Increasing Cardiomyopathy Screening in Childhood Cancer Survivors: A Cost Analysis of Advanced Practice Nurse Phone Counseling
Purpose/Objectives: To document the per survivor and per additional survivor screening costs of a mailed survivorship care plan (SCP) with advanced practice nurse (APN) telephone counseling (SCP+C) or without APN telephone counseling (SCP).
Design: Randomized, longitudinal clinical trial.
Setting: St. Jude Children’s Research Hospital in Memphis, Tennessee.
Sample: 411 at-risk pediatric cancer survivors (aged 26–59 years), stratified by age (younger than 30 years versus 30 years or older), recommended screening frequency (every one, two, or five years), gender, and cancer diagnosis (hematologic versus solid tumor).
Methods: Clinical and resource data costs were derived from trial data and external estimates.
Main Research Variables: The cost-effectiveness of left ventricular systolic function screening per survivor and per each additional survivor screened.
Findings: The per-survivor costs of SCP (n = 206) and SCP+C (n = 205) were $74.91 and $224.69, respectively. The estimated costs of SCP and SCP+C per additional survivor screened for two years disseminated in a medium-sized clinic (n = 101 survivors annually) were $345.41 and $293.85, respectively.
Conclusions: Adding APN counseling to a printed SCP may help preserve cardiac health at little or no cost per additional survivor screened.
Implications for Nursing: APN counseling is cost-effective and superior to the standard of care in supporting at-risk survivors’ cardiac screening participation.
Jump to a section
Despite the contribution of anthracyclines to childhood cancer survival, these chemotherapy drugs confer a high risk of asymptomatic left ventricular (LV) dysfunction, cardiomyopathy, congestive heart failure, and death (Lipshultz et al., 2013; Mulrooney et al., 2009; Pein et al., 2004; van Dalen, van der Pal, Kok, Caron, & Kremer, 2006). Radiation of cardiovascular (CV) structures is associated with various adverse outcomes, including cardiomyopathy, constrictive pericarditis, and accelerated atherosclerosis, predisposing survivors to early onset coronary artery disease, myocardial infarction, and stroke (van der Pal, van Dalen, Kremer, Bakker, & van Leeuwen, 2005; van der Pal et al., 2012). Unfortunately, these CV effects can be progressive and frequently subclinical in the early stages (Mulrooney et al., 2009; van Dalen et al., 2006; van der Pal et al., 2005).
All available long-term follow-up (LTFU) guidelines for pediatric cancer survivors (Armenian et al., 2015; Children’s Oncology Group [COG], 2010; Dutch Childhood Oncology Group, 2010; Scottish Intercollegiate Guidelines Network, 2013; Skinner, Wallace, & Levitt, 2005) recommend evaluating LV systolic function through echocardiography or comparable imaging. Screening frequency is determined based on a patient’s age at cancer diagnosis and the cumulative dose of cardiotoxic therapies. Recommendations range from annual screening to screening every five years (COG, 2010); however, most childhood cancer survivors are not receiving risk-based preventive care for cardiac disease. The Childhood Cancer Survivor Study (CCSS) demonstrated that only 511 (28%) of 1,810 childhood cancer survivors at high risk for cardiomyopathy reported undergoing screening in the previous 24 months (Nathan et al., 2008). Cancer treatment centers are encouraged to provide all patients with survivorship care plans (SCPs) that summarize treatment information and outline health-screening recommendations for late effects. However, the single prospective pilot study evaluating the effect of SCPs on adherence to cardiac screening among survivors demonstrated that, even when patients received care plans detailing risks and follow-up recommendations, only 20% underwent cardiac screening within the next two years (Oeffinger et al., 2010).
A randomized, controlled trial by Hudson et al. (2014) revealed that adding telephone counseling by advanced practice nurses (APNs) to printed SCPs substantially increased cardiac screening participation. Hudson et al. (2014) described the costs associated with providing personalized care plans and APN counseling to improve CV screening participation among at-risk survivors. The authors of the current study used a cost-effectiveness analysis (CEA) of a distance-based strategy to address cardiomyopathy screening in adult pediatric cancer survivors. A CEA describes the costs of achieving a specific effect or benefit through interventions. In this study, the cost of two different interventions (SCP and SCP plus counseling) were compared in terms of the number of survivors screened for cardiomyopathy.
