Chemotherapy-Induced Peripheral Neuropathy Assessment Tools: A Systematic Review
Problem Identification: Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting chemotherapy toxicity, which has a long-lasting effect and decreases quality of life. Although several tools have been developed to detect CIPN, the study quality, psychometric properties, and practicality of CIPN assessment tools have not been systematically reviewed.
Literature Search: Electronic searches using keywords were conducted in Medline, PubMed, CINAHL®, and Cochrane Library for articles published from 1980–2015. Eligible studies were included if they involved patients with cancer receiving chemotherapy, provided CIPN assessment tools with psychometric properties, and were published in English.
Data Evaluation: Data were extracted, and study quality was assessed. CIPN tools were evaluated in terms of psychometric properties and practicality.
Synthesis: A total of 19 studies describing 20 tools were reviewed. The quality of studies varied from strong to weak. The validity ranged from low to high, and the reliability with internal consistency ranged from 0.56–0.96. Poor inter-rater agreement was found. Not all of the tools were deemed practical.
Conclusions: Considering the psychometric properties and practicality, two tools (Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity [FACT/GOG-Ntx] and Total Neuropathy Score [TNS]) are recommended for assessing CIPN.
Implications for Nursing: Routine assessment of CIPN and choosing appropriate assessment tools are imperative. The FACT/GOG-Ntx and TNS are recommended for clinical use.
Jump to a section
Chemotherapy-induced peripheral neuropathy (CIPN) is a type of neuropathic pain that results from chemotherapy toxicity. A systematic review and meta-analysis involving 4,179 patients revealed a CIPN prevalence of 68% in the first month after chemotherapy, 60% within three months, and 30% within six months or longer, with prevalence associated with different chemotherapy drugs (Seretny et al., 2014). Several chemotherapy agents lead to CIPN, including platinum-based agents, taxanes, epothilones, and vinca alkaloids, as well as more recent agents like bortezomib (Velcade®) and lenalidomide (Revlimid®) (Hershman et al., 2014). Sensory and motor nerve damages are common features of CIPN that influence individuals’ quality of life (Hausheer, Schilsky, Bain, Berghorn, & Lieberman, 2006). Sensory damages are the predominant symptoms of CIPN, including paresthesia, numbness and tingling, dulled sensations in the peripheral nerves, burning and shooting pain, or electric shock–like pain (Cavaletti & Marmiroli, 2015; Visovsky, Collins, Abbott, Aschenbrenner, & Hart, 2007). Motor damage can be manifested as weakness, gait and balance disturbance, and difficulty with fine motor skills (Visovsky et al., 2007). The incidence of CIPN is influenced by age, type of chemotherapy, dose, intensity, cumulative dose, therapy duration, and preexisting diseases (e.g., diabetes) (Wolf et al., 2012). CIPN has long-lasting effects; 30% of patients with cancer who receive taxanes still suffer from CIPN several years after the treatment (Ewertz, Qvortrup, & Eckhoff, 2015). In one study, more than half of patients with ovarian cancer who received platinum-based chemotherapy agents and taxanes suffered from neuropathy symptoms for as many as 12 years after the end of treatment, which caused a considerable impact on quality of life (Ezendam et al., 2014).
Early detection of CIPN is important for early symptom management. Several assessment tools for CIPN have been developed, including tools that can be completed by the patient and tools that are completed by healthcare providers. Four assessment tools are completed by healthcare providers: National Cancer Institute Common Toxicity Criteria (NCI-CTC), Eastern Cooperative Oncology Group (ECOG) neuropathy scale, World Health Organization (WHO) guidelines, and the Ajani scale (Hausheer et al., 2006). Those tools employ ordinal rating scales, which become a limitation in detecting small changes in CIPN. In addition, the inter-rater reliability of the tools is low (Park, Lin, & Kiernan, 2012).
The lack of universal agreement on and standardized assessment of tools for CIPN impedes detection. Procedures for quantitative sensory testing still lack consistency between institutions and laboratories (Paice, 2009). In addition, few CIPN assessment tools have been validated (Hausheer et al., 2006).
A previous systematic review of CIPN assessment tools identified 15 studies (Griffith, Merkies, Hill, & Cornblath, 2010). The quality of the studies in Griffith et al.’s (2010) review was assessed using the modified Quality of Diagnostic Accuracy Study (QUADAS) tool. The modified QUADAS contains survey items that assess participant characteristics, sampling, reliability, construct validity, reference standard, study procedure, and scoring method (Griffith et al., 2010). Each item of the modified QUADAS is rated based on dichotomous responses of “yes” (score of 1) and “no” (score of 0). The quality of the studies was determined by a total score of the seven items, with scores of 0–3 indicating poor quality, 4–5 indicating moderate quality, and 6–7 indicating high quality. However, aggregating individual items to reflect the quality of the studies may ignore the value of individual items and can lead to potential bias associated with these items (Whiting, Rutjes, Reitsma, Bossuyt, & Kleijnen, 2003).
For early detection of CIPN symptoms, healthcare providers should use a valid assessment tool. This systematic review aims to identify CIPN assessment tools that are currently available, as well as their psychometric properties, and to determine the quality of studies reporting those tools.
Methods
Electronic searches were conducted using Medline, PubMed, CINAHL®, and Cochrane Library for articles published from 1980–2015. The search terms included cancer, chemotherapy, assessment tool, evaluation tool, measurement tool, scores tool, and tests tool, as well as peripheral neuropathy, peripheral nerve diseases, peripheral nerve disorders, peripheral nervous system diseases, and peripheral nervous system disorders. After removing duplicates, titles and abstracts were examined for their eligibility. Two reviewers independently reviewed the full texts of studies based on the inclusion and exclusion criteria. If there were any conflicting opinions, the reviewers discussed the opinions and consulted an independent researcher to attain a consensus. The reference list of each eligible study was checked for any additional studies that meet the criteria of this review.
Studies were considered eligible if they included patients with cancer receiving chemotherapy, described the CIPN assessment tools, reported psychometric properties, and were published in English. Studies were excluded if diabetes was the source of neuropathic pain. Case reports were also excluded.
A data extraction tool was used to record information about study purpose, design, sample, tools, and psychometric properties. The first author independently extracted the data. Then, the data were reviewed by the second author to promote extraction accuracy.
