional Research Fellowship (APP1070997). All authors contributed to the conceptualization and design and the manuscript preparation. Chan completed the data collection and analysis. Chan and Yates provided statistical support. Chan can be reached at raymond.chan@qut.edu.au, with copy to editor at ONFEditor@ons.org. Submitted September 2015. Accepted for publication February 7, 2016.