Methods
Study Setting and Sample
The intervention and evaluation methods are described in detail elsewhere (Hudson et al., 2014). The study was initiated after approval by the St. Jude Children’s Research Hospital’s (SJCRH) institutional review board in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. The study was conducted from April 2010 to August 2013, and the population was recruited from long-term childhood cancer survivors who were participating in the CCSS, a 27-institution cohort study that is following more than 10,000 long-term survivors of childhood cancer diagnosed from 1970–1986. Survivors were eligible for the study if they met the following criteria: (a) they were CCSS participants, (b) were aged 25 years or older, (c) had no cardiomyopathy screening in the past five years, (d) received anthracyclines and/or radiation to CV structures, (e) were not followed by a LTFU survivorship program, and (f) had a previous history of successful independent (nonsurrogate) response to CCSS surveys. Eligible participants were randomized to either the SCP group or to the SCP plus APN telephone counseling (SCP+C) group. The total sample was stratified by age (younger than 30 years, 30 years or older), COG–recommended screening frequency (every one, two, or five years), sex, and cancer diagnosis (hematologic malignancy versus solid tumor) (see Tables 1 and 2).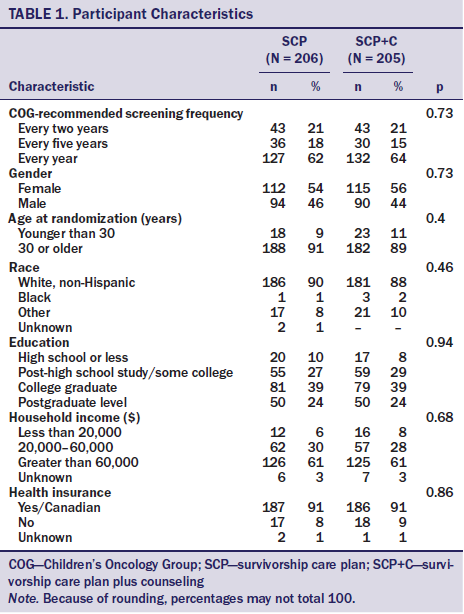
Survivorship Care Plan
After baseline assessment and randomization, survivors were mailed a printed SCP outlining their specific cancer treatments, health risks, and behavioral recommendations to maintain health as per the COG guidelines. The SCPs were personalized by using the term you or the survivors’ first names throughout the document, offering specific treatments rather than general treatment exposures, and suggesting specific recommendations for follow-up and screening. The packets also included laminated cards with treatment exposures, future health risks, and recommendations for follow-up that survivors could take to their primary care providers (PCPs).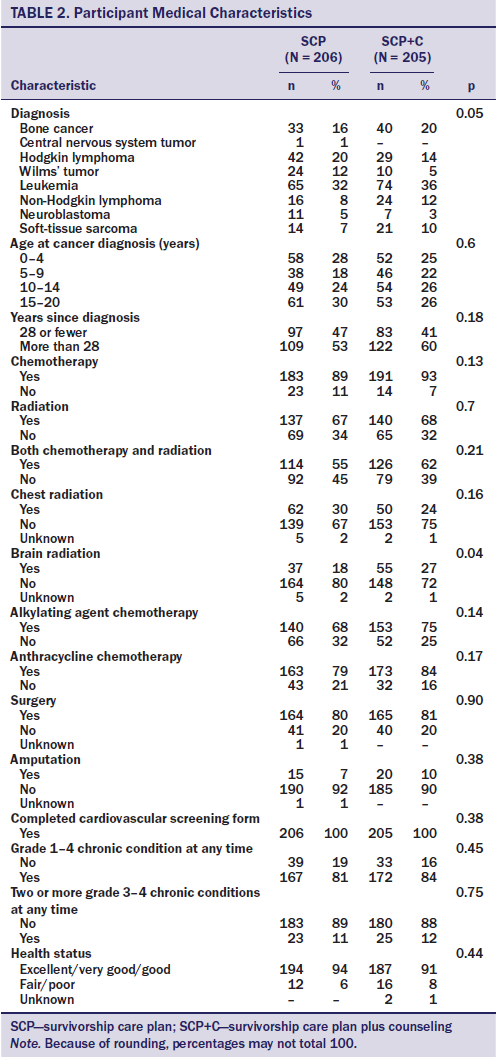
Survivorship Care Plan Plus Counseling