Appraisal of Study Quality
The quality of each study was assessed by the QUADAS-2 (Whiting et al., 2011). QUADAS-2 is a revision of the original QUADAS developed by Whiting et al. (2003) to assess the unique features of the study design regarding diagnostic accuracy. The original QUADAS is a 14-item checklist with three options (“yes,” “no,” and “unclear”). The items evaluate the quality of design, including sample, reference standard, disease progression bias, verification bias, review bias, clinical bias, test execution, withdrawals, and indeterminate results. Difficulties in rating items resulted in a revision of the original QUADAS to the QUADAS-2 (Whiting et al., 2011). The QUADAS-2 covers four domains of bias risks: patient selection, index test, reference standard, and flow and timing. Different from the original QUADAS, QUADAS-2 mainly assesses the applicability of studies. Applicability is to assess whether retrieved studies match the review questions of a systematic review or not. Signaling questions are used to flag the potential of bias risk judged as low, high, or unclear. If the study matches the review questions, it can be rated as “high.” If the study does not match the review questions, it can be rated as “low.” “Unclear” refers to a case where no sufficient data are available to determine whether the study is “high” or “low.” Appraisal quality using QUADAS-2 was evaluated independently by the two researchers through discussions to resolve differences.
Psychometric Evaluation
The CIPN assessment tools were evaluated for their reported psychometric properties, including validity, reliability, sensitivity, specificity, responsiveness, and practicality. The frames of reference for the psychometric properties are based on the criteria proposed by DeVon et al. (2007) and Peacock and Peacock (2011). Reliability was evaluated by the following indexes: internal consistency (Cronbach alpha coefficient = 0.7 or greater), test-retest reliability (correlation = 0.7 or greater), inter-rater agreement (kappa = 0.6–0.8). Validity involves content, construct, and criterion validity. The accepted correlation coefficient was 0.45 or greater for criterion validity (i.e., concurrent, predictive, and convergent) and 0.45 or less for discriminant validity.