Survivors assigned SCP+C received the same mailed summary and laminated card as those in the SCP group, but participated in two counseling sessions via telephone at one and three weeks after receiving the treatment summary. Each survivor was sent a follow-up letter summarizing the content of each call. The APNs focused on supporting survivors’ competency by providing information about behavior-outcome contingencies; facilitating realistic expectations and self-selected goals; and providing positive, nonjudgmental feedback. APNs supported survivors’ autonomy by avoiding confrontation and coercion, exploring behavioral options, identifying the discrepancy between current behaviors and behaviors needed for optimal CV health, and encouraging decisions on course of action. Last, APNs supported survivors’ need for relatedness by expressing genuine interest and warmth; offering empathic, unconditional support; and avoiding criticism and blame (Cox, 2003; Deci, Eghrari, Patrick, & Leone, 1994; Williams & Deci, 2001). APN counseling intervention activities are summarized in Figure 1.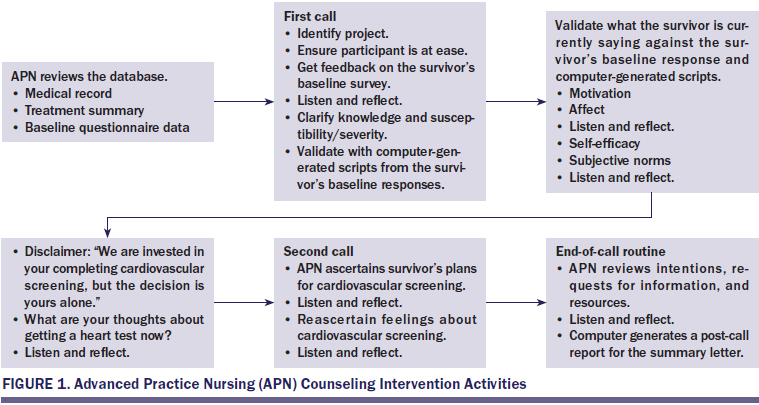
The APNs relied on an interactive computer program developed specifically for the information technology staff at SJCRH. The program not only supported standardized delivery of the intervention but allowed for complete access to (a) previously collected CCSS questionnaire data; (b) the most recent CCSS-abstracted medical records; (c) printed standardized care plans; (d) a baseline questionnaire; and (e) scripts for the targeted behavioral indicators (e.g., knowledge, beliefs, motivations, fears or worries). The program generated scripts based on survivors’ specific responses to the behavioral indicators on the baseline questionnaire. The APNs then used these scripts in interactions with the survivors.
Twelve months after baseline assessment and completion of each study arm, the medical records of all survivors were requested to document physician encounters and adherence or nonadherence to CV screening during the year before and after the intervention. The SCP and SCP+C interventions, respectively, motivated 22% and 52% of the participants to complete a LV function study. Survivors in the SCP+C group were more than twice as likely to have the recommended cardiomyopathy screening than those in the SCP group (relative ratio = 2.31; 95% confidence interval [1.74, 3.07]) (Hudson et al., 2014).