Results
A total of 1,712 studies were identified from the initial search. From the 1,712 studies, 1,304 were duplicates, leaving 408 potential studies. After reviewing the abstracts of the 408 potential studies, 38 studies met the inclusion criteria for full-text review. From the 38 studies, 22 studies were excluded, yielding 16 studies. A hand search of the reference list of the 16 eligible studies revealed 3 additional studies that met the inclusion criteria. A total of 19 studies were included in the systematic review (see Figure 1).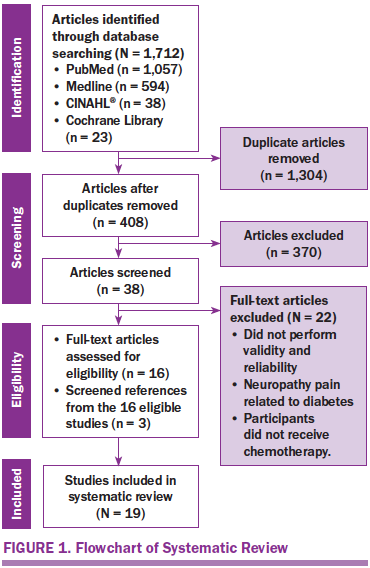
Study Characteristics
The characteristics of the 19 studies are presented in Table 1. The studies were conducted in the United States (n = 10), Italy (n = 3), Japan (n = 3), Canada (n = 1), Norway (n = 1), and the Netherlands (n = 1). The studies employed longitudinal designs (n = 11), clinical trial designs (n = 4), cross-sectional designs (n = 3), and a case-control design (n = 1). A total of 3,329 participants were involved in the 19 studies, and cancer types included breast, ovarian, colon, testicular, and endometrial. The sample size ranged from 20–684 participants, and age ranged from 18–83 years. Most participants were female. A variety of chemotherapy agents were reported, including carboplatin (Paraplatin®), cisplastin (Platinol®), oxaliplatin (Eloxatin®), paclitaxel (Taxol®), docetaxel (Taxotere®), and vincristine (Oncovin®).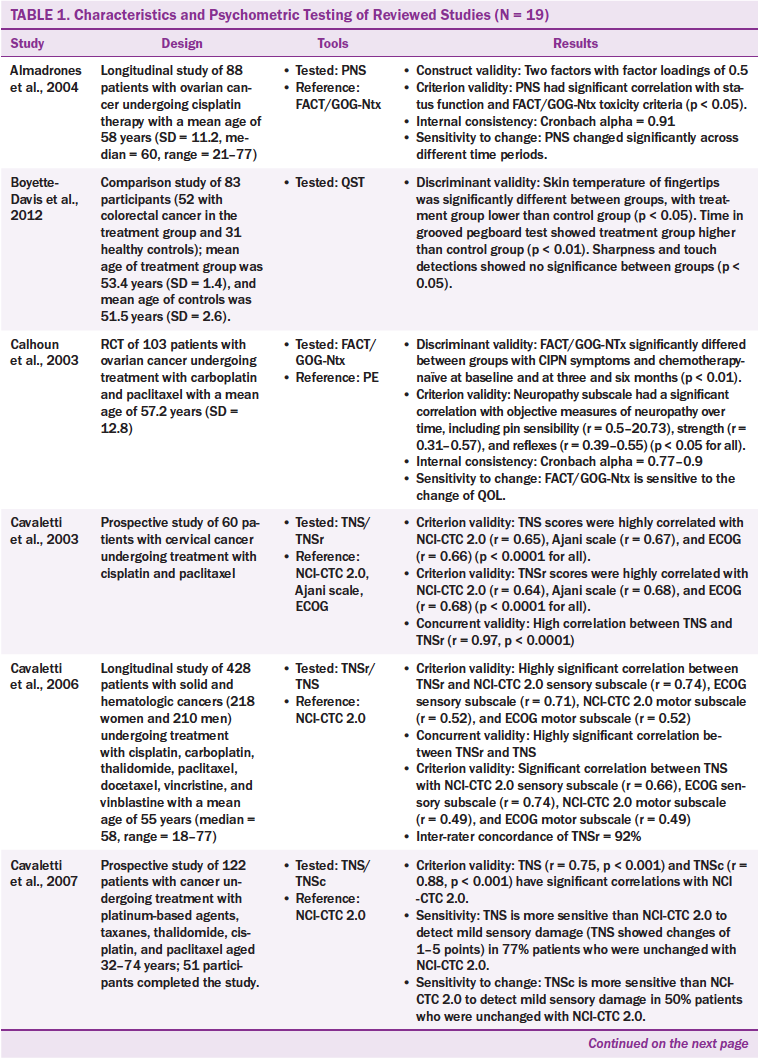
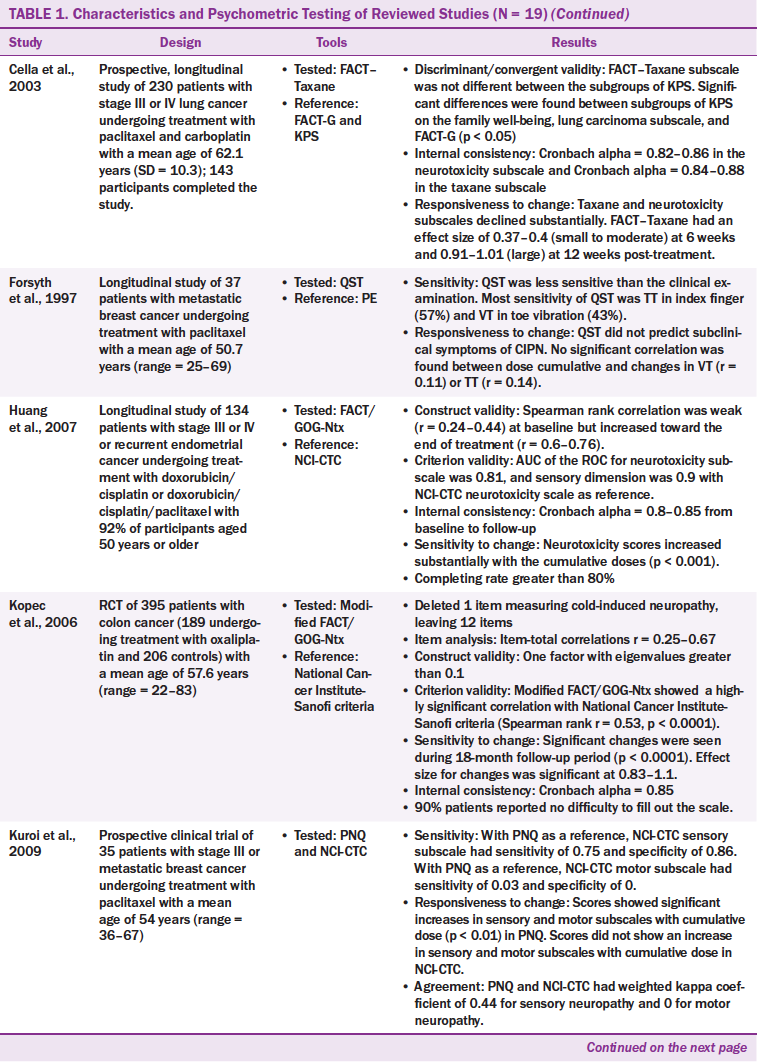
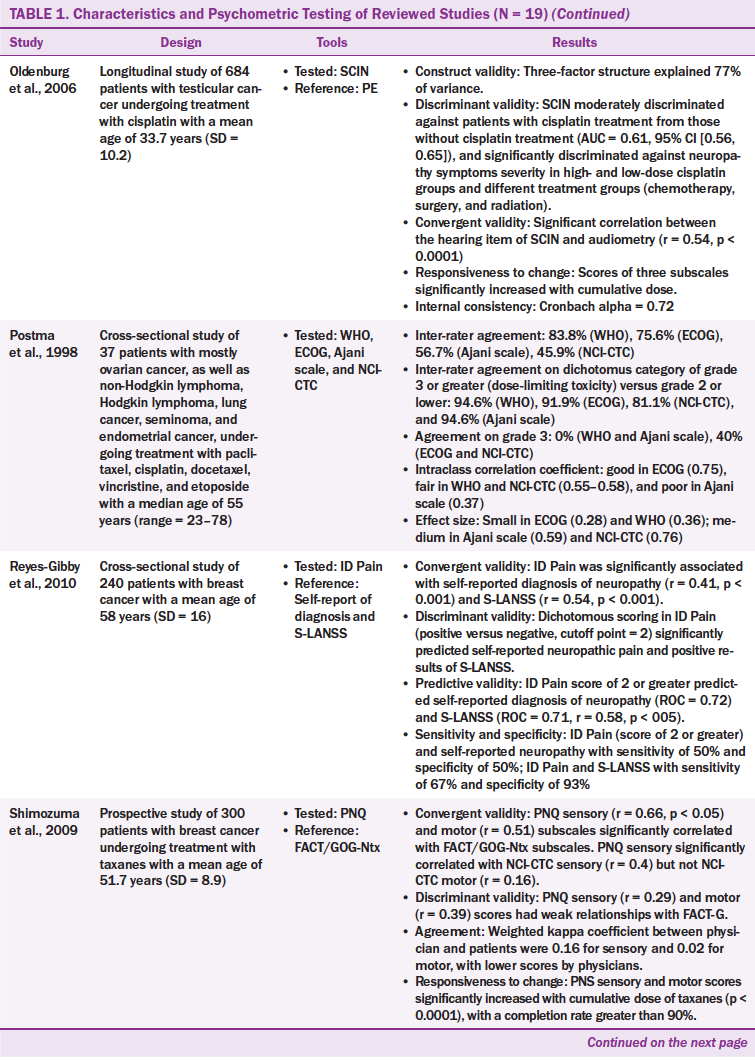
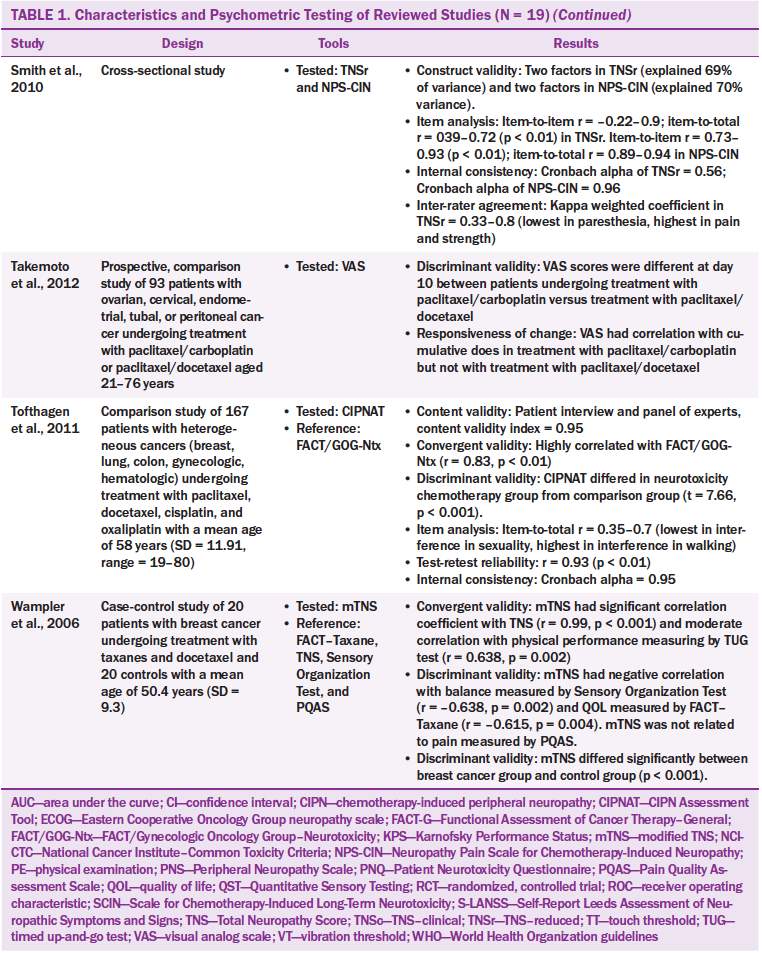
Twenty CIPN assessment tools were administered across the 19 studies that used from one to four tools. Of the 20 CIPN assessment tools, seven were to be completed by healthcare providers, nine were to be completed by patients, and four were to be completed by healthcare providers and patients. The patient-based tools required patients to report their subjective neuropathic symptoms. The healthcare provider–based tools required healthcare providers to assess patients’ symptoms and conduct physical examinations, such as motor, pin sensibility, vibration, strength, and tendon reflex.
The most commonly used tool was the Total Neuropathy Score (TNS) and its modifications, such as the reduced TNS (TNSr), the TNS clinical version (TNSc), and the modified TNS (mTNS). The TNS is a tool combining patient- and healthcare provider–based reports. The TNS contains two components: (a) subjective reports of sensory, motor, or autonomic symptoms and (b) neurologic examinations of deep tendon reflexes, pin sensibility, vibration sensibility, and nerve conduction. The neurologic examinations should be performed by trained healthcare providers.
The Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT/GOG-Ntx) has been examined in three studies and is used to assess the subjective symptoms of CIPN (Calhoun et al., 2003; Huang, Brady, Cella & Fleming, 2007; Kopec et al., 2006). This tool has been modified to assess CIPN caused by specific chemotherapy agents. For example, the modified FACT/GOG-Ntx was used to assess platinum-based chemotherapy/paclitaxel/oxaliplatin-induced neurologic symptoms, and the FACT–Taxane subscale was used to detect taxane-induced neuropathy. In the 19 studies, one study used the FACT–Taxane subscale to detect platinum-based chemotherapy/paclitaxel-induced neuropathy (Cella, Peterman, Hudgens, Webster, & Socinski, 2003), four studies used tools containing items related to activities of daily living to assess the effects of CIPN on quality of life (Calhoun et al., 2003; Cella et al., 2003; Huang et al., 2007; Kuroi et al., 2009; Shimozuma et al., 2009; Tofthagen, McMillan, & Kip, 2011). Tools that assess activities of daily living include FACT/GOG-Ntx and its modified version, FACT–Taxane, and CIPN Assessment Tool (CIPNAT).
Most of the tools (n = 14) used multiple-choice responses to assess CIPN symptoms with Likert-type scales ranging from 0 (no symptoms) to 4 or 5 (severe symptoms). Only one tool (the ID Pain) required “yes” or “no” responses (Reyes-Gibby, Morrow, Bennett, Jensen, & Shete, 2010). The ID Pain is a questionnaire based on six interview questions. A visual analog scale (VAS) was used to measure patients’ reports of pain and numbness (Takemoto et al., 2012).
Study Quality
Study quality was determined by the QUADAS-2 and revealed that most studies (n = 12) had good patient selection, with less than half indicating low risk of bias (see Table 2). High risk of bias in the index test was found in 14 studies, in which the index tests were conducted and interpreted after the reference standard. Therefore, the interpretation of the index test result may be affected by the reference test result. Nine studies presented high risk of bias because the reference standards were not consistent with the index test. Fifty percent of the studies clearly presented patient flow and had an appropriate interval between the index test and reference standard.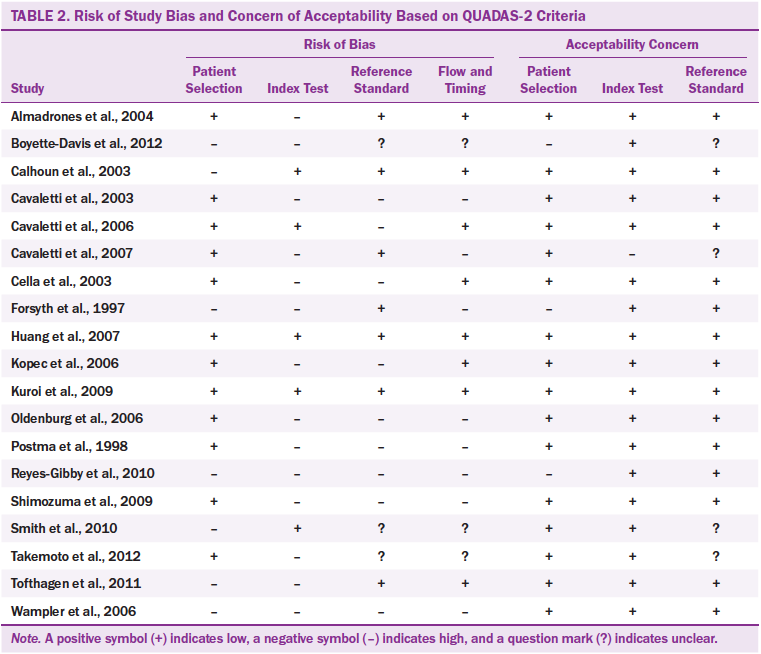
Two studies were judged as highly satisfactory in terms of all four risks of bias (i.e., patient selection, index text, reference standard, and flow and timing domains) (Huang et al., 2007; Kuroi et al., 2009). However, two studies were judged unsatisfactory on the four risks of bias (Reyes-Gibby et al., 2010; Wampler et al., 2006). Based on the QUADAS-2 applicability criteria, 16 of the studies demonstrated satisfactory applicability, particularly in the index test, which reached 100% low applicability concern. A low applicability concern means the index test was conducted to match the study questions.
Psychometric Properties
Validity: The validity of CIPN tools was examined through a variety of approaches, including convergent, concurrent, construct, criterion, and discriminant validity. Validity was examined in 15 of 20 tools. Tools that did not report validity include the Ajani scale, ECOG neuropathy scale, VAS, WHO guidelines, and Quantitative Sensory Testing (QST). The Ajani scale, ECOG neuropathy scale, and QST were used in one study mainly to examine the agreement between raters (Postma et al., 1998).
Criterion validity was provided for nine tools that had moderate to high correlations with their criterion reference (r = 0.31–0.97). The nine tools were the FACT/GOG-Ntx, modified FACT/GOG-Ntx, NCI-CTC, Patient Neurotoxicity Questionnaire (PNQ), Peripheral Neuropathy Scale (PNS), Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN), TNS, TNSr, and TNSc.