Cost Data Collection
Intervention cost data were collected using standard methods (Gold, Siegel, Russell, & Weinstein, 1996; Graham, Corso, Morris, Sequi-Gomez, & Weinstein, 1998) and included accounting records, timers and time logs, and data-based estimates. Accounting records were used to estimate payroll and expenditures. Staff entered expenditures, such as capital equipment and office supplies, at the time of purchase. Payroll data were used to calculate average salaries on a per-minute basis for those providing the intervention (computer programmers, APNs, personnel training the nurses, office staff who sent questionnaires and performed data entry). The costs were calculated separately for all study personnel on the basis of their 2011 salaries and fringe costs, and salary costs were averaged when multiple individuals worked on a single task. A timer was built into the study database to collect time spent on tasks related to the intervention (see Table 3). Times for making each phone call, entering data into the database, and updating participant statuses were recorded by pressing start and stop on the database recorder, indicating that the task had been performed. This timer was also used to calculate the time spent on larger tasks, such as compiling and mailing materials. If task time for an activity could not be adequately captured in the database, then staff members manually tracked time expended per task (e.g., social worker assistance). The time per item (e.g., time to compile an introductory packet) was then calculated as the total time to complete the task divided by the total number of items. In the few cases (n = 11) the timers were not started or turned off, staff estimates of the average times for those activities were used. Overhead costs for office space, heat, lights, and computer and phone access were not included, as they were provided and were assumed to be available at other institutions that would seek to offer these services. 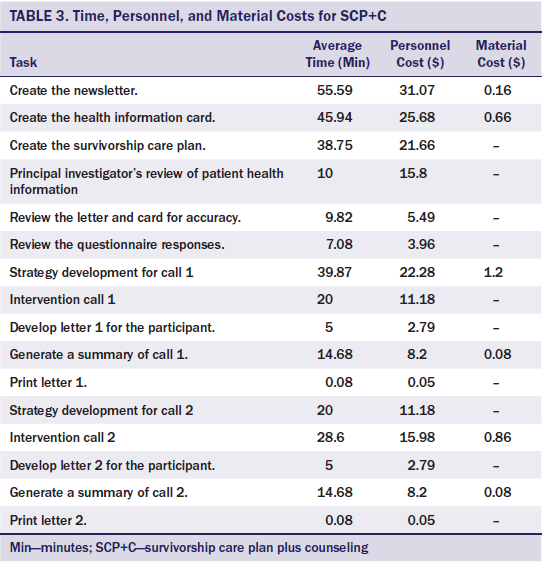
Cost Classification
Both fixed and variable costs for the provision of SCP or SCP+C were documented for the base model. Fixed, onetime intervention costs for SCP included laminating equipment, while fixed costs for SCP+C included the laminator, the development of the interactive computer program, and APN training. The fixed, onetime costs unique to the SCP+C intervention (e.g., programming, nurse training) were divided among the participants in the study arm to provide the cost per participating survivor. APN training costs in the base cost model included the salary of the investigator providing the training and the cost and time for regular meetings with the trainer to refresh the intervention approach and to review periodic audio-recorded counseling sessions (nurse dialogue only) to ensure optimal performance.
Variable costs related to personnel in the base model (see Table 4) included supplies and the time for the personnel who delivered the interventions. The unit cost of materials for both study arms (e.g., stationery, stamps, laminating materials) was obtained from invoices. Personnel costs included time spent reviewing survivor baseline questionnaires, medical records, and CCSS questionnaires; entering progress notes; and preparing postcounseling follow-up letters. Personnel costs for tasks were multiplied by the salary-per-minuteof the required personnel.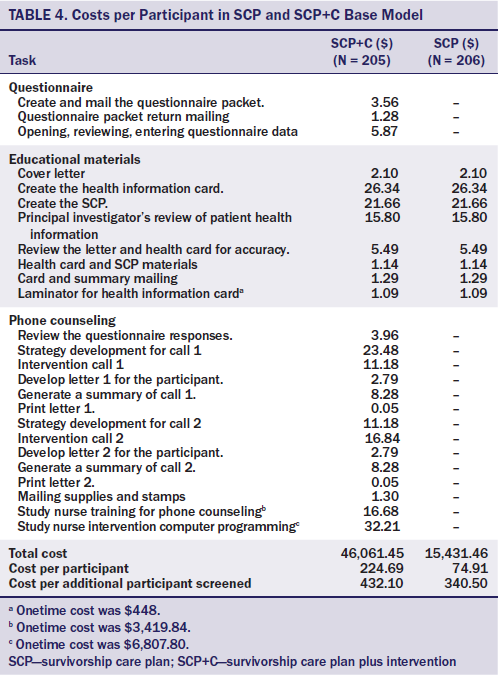
Cost Modeling
The authors described the costs associated with replicating the intervention at medium-sized or larger LTFU clinics (Eshelman-Kent et al., 2011). For this purpose, they modeled two different clinic populations: 202 and 1,000 survivors for a two-yearprogram. Differences in fixed costs and variable costs in year 1 and in subsequent years were noted. The fixed costs associated with developing the interactive computer program were dropped because clinics could download the program from the CCSS website at no charge. APN training costs were left as in the base model, allowing for onetime training of staff as they entered the project and for staff turnover every two years, as necessary. The laminator was budgeted for use during a two-year period for 202 survivors in the medium-sized clinic model and for 1,000 survivors in the larger-sized clinic model, but would be available to use for other purposes and likely could be used for a considerably longer period of time in either case.