Construct validity was reported for five tools (PNS, FACT/GOG-Ntx, modified FACT/GOG-Ntx, SCIN, and TNSr), which reported acceptable Eigenvalues of greater than 1 and factor loadings of greater than 0.4. The number of factors in the tools ranged from 1–3, with 61%–77% of the variance explained (Almadrones, McGuire, Walczak, Florio, & Tian, 2004; Huang et al, 2007; Kopec et al., 2006; Oldenburg, Fosså, & Dahl, 2006; Smith, Cohen, Pett, & Beck, 2010). For example, the PNS had two-factor solutions (hand neuropathy and foot neuropathy) (Almadrones et al., 2004), and the TNSr had two-factor solutions (neuropathy and neuropathy pain) (Smith et al., 2010).
Discriminant validity had been frequently used to examine the validity of CIPN tools. From the 20 CIPN tools, eight were evaluated for discriminant validity (CIPNAT, FACT/GOG-Ntx, ID Pain, mTNS, PNQ, QST, SCIN, and VAS). The FACT/GOG-Ntx significantly discriminated (area under the curve = 0.81) between chemotherapy-naïve groups and patients who had CIPN (Calhoun et al., 2003). The SCIN significantly discriminated neuropathy symptom severity not only in high- and low-dose cisplatin groups, but also in diverse treatment groups (chemotherapy, surgery, and radiation) (Oldenburg et al., 2006). The CIPNAT mean scores were different between the neurotoxicity chemotherapy group and the chemotherapy-naïve group (t = 7.66, p < 0.001) (Tofthagen et al., 2011). The mTNS showed significant differences between the breast cancer group and the control group (p < 0.001) (Smith et al., 2010). The QST is a quantitative measurement using serial neurologic examinations. The QST could detect a lower skin temperature in the treatment group than in the control group; however, the QST could not detect differences in sharpness and touch threshold (Boyette-Davis et al., 2012).
One study reported convergent/discriminant validity for two tools (FACT–Taxane and ID Pain). The study, conducted by Cella et al. (2003), revealed that the FACT–General showed a significant difference between the subgroups of Karnofsky Performance Status (KPS) before chemotherapy, but not in the taxane subscale. Convergent validity was reported in five tools (SCIN, ID Pain, PNQ, CIPNAT, and FACT/GOG-Ntx), and all of those tools are based on patient report. Those five tools had low to high correlation with their reference tools (r = 0.16–0.83).
Reliability: The reliability of several CIPN tools was examined through the approaches of internal consistency, inter-rater agreement, or test-retest reliability. The internal consistency of Cronbach alpha was reported in eight tools (i.e., the CIPNAT, FACT/GOG-Ntx, FACT–Taxane, modified FACT/GOG-Ntx, Neuropathy Pain Scale–Chemotherapy-Induced Neuropathy [NPS-CIN], PNS, SCIN, TNSr) and ranged from 0.56–0.96 (Almadrones et al., 2004; Calhoun et al., 2003; Cella et al., 2003; Huang et al., 2007; Kopec et al., 2006; Oldenberg et al., 2006; Smith et al., 2010; Tofthagen et al., 2011). Among the eight tools, NPS-CIN had the highest Cronbach alpha at 0.96, and TNSr had the lowest at 0.56.
Inter-rater agreement was commonly examined in the CIPN tools. It had been reported for seven tools (Ajani scale, ECOG neuropathy scale, NCI-CTC, PNQ, TNS, TNSr, and WHO guidelines), ranging from 46%–92% (Cavaletti et al., 2006; Kuroi et al., 2009; Postma et al., 1998; Shimozuma et al., 2009; Smith et al., 2010). The inter-rater agreement included the agreement between healthcare providers, and between healthcare providers and patients, which had varied agreement of coefficients. For example, Postma et al. (1998) found that the agreement coefficient between physicians ranged from r = 0.45 in NCI-CTC to r = 0.83 in the WHO guidelines. The study also showed that grading system influenced interrater agreement, with the highest agreement coefficients (r = 0.81–0.95) in a dichotomous scoring system and the lowest agreement coefficients (r = 0–0.4) in a multiple-choice grading system. Smith et al. (2010) reported similar findings in the TNSr tool, which had weighted kappa coefficients between physicians and nurse practitioners ranging from 0.33 in paresthesia to 0.8 in neuropathy pain and strength.
Agreement between healthcare providers and patients in the PNQ is low and divergent, with a kappa coefficient of 0.16 for sensory symptoms and 0.02 for motor symptoms (Shimozuma et al., 2009). Intraclass correlation coefficients (ICCs) were reported in the Postma et al. (1998) study, which assessed the inter-rater agreement in four tools (WHO guidelines, ECOG neuropathy scale, Ajani scale, NCI-CTC), with poor agreement (ICC = 0.37) in the Ajani scale and good agreement in the ECOG neuropathy scale (ICC = 0.75). Only one tool (CIPNAT) had a test-retest reliability with a high correlation of r = 0.93 within a two-week duration (Tofthagen et al., 2011).
Sensitivity, specificity, and responsiveness: Sensitivity and specificity of tools were reported in only four CIPN tools. Two tools (NCI-CTC and ID Pain) were tested for sensitivity and specificity simultaneously (Kuroi et al., 2009; Reyes-Gibby et al., 2010). The NCI-CTC had predominantly divergent sensitivity and specificity in sensory and motor symptom subscales (Kuroi et al., 2009). When the PNQ was considered as a standard, the sensitivity and specificity in NCI-CTC sensory subscale (cutoff = absence/presence) were 0.75 and 0.86, respectively; the sensitivity and specificity in NCI-CTC motor subscale (cut off = absence/presence) were 0.03 and 0, respectively. Sensitivity and specificity in ID Pain were both 50% when it was compared with self-reported neuropathy among women with breast cancer (Reyes-Gibby et al., 2010). The ID Pain as a patient-report assessment tool has been used in patients with neuropathic pain; 50% of them had positive ID Pain score (score of 2 or greater), and 86% not diagnosed with neuropathic pain had negative ID Pain (Reyes-Gibby et al., 2010).
Two tools (TNS and QST) were only assessed in terms of sensitivity (Cavaletti et al., 2007; Forsyth et al., 1997). Cavaletti et al. (2007) reported that TNS was more sensitive than NCI-CTC, version 2.0, in which 77% of the patients who had TNS scoring changes did not have any significant scoring changes with the NCI-CTC. However, the QST, as quantitative measurement based on serial neurology examination conducted by physicians, did not predict subclinical CIPN. The QST had been reported to have less sensitivity than clinical examination, with a particularly low sensitivity in the vibration threshold item (Forsyth et al., 1997).