Cost-Effectiveness Analysis
To assess cost-effectiveness, costs of each study arm were calculated on a per-survivor basis and then divided by the effectiveness of that arm (Gold et al., 1996). To determine the incremental treatment effect on costs, the authors calculated two types of predicted expenditures, including the costs related to SCP and SCP+C. The only incrementalexpenses were those associated with the counseling portion of the intervention. This approach provided information on the cost per participant of each arm and the cost of the additional intervention activities associated with counseling, and can be used with study-arm effectiveness to calculate the cost basis and the cost per additional survivors screened—the increased number of survivors who obtained echocardiograms in the SCP+C group compared to the SCP group (Andersen, Hager, Su, & Urban, 2002; Andersen, Urban, Ramsey, & Briss, 2004).
Results
The total costs were $15,431.46 for the SCP arm and $46,061.45 for the SCP+C, resulting in a cost per survivor of $74.91 to implement SCP and $224.69 to implement SCP+C. Subtracting the SCP intervention cost from the SCP+C intervention cost revealed an additional cost of $149.78 per survivor for APN-led counseling. The SCP and SCP+C interventions encouraged 22% and 52%, respectively, of previously nonadherent survivors to participate in screening, making the costs per additional survivor screened $340.50 (SCP) and $432.10 (SCP+C). The addition of telephone counseling increased the rate of screening by 30%, decreasing the difference in costs between the two interventions per additional survivor screened.
Dissemination Models
Dissemination models were created to better understand the likely costs of each intervention when implemented in actual survivorship care settings. Variable costs were similar to those of the base case model but did not include costs associated with sending, receiving, reviewing, and entering data of baseline questionnaires. Although the questionnaires were used for both research and counseling intervention purposes, secondary analyses of the intervention revealed the most influential variables to tailor. For example, using the computer program during the call and on the basis of the participants’ responses could eliminate the need for a precall questionnaire, which would significantly reduce the APN and office staff efforts required for review, mailing, and data entry.
The estimated dissemination costs of SCP and SCP+C for two years in a medium-sizedclinic (n = 101 survivors seen each year) were $75.99 and $152.80, respectively. After dividing by the effectiveness of the two interventions, the CEA per additional survivor screened were $345.41 (SCP) and $293.85 (SCP+C). In a larger clinic (500 at-risk patients seen annually for two years), the CEA was projected to be $337.59 for SCP and $264.54 for SCP+C per additional survivor screened (see Table 5).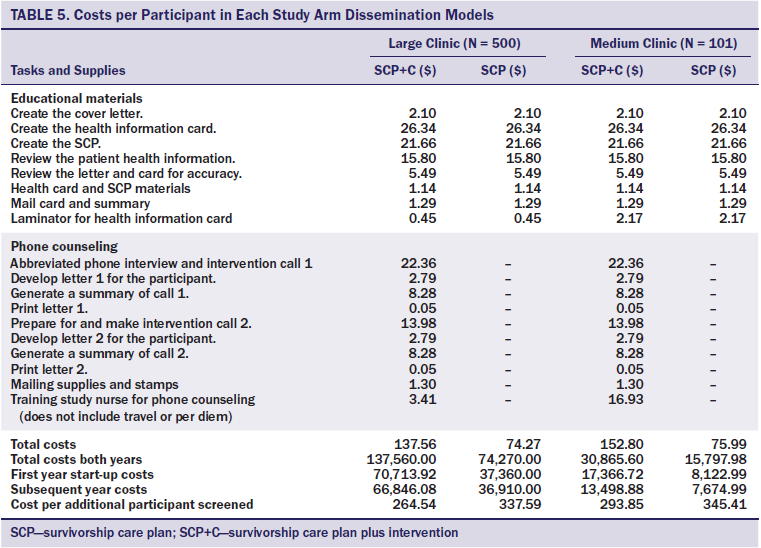
Discussion
The clinical trial from which the cost data were derived demonstrated that the addition of two autonomy-supportive APN phone counseling sessions substantially increased the rate of cardiomyopathy screening among previously nonadherent, at-risk adult survivors of pediatric cancer. APNs were selected as the interventionists based on the long-termcare model at SJCRH and their familiarity with the implementation of research protocols. APNs assumed primary responsibility for ensuring that the LTFU guidelines were the standard of long-term care; in addition, they assisted physicians who rotated through long-term care clinics in learning and implementing the COG long-term care guidelines. The APNs had many years of experience in caring for survivors using their advanced assessment skills and implementing behavioral change strategies. In telephone interactions, survivors often asked questions about symptoms and current and previous therapies, and APNs occasionally provided screening recommendations beyond cardiomyopathy screening. In addition, they provided the most current literature to PCPs who sought to gain greater understanding of survivors’ long-term care issues. All APN training was related to motivational communication strategies; additional training relative to long-term care of survivors was not necessary. With this training, RNs with similar experience in long-term management of adult survivors of pediatric malignancies and knowledge of presenting symptomatology, management, and the COG guidelines could likely implement the intervention with equal success.
Although adding telephone counseling to a printed care plan increases a clinic’s costs, the expense is substantially offset by the larger percentage of survivorswho participate in screening, reducing the cost per survivor screened. In addition, dissemination costs for the counseling intervention could be reduced substantially related to the size of the LTFU clinic and the approach to implementation, potentially making SCP+C more affordable than SCP per survivor screened. Additional strategies to further reduce the costs associated with the intervention include assigning non-APN staff to perform support activities (writing letters to survivors, abstracting medical records, scheduling calls) and training APNs partially through teleconferencing.
Assuming that they screened 101 survivors each year and had a 35-hour work week and 45-week work year, APNs would need to devote less than five hours a week to the counseling intervention. This estimate is based on initial and second phone calls of about 40 and 25 minutes, respectively, per patient and some additional time for call preparation and completion of call summary letters. In a larger clinic with 1,000 patients per year to be contacted, the counseling intervention might require 55% of an APN’s time but could likely be divided among a group of APNs so that one clinician would not have to devote most of his or her time to phone counseling.
Participation rates may be higher at other LTFU clinics than what was demonstrated in the current clinical trial. None of the patients in this trial was treated at SJCRH; in addition, patients had to depend on their PCPs to order echocardiograms, and some declined to order them because of an absence of symptoms and their own unfamiliarity with the COG guidelines (Hudson et al., 2014). Calls and follow-up from survivors’ own treating institutions would likely substantially increase participation. Clinicians may come across instances in which patients who were originally treated for cancer at their facilities are now monitored primarily by PCPs. In these cases, clinicians should call the PCPs to request support for screening, which would more likely result in screening participation.
Screening Recommendations
Survivors exposed to anthracyclines and chest radiation should begin LV function screening no later than two years after completion of therapy and continue to be screened at least every five years (Hunt et al., 2009). Some debate exists as to the frequency of screening needed for patients at high, moderate, or low risk. Wong et al. (2014) found that, based on risk profile, changing from every 1-, 2-, or 5-year screening intervals to 2-, 5-, and 10-year intervals resulted in maintaining 80% of the health benefits of the recommended schedule from the COG guidelines and decreasing costs by 50%. Yeh, Nohria, and Diller (2014) proposed that survivors at high risk (treated with anthracyclines at 250 mg/m2 or greater) should have screening echocardiography every two years or cardiac magnetic resonance imaging (cMRI) every five years. The preferred strategy for patients at low risk was either no echocardiography or cMRI every 10 years (Yeh et al., 2014). Decreased screening frequency would reduce the number of annual survivor contacts, thereby reducing the overall costs of intervention implementation; and longer intervals between screenings might contribute to increased survivor follow-up attrition, reinforcing the need for the counseling intervention.
Limitations
The data are not generalizable to survivors who did not participate in CCSS. Cost data were based on a single institution’s personnel and material costs, and therefore may not be representative of costs at other long-term care facilities or oncology practices. The authors made every effort to be transparent in their analysis by providing both a base case and dissemination model; however, varying degrees of uncertainty and assumptions exist in all cost analyses (Raftery, 1999). To minimize uncertainty and assumptions, the authors relied on objective data or databased estimates, and described their implementation of the current standard of care in their cost analysis.
Implications for Nursing
APNs are uniquely positioned to take leadership roles in the long-term management of adult survivors of pediatric malignancies. Although survivorship clinics are increasing in number and capacity nationwide, a significant gap exists between survivors who need LTFU care and providers who understand it. Oncology APNs and RNs who know long-term care guidelines and are trained in the modeled intervention approach could influence many other institutions. This includes direct interactions with survivors who are no longer followed at the treating institution and with regional PCPs who are not familiar with follow-up guidelines. Implementing this approach would address a much larger at-risk population, potentially supporting screening participation at a level far exceeding the current standard of care, and could have far-reaching implications for the long-term health and wellbeing of cancer survivors.