Eleven of the CIPN tools were assessed in terms of their responsiveness (sensitivity) to change. The 11 tools tested for their responsiveness were the PNS, QST, FACT/GOG-Ntx, modified FACT/GOG-Ntx, FACT–Taxane, PNQ, NCI-CTC, SCIN, TNS, TNSc, and VAS (Almadrones et al., 2004; Boyette-Davis et al., 2012; Calhoun et al., 2003; Cavaletti et al., 2007; Cella et al., 2003; Kopec et al., 2006; Kuroi et al., 2009; Oldenburg et al., 2006; Takemoto et al., 2012). The indicators of changes consisted of longitudinal trends of symptoms, changes in quality of life, and the cumulative doses of chemotherapy agents. All of those tools significantly responded to the changes of the indicators over time except NCI-CTC.
The PNS score changed significantly over time (Almadrones et al., 2004). The FACT/GOG-Ntx was sensitive to changes in global quality of life (p = 0.017) (Calhoun et al., 2003). The modified FACT/GOG-Ntx significantly changed during 18 months, with increasing effect size from 0.83 to 1.1 (Kopec et al., 2006). The PNQ sensory and motor scores significantly increased with cumulative doses of taxane (p < 0.01) (Kuroi et al., 2009). The three subscales (i.e., neuropathy, Raynaud’s disease symptoms, and toxicity) of the SCIN significantly increased with cumulative doses of cisplatin (Oldenburg et al., 2006). However, the NCI-CTC was not sensitive to detect changes in neuropathic symptoms over time (Cavaletti et al., 2007; Kuroi et al., 2009).
Practicality: Only patient-report tools were assessed in terms of practicality. Three studies reported the completion rate; all rates were more than 80% (Huang et al., 2007; Kopec et al., 2006; Shimozuma et al., 2009). Eighty percent of patients (n = 316 of 395) completed the 12-item modified FACT/GOG-Ntx tool (Kopec et al., 2006). The completion rates of the FACT/GOG-Ntx and PNQ were 80% and 90%, respectively, indicating that both tools had practicality (Huang et al., 2007; Shimozuma et al., 2009).
Discussion
This systematic review offers new insights for evaluating 20 CIPN assessment tools retrieved from 19 studies. The contents of the studies and the psychrometric properties of CIPN tools were reviewed. In addition, the quality of the studies was assessed using QUADAS-2 (Whiting et al., 2011). Study quality, risk of bias on patient selection, index tests, reference standards, flow and timing, and applicability were considered (Whiting et al., 2011). In the systematic review, only two studies were judged as satisfactory on the four risks of bias. Much more attention should be paid when results from low-quality studies are applied in clinical practice.
The review reveals that CIPN tools are useful when patients with cancer are treated with platinum-based agents, vinca alkaloids, or taxanes. The main characteristics of the tools to detect CIPN can be categorized as common toxicity features, functional status, quality of life, and composite scales. A similarity in the patterns and CIPN symptoms across different chemotherapy agents has been found, and CIPN is predominantly manifested by sensory and motor symptoms (Hausheer et al., 2006). The features and symptoms of neurotoxicity should be contained in any CIPN assessment tool.
Because CIPN constitutes subjective experience, CIPN assessment tools should consist of subjective and objective items. In addition, this systematic review reveals poor inter-rater agreement between healthcare providers or between healthcare providers and patients. For this reason, it is ideal to combine healthcare provider–based and patient-based tools for a comprehensive CIPN assessment. The TNS and its modifications (TNSr, TNSc, and mTNS) use patient report and healthcare provider assessment. The TNS and its modifications may be considered as appropriate tools to assess those receiving certain chemotherapy agents, such as platinum-based agents, taxanes, epothilones, vinca alkaloids, bortezomib, and lenalidomide. The Ajani scale, WHO guidelines, ECOG neuropathy scale, and NCI-CTC are healthcare provider–based assessment tools that have limited psychometric properties (Postma et al., 1998).
The adequacy of an instrument to measure the symptoms of CIPN can be evaluated based on psychometric parameters. The psychometric properties (reliability, validity, sensibility/specificity, and responsiveness) were assessed in the 20 CIPN tools. Hausheer et al. (2006) proposed five parameters of CIPN assessment tools: (a) a tool must be sensitive to detect the presence or absence of CIPN; (b) a tool should differentiate CIPN from other neurologic disorders; (c) a tool should be responsive to changes in symptoms related to dose, duration, and number of drugs; (d) impairments of functional activities of daily living or quality of life should be reflected in the tool and considered for treatment decisions; and (e) a tool should be easy to use, as reflected by a patient participation rate greater than 80%.
The sensitivity to detect CIPN can be reflected in the sensitivity and specificity of the tool. In this systematic review, TNS, TNSc, NCI-CTC, QST, PNQ, and ID Pain were examined in terms of their sensitivity and specificity. Most of the tools had satisfactory results in detecting the presence or absence of CIPN, particularly sensory neuropathic symptoms. Cavaletti et al. (2007) reported that the TNS, TNSr, and TNSc are reliable tools for assessing the severity of CIPN.
The second parameter is that tools should differentiate CIPN from other neurologic disorders, such as diabetic neuropathy, carpal tunnel syndrome, and metabolic neuropathy. This can be shown by the discriminant validity. Several CIPN tools in this review showed discriminant validity. Discriminant validity is vital for CIPN assessment tools because healthcare providers need to apply the tools to detect the signs and symptoms of CIPN in clinical practice.
The third parameter can be reflected in the responsiveness of change. Chemotherapy is administered within a specific period. The CIPN symptoms may occur in the initial stage of treatment or after the end of treatment (Ewertz et al., 2015; Ezendam et al., 2014). Healthcare providers should choose CIPN assessment tools that have been approved with regard to the responsiveness during treatment and survival.
The parameter of functional activity assessment involves the questions covering the impact of CIPN symptoms on activities of daily living. Improvement in cancer treatment results in an increasing number of cancer survivors; therefore, assessment of quality of life related to treatment for patients with cancer becomes a concern (Cavaletti et al., 2010). Accurate assessment of functional status and quality of life can help healthcare providers understand the impact of treatment on patients’ lives. With proper assessment, healthcare providers could make decisions regarding treatment regimens based on the impact on quality of life (Calhoun et al., 2003; Paice, 2009). The FACT/GOG-Ntx, PNS, PNQ, and SCIN can be categorized as functional assessment tools because they contain items to assess patients’ functional status or quality of life. The FACT/GOG-Ntx measures CIPN symptoms and the impact of CIPN symptoms on daily activities. The FACT/GOG-Ntx has been tested on drug-specific symptoms, with the FACT–Taxane and the mFACT/GOG-Ntx assessing oxaliplatin-related CIPN. The four studies using the FACT/GOG-Ntx meet the criteria of high applicability proposed in the QUADAS-2 (Calhoun et al., 2003; Cella et al., 2003; Huang et al., 2007; Kopec et al., 2006). Risk of bias of the studies under the FACT/GOG-Ntx is also determined as low. Therefore, the FACT/GOG-Ntx may be applied to assess CIPN comprehensively.
The last parameter can be reflected by patients’ participation rate. The studies in this systematic review rarely reported the participation rate, but in all three that reported it, the rate was greater than 80%. In the future, the participation rate and participants’ responses should be emphasized.
For practical perspectives, composite tools have recently been proposed to improve the accuracy and reliability of CIPN assessment (Cavaletti et al., 2007; Smith et al., 2010). Composite tools refer to the combination of patient-reported CIPN symptoms, neurologic examination performed by a physician, and use of healthcare provider–based assessment tools. The TNS, TNSr, and TNSc are regarded as composite tools to assess CIPN. Those tools require patients and healthcare providers to complete them. The TNS includes a patient self-report of CIPN symptoms, followed by physician examinations, including tests of pin and vibration sensation, strength, deep tendon reflex, and nerve conduction. Although the TNS and its modified versions have been shown to have good psychometric properties, the TNS contains CIPN symptoms but not the impact of symptoms on activities of daily living or quality of life.
In addition, healthcare providers must be well trained in neurologic assessment because inadequate rating experience may result in assessment bias (Smith et al., 2010). Barriers in completing the CIPN tools have been proposed by oncologists, including complicated procedures, their time-consuming nature, and required availability and standardized instruments (Cavaletti et al., 2010). For example, the QST requires well-trained healthcare providers and standardized equipment for completion.
From the available literature, the existing tools being developed may be less satisfactory for evaluating CIPN. In addition, healthcare providers tend to underestimate and underreport the severity and frequency of CIPN, particularly the subjective symptoms that affect patients’ function and quality of life. Despite the limitations of the existing tools, the authors suggest that oncology care providers should consider patient-reported assessment as a first assessment because CIPN is subjective in nature, followed by provider assessment. The authors recommend the FACT/GOG-Ntx as the first assessment; then, providers validate the patient report using the TNSc. This might allow the most effective assessment of the type and severity of CIPN.
Limitations
Some limitations can be drawn from this systematic review. This systematic review only included articles published in English, which may lead to publication bias. The tools in the systematic reviewed are limited to measuring CIPN; therefore, the findings of the systematic review could not be applied to the scales that are related to peripheral neuropathy caused by other comorbidities, such as diabetes, that might conflate assessment.
Implications for Nursing
Early detection of CIPN is valuable in designing interventions to prevent or decrease deterioration caused by CIPN. Setting a regular CIPN assessment schedule is needed because CIPN can occur at the beginning of chemotherapy treatment or after the end of treatment. Lastly, the burden to the patient and the cost-effectiveness of examination should also be considered. 
Conclusion
CIPN is a debilitating symptom experienced by patients with cancer receiving neurotoxic chemotherapy agents. Assessment and evaluation of CIPN symptoms using standardized and practical tools in routine practice are important. This systematic review offered new insights by evaluating 20 assessment tools related to CIPN retrieved from 19 studies. The QUADAS-2 was applied to evaluate the study quality of the 19 studies. After considering the psychometric properties, practicality, and comprehensive issues, two CIPN assessment tools are recommended: the FACT/GOG-Ntx and the TNS and its modifications.
About the Author(s)
Haryani is a PhD candidate at the Institute of Allied Health Sciences in the College of Medicine at National Cheng Kung University in Tainan City, Taiwan, and a lecturer in the School of Nursing Faculty of Medicine at Universitas Gadjah Mada in Yogyakarta, Indonesia; Fetzer is a professor in the Department of Nursing of the College of Health and Human Services at the University of New Hampshire in Durham; Wu is a case manager at the National Cheng Kung University Hospital; and Hsu is an associate professor in the Department of Nursing and Institute of Allied Health Sciences of the College of Medicine at National Cheng Kung University. No financial relationships to disclose. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Haryani and Hsu contributed to the conceptualization and design and provided statistical support. Haryani, Wu, and Hsu completed the data collection. Haryani, Fetzer, and Hsu provided the analysis and contributed to the manuscript preparation. Hsu can be reached at yuht12@mail.ncku.edu.tw, with copy to editor at ONFEditor@ons.org. Submitted March 2016. Accepted for publication September 27, 2016.
References
Almadrones, L., McGuire, D.B., Walczak, J.R., Florio, C.M., & Tian, C. (2004). Psychometric evaluation of two scales assessing functional status and peripheral neuropathy associated with chemotherapy for ovarian cancer: A gynecologic oncology group study. Oncology Nursing Forum, 31, 615–623. doi:10.1188/04.ONF.615-623
Boyette-Davis, J.A., Eng, C., Wang, X.S., Cleeland, C.S., Wendelschafer-Crabb, G., Kennedy, W.R., . . . Dougherty, P.M. (2012). Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clinical Cancer Research, 18, 3180–3187. doi:10.1158/1078-0432.ccr-12-0205
Calhoun, E.A., Welshman, E.E., Chang, C.H., Lurain, J.R., Fishman, D.A., Hunt, T.L., & Cella, D. (2003). Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. International Journal of Gynecological Cancer, 13, 741–748.
Cavaletti, G., & Marmiroli, P. (2015). Chemotherapy-induced peripheral neurotoxicity. Current Opinion in Neurology, 28, 500–507. doi:10.1097/wco.0000000000000234
Cavaletti, G., Bogliun, G., Marzorati, L., Zincone, A., Piatti, M., Colombo, N., . . . Zanna, C. (2003). Grading of chemotherapy-induced peripheral neurotoxicity using the total neuropathy scale. Neurology, 61, 1297–1300.
Cavaletti, G., Frigeni, B., Lanzani, F., Mattavelli, L., Susani, E., Alberti, P., . . . Bidoli, P. (2010). Chemotherapy-induced peripheral neurotoxicity assessment: A critical revision of the currently available tools. European Journal of Cancer, 46, 479–494. doi:10.1016/j.ejca.2009.12.008
Cavaletti, G., Frigeni, B., Lanzani, F., Piatti, M., Rota, S., Briani, C., . . . Zanna, C. (2007). The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute-Common Toxicity Scale. Journal of the Peripheral Nervous System, 12, 210–215. doi:10.1111/j.1529-8027.2007.00141.x
Cavaletti, G., Jann, S., Pace, A., Plasmati, R., Siciliano, G., Briani, C., . . . Giussani, G. (2006). Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. Journal of the Peripheral Nervous System, 11, 135–141. doi:10.1111/j.1085-9489.2006.00078.x
Cella, D., Peterman, A., Hudgens, S., Webster, K., & Socinski, M.A. (2003). Measuring the side effects of taxane therapy in oncology: The Functional Assessment of Cancer Therapy–Taxane (FACT–Taxane). Cancer, 98, 822–831. doi:10.1002/cncr.11578
DeVon, H.A., Block, M.E., Moyle-Wright, P., Ernst, D.M., Hayden, S.J., Lazzara, D.J., . . . Kostas-Polston, E. (2007). A psychometric toolbox for testing validity and reliability. Journal of Nursing Scholarship, 39, 155–164. doi:10.1111/j.1547-5069.2007.00161.x
Ewertz, M., Qvortrup, C., & Eckhoff, L. (2015). Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncologica, 54, 587–591. doi:10.3109/0284186x.2014.995775
Ezendam, N.P., Pijlman, B., Bhugwandass, C., Pruijt, J.F., Mols, F., Vos, M.C., . . . van de Poll-Franse, L.V. (2014). Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: Results from the population-based PROFILES registry. Gynecologic Oncology, 135, 510–517. doi:10.1016/j.ygyno.2014.09.016
Forsyth, P.A., Balmaceda, C., Peterson, K., Seidman, A.D., Brasher, P., & DeAngelis, L.M. (1997). Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. Journal of Neuro-Oncology, 35, 47–53.