Additional cost-effectiveness research addressing adult cancer survivors’ participation in other high-priority screening could be modeled on studies of the cost-effectiveness of cancer-screening promotion (Andersen et al., 2002, 2004). Studies should compare the screening participation of patients who participate in APN counseling versus RN counseling. The equivalence of care and outcomes among APNs and RNs would have significant implications for reducing the overall costs of delivering the counseling intervention.
Conclusion
The results indicate that adding APN counseling is no more expensive—and perhaps less expensive—per additional survivor screened than a printed SCP alone for increasing cardiomyopathy screening among at-risk survivors of pediatric malignancies. This intervention approach very likely can be extended to other at-risk childhood cancer survivors (e.g., screening mammograms of women exposed to chest radiation), survivors of adult cancers, and patients with other chronic illnesses that require regular screening.
References
Andersen, M.R., Hager, M., Su, C., & Urban, N. (2002). Analysis of the cost-effectiveness of mammography promotion by volunteers in rural communities. Health Education and Behavior, 29, 755–770.
Andersen, M.R., Urban, N., Ramsey, S., & Briss, P.A. (2004). Examining the cost-effectiveness of cancer screening promotion. Cancer, 101(Suppl. 5), 1129–1138.
Armenian, S.H., Hudson, M.M., Mulder, R.L., Chen, M.H., Constine, L.S., Dwyer, M., . . . Kremer, L.C. (2015). Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncology, 16, E123–E136. doi:10.1016/S1470-2045(14)70409-7
Children’s Oncology Group. (2010). Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers (version 3.0). Retrieved from http://bit.ly/2ccZ71l
Cox, C.L. (2003). A model of health behavior to guide studies of childhood cancer survivors [Online exclusive]. Oncology Nursing Forum, 30, E92–E99. doi:10.1188/03.ONF.E92-E99
Deci, E.L., Eghrari, H., Patrick, B.C., & Leone, D.R. (1994). Facilitating internalization: The self-determination theory perspective. Journal of Personality, 62, 119–142. doi:10.1111/j.1467-6494.1994.tb00797.x
Dutch Childhood Oncology Group. (2010). Richtlijn follow-up na kinderkanker [Directive follow-up in childhood]. Retrieved from https://www.skion.nl/voor-patienten-en-ouders/late-effecten/533/richtli…
Eshelman-Kent, D., Kinahan, K.E., Hobbie, W., Landier, W., Teal, S., Friedman, D., . . . Freyer, D.R. (2011). Cancer survivorship practices, services, and delivery: A report from the Children’s Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. Journal of Cancer Survivorship, 5, 345–357. doi:10.1007/s11764-011-0192-8
Gold, M.R., Siegel, J.E., Russell, L.B., & Weinstein, M.C. (1996). Cost-effectiveness in health and medicine. New York, NY: Oxford University Press.
Graham, J.D., Corso, P.S., Morris, J.M., Sequi-Gomez, M., & Weinstein,M.C. (1998). Evaluating the cost-effectiveness of clinical and public health measures. Annual Review of Public Health, 19, 125–152. doi:10.1146/annurev.publhealth.19.1.125
Hudson, M.M., Leisenring, W., Stratton, K.K., Tinner, N., Steen, B.D., Ogg, S., . . . Cox, C.L. (2014). Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: A randomized controlled trial. Journal of Clinical Oncology, 32, 3974–3981. doi:10.1200/JCO.2014.57.3493
Hunt, S.A., Abraham, W.T., Chin, M.H., Feldman, A.M., Francis, G.S., Ganiats, T.G., . . . Tarkington, L.G. (2009). 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Journal of the American College of Cardiology, 119, E391–E479.