Griffith, K.A., Merkies, I.S., Hill, E.E., & Cornblath, D.R. (2010). Measures of chemotherapy-induced peripheral neuropathy: A systematic review of psychometric properties. Journal of the Peripheral Nervous System, 15, 314–325. doi:10.1111/j.1529-8027.2010.00292.x
Hausheer, F.H., Schilsky, R.L., Bain, S., Berghorn, E.J., & Lieberman, F. (2006). Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Seminars in Oncology, 33, 15–49. doi:10.1053/j.seminoncol.2005.12.010
Hershman, D.L., Lacchetti, C., Dworkin, R.H., Lavoie Smith, E.M., Bleeker, J., Cavaletti, G., . . . Loprinzi, C.L. (2014). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guidelines. Journal of Clinical Oncology, 32, 1941–1967. doi:10.1200/JCO.2013.54.0914
Huang, H.Q., Brady, M.F., Cella, D., & Fleming, G. (2007). Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: A Gynecologic Oncology Group study. International Journal of Gynecological Cancer, 17, 387–393. doi:10.1111/j.1525-1438.2007.00794.x
Kopec, J.A., Land, S.R, Cecchini, R.S., Ganz, P.A., Cella, D., Costantino, J.P., . . . Wolmark, N. (2006). Validation of a self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. Journal of Supportive Oncology, 4(8), W1–W8.
Kuroi, K., Shimozuma, K., Ohashi, Y., Hisamatsu, K., Masuda, N., Takeuchi, A., . . . Hausheer, F.H. (2009). Prospective assessment of chemotherapy-induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP-HOR 02 study). Supportive Care in Cancer, 17, 1071–1080. doi:10.1007/s00520-008-0550-x
Oldenburg, J., Fosså, S.D., & Dahl, A.A. (2006). Scale for chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics, validation, and findings in a large sample of testicular cancer survivors. Quality of Life Research, 15, 791–800. doi:10.1007/s11136-005-5370-6
Paice, J.A. (2009). Clinical challenges: Chemotherapy-induced peripheral neuropathy. Seminars in Oncology Nursing, 25(Suppl. 1), S8–S19. doi:10.1016/j.soncn.2009.03.013
Park, S.B., Lin, C.S., & Kiernan, M.C. (2012). Nerve excitability assessment in chemotherapy-induced neurotoxicity. Journal of Visualized Experiments, (62), e3439. doi:10.3791/3439
Peacock, J., & Peacock, P.J. (2011). Oxford handbook of medical statistics. New York, NY: Oxford University Press.
Postma, T.J., Heimans, J.J., Muller, M.J.., Ossenkoppele, G.J., Vermorken, J.B., & Aaronson, N.K. (1998). Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Annals of Oncology, 9, 739–744.
Reyes-Gibby, C., Morrow, P.K., Bennett, M.I., Jensen, M.P., & Shete, S. (2010). Neuropathic pain in breast cancer survivors: Using the ID pain as a screening tool. Journal of Pain and Symptom Management, 39, 882–889. doi:10.1016/j.jpainsymman.2009.09.020
Seretny, M., Currie, G.L., Sena, E.S., Ramnarine, S., Grant, R., MacLeod, M.R., . . . Fallon, M. (2014). Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain, 155, 2461–2470. doi:10.1016/j.pain.2014.09.020
Shimozuma, K., Ohashi, Y., Takeuchi, A., Aranishi, T., Morita, S., Kuroi, K., . . . Hausheer, F.H. (2009). Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Supportive Care in Cancer, 17, 1483–1491. doi:10.1007/s00520-009-0613-7
Smith, E.M., Cohen, J.A., Pett, M.A., & Beck, S.L. (2010). The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nursing, 33, 173–183. doi:10.1097/NCC.0b013e3181c989a3
Takemoto, S., Ushijima, K., Honda, K., Wada, H., Terada, A., Imaishi, H., & Kamura, T. (2012). Precise evaluation of chemotherapy-induced peripheral neuropathy using the visual analogue scale: A quantitative and comparative analysis of neuropathy occurring with paclitaxel-carboplatin and docetaxel-carboplatin therapy. International Journal of Clinical Oncology, 17, 367–372. doi:10.1007/s10147-011-0303-6
Tofthagen, C.S., McMillan, S.C., & Kip, K.E. (2011). Development and psychometric evaluation of the chemotherapy-induced peripheral neuropathy assessment tool. Cancer Nursing, 34(4), E10–E20. doi:10.1097/NCC.0b013e31820251de
Visovsky, C., Collins, M., Abbott, L., Aschenbrenner, J., & Hart, C. (2007). Putting evidence into practice: Evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clinical Journal of Oncology Nursing, 11, 901–913. doi:10.1188/07.CJON.901-913
Wampler, M.A., Miaskowski, C., Hamel, K., Byl, N., Rugo, H., & Topp, K.S. (2006). The modified Total Neuropathy Score: A clinically feasible and valid measure of taxane induced peripheral neuropathy in women with breast cancer. Journal of Supportive Oncology, 4(8), W9–W16.
Whiting, P., Rutjes, A.W., Reitsma, J.B., Bossuyt, P.M., & Kleijnen, J. (2003). The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology, 3, 25. doi:10.1186/1471-2288-3-25
Whiting, P.F., Rutjes, A.W., Westwood, M.E., Mallett, S., Deeks, J.J., Reitsma, J.B., . . . Bossuyt, P.M. (2011). QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine, 155, 529–536. doi:10.7326/0003-4819-155-8-201110180-00009
Wolf, S.L., Barton, D.L., Qin, R., Wos, E.J., Sloan, J.A., Liu, H., . . . Loprinzi, C.L. (2012). The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Supportive Care in Cancer, 20, 625–632. doi:10.1007/s00520-011-1141-9