Lipshultz, S.E., Adams, M.J., Colan, S.D., Constine, L.S., Herman, E.H., Hsu, D.T., . . . Wilkinson, J.D. (2013). Long-term cardiovasculartoxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management,prevention, and research directions: A scientific statement from the American Heart Association. Circulation, 128, 1927–1995. doi:10.1161/CIR.0b013e3182a88099
Mulrooney, D.A., Yeazel, M.W., Kawashima, T., Mertens, A.C., Mitby, P., Stovall, M., . . . Leisenring, W.M. (2009). Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ, 339, b4606. doi:10.1136/bmj.b4606
Nathan, P.C., Greenberg, M.L., Ness, K.K., Hudson, M.M., Mertens, A.C., Mahoney, M.C., . . . Oeffinger, K.C. (2008). Medical care in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. Journal of Clinical Oncology, 26, 4401–4409. doi:10.1200/JCO.2008.16.9607
Oeffinger, K.C., Hudson, M.M., Mertens, A.C., Smith, S.M., Mitby, P.A., Eshelman-Kent, D.A., . . . Robison, L.L. (2010). Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatric Blood and Cancer, 56, 818–824. doi:10.1002/pbc.22696
Pein, F., Sakiroglu, O., Dahan, M., Lebidois, J., Merlet, P., Shamsaldin, A., . . . Hartmann, O. (2004). Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. British Journal of Cancer, 91, 37–44. doi:10.1038/sj.bjc.6601904
Raftery, J. (1999). Methodological limitations of cost-effectiveness analysis in health care: Implications for decision making and service provision. Journal of Evaluation in Clinical Practice, 5, 361–366.
Scottish Intercollegiate Guidelines Network. (2013). Long term follow up of survivors of childhood cancer: A national clinical guideline. Retrieved from http://www.sign.ac.uk/pdf/sign132.pdf
Skinner, R., Wallace, W.H., & Levitt, G.A. (2005). Therapy based long term follow up practice statement, second edition. Retrieved from http://www.birminghamcancer.nhs.uk/uploads/document_file/document/4ea01…
van Dalen, E.C., van der Pal, H.J., Kok, W.E., Caron, H.N., & Kremer, L.C. (2006). Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. European Journal of Cancer, 42, 3191–3198. doi:10.1016/j.ejca.2006.08.005
van der Pal, H.J., van Dalen, E.C., Kremer, L.C., Bakker, P.J., & van Leeuwen, F.E. (2005). Risk of morbidity and mortality from cardiovascular disease following radiotherapy for childhood cancer: A systematic review. Cancer Treatment Reviews, 31, 173–185. doi:10.1016/j.ctrv.2005.03.008
van der Pal, H.J., van Dalen, E.C., van Delden, E., van Dijk, I.W., Kok, W.E., Geskus, R.B., . . . Kremer, L.C. (2012). High risk of symptomatic cardiac events in childhood cancer survivors. Journal of Clinical Oncology, 30, 1429–1437. doi:10.1200/JCO.2010.33.4730
Williams, G.C., & Deci, E.L. (2001). Activating patients for smoking cessation through physician autonomy support. Medical Care, 39, 813–823.
Wong, F.L., Bhatia, S., Landier, W., Francisco, L., Leisenring, W., Hudson, M.M., . . . Armenian, S.H. (2014). Cost-effectiveness of the Children’s Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-relatedheart failure. Annals of Internal Medicine, 160, 672–683. doi:10.7326/M13-2498
Yeh, J.M., Nohria, A., & Diller, L. (2014). Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: A model-based estimation of the clinical and economic effects. Annals of Internal Medicine, 160, 661–671.
About the Author(s)
Cox is a member in the Department of Epidemiology and Cancer Control at St. Jude Children’s Research Hospital in Memphis, TN; Andersen is a member in the Division of Public Health Sciences at the Fred Hutchinson Cancer Research Center in Seattle, WA; and Santucci is a clinical research scientist and Robison is a member and chair, both in the Department of Epidemiology and Cancer Control, and Hudson is a member and director of cancer survivorship in the Department of Oncology, all at St. Jude Children’s Research Hospital. This research was supported by grants (R01NR011322 [Co-Principal Investigators (PIs): Cox and Hudson], 5U24CA55727 [PI: Armstrong], and P30CA21765 [PI: Roberts]) from the National Institutes of Health and by the American Lebanese Syrian Associated Charities. Cox and Andersen contributed to the conceptualization and design and analysis. Santucci completed the data collection. Andersen provided statistical support. All authors contributed to the manuscript preparation. Cox can be reached at cox1072@bellsouth.net, with copy to editor at ONFEditor@ons.org. Submitted October 2015. Accepted for publication February 19, 2016.

