Exercise Among Women With Ovarian Cancer: A Feasibility and Pre-/Post-Test Exploratory Pilot Study
Purpose/Objectives: To establish the feasibility and acceptability of completing a higher dose of the planned physical activity volume among women with ovarian cancer, including those undergoing active treatment.
Design: A pre-/post-test exercise intervention. All participants were asked to complete 225 minutes per week of physical activity for 26 weeks. Multiple supports were provided, including exercise DVDs, self-reported logs, and an objective physical activity tracker (Fitbit®).
Setting: Home-based exercise intervention with in-person training and telephone follow-ups.
Sample: 10 women with ovarian cancer who were treated within Penn Medicine in Philadelphia, Pennsylvania.
Methods: Home-based, in-person exercise counseling was provided by an exercise trainer weekly for the first six weeks and then monthly for a total of 26 weeks. Weekly follow-up telephone calls were used to assess exercise adherence and barriers to completing exercise, review symptom changes, and provide behavioral support.
Main Research Variables: Feasibility and acceptability.
Findings: Eight participants completed the study and achieved at least 80% of the prescribed exercise dose. Five participants were undergoing chemotherapy simultaneously. Participants experienced no adverse events during the 26-week intervention. Compared to baseline, average steps increased by 1,593 per day and moderate-intensity physical activity increased by 15 minutes per day.
Conclusions: A 225-minutes-per-week exercise program is feasible and acceptable in a population of patients with ovarian cancer. Participants significantly improved their physical activity during the 26-week intervention.
Implications for Nursing: The findings suggest that nursing professionals could recommend that women with ovarian cancer exercise 225 minutes per week regardless of cancer and/or treatment trajectory. For those experiencing aches and pains, behavioral supports and suggestions of a lower exercise dose are needed to maintain physical activity.
Jump to a section
As the number of ovarian cancer survivors increases, concerns have shifted toward improving or maintaining quality of life (Smits, Lopes, et al., 2015). Women with ovarian cancer may experience deleterious physical and psychological sequelae resulting from cancer treatment, such as nausea, vomiting, loss of appetite, anemia, neuropathy, poor sleep, cancer-related fatigue, depression, and anxiety (Moonsammy et al., 2013). Therefore, women may become sedentary during and after ovarian cancer treatment, which may impair quality of life (Mizrahi, Naumann, et al., 2015; Thigpen et al., 2010). Physical activity can provide improvements in cardiopulmonary fitness, muscle strength, aerobic capacity, fatigue, and quality of life among women with ovarian cancer (Mizrahi, Naumann, et al., 2015; Moonsammy et al., 2013). In addition, observational evidence suggests that physical activity may prolong survival and lower the risk of mortality among women with ovarian cancer (Moorman, Jones, Akushevich, & Schildkraut, 2011; Zhou et al., 2014).
Despite the benefits of physical activity, only 20% of women with ovarian cancer reported meeting the physical activity guidelines of 150 minutes of moderate physical activity per week (Mizrahi, Broderick, et al., 2015; Smits, Lopes, et al., 2015). A prior exercise intervention has shown that it is feasible to increase physical activity levels from 31 minutes per week to 132.8 minutes per week during six months among women with ovarian cancer (Zhou et al., 2015). In addition, women with recurrent ovarian cancer are also able to exercise, with the majority being able to complete at least 90 minutes of exercise per week during chemotherapy (Mizrahi, Broderick, et al., 2015). Previous studies corroborated the feasibility of prescribing exercise to women with ovarian cancer (Hwang, Cho, & Yoo, 2016; Moonsammy et al., 2013; Newton et al., 2011). However, no prior study has examined the feasibility of prescribing a larger dose of exercise (greater than 150 minutes per week) in this population, and no study has examined if women with advanced ovarian cancer (stages III–IV) are able to complete a 26-week exercise intervention. To assess if women with ovarian cancer, particularly those with advanced disease, are able to complete a large-dose exercise intervention, the current study aimed to examine the feasibility and acceptability of a 26-week home-based exercise program of 225 minutes per week among women with advanced ovarian cancer.
Methods
The primary aim of this nonrandomized, pre-/post-test pilot study was to assess the feasibility and acceptability of a 26-week home-based, high-dose exercise intervention among women with advanced-stage ovarian cancer. The accrual goal of the current pilot study was 10 participants. This study was approved by the University of Pennsylvania Institutional Review Board, and the trial was registered with ClinicalTrials.gov as NCT02529150.
Participants were recruited through three approaches: recruitment letters, physician referrals, and flyers placed in a clinic waiting room. A recruitment letter with an invitation to participate, a one-page flyer describing the study, and the name and contact information of the study coordinator were mailed to women with ovarian cancer identified from the Penn Medicine Cancer Registry. An ICD-9-CM code was used to identify women with ovarian cancer (183.0). The Penn Medicine Cancer Registry is a systematic information system that captures all people who are diagnosed and/or treated for a diagnosis of cancer within Penn Medicine. This recruitment approach has been used by the current research group in prior studies among breast and colon cancer survivors (Brown et al., 2016; Rogerino, Grant, Wilcox, & Schmitz, 2009). In addition, gynecologic oncologists at Penn Medicine introduced the study to potentially eligible participants and referred those who were interested to the study coordinator. Flyers describing the study were also distributed in the waiting room at the Jordan Center for Gynecologic Cancers at the Abramson Cancer Center at Penn Medicine in Philadelphia, Pennsylvania.
Participants were eligible for the study if they were diagnosed with stages III–IV ovarian cancer and underwent surgery at Penn Medicine from 2010–2014, estimated by their oncologists to have 12 months or greater life expectancy, were able to walk 15 minutes without stopping, spoke English, and lived within a 45-minute radius from the University of Pennsylvania (given the goal to deliver home-based, in-person exercise training sessions). There were no age limits or exclusions based on prior and current cancer treatments. Exclusions included an Eastern Cooperative Oncology Group Performance Status score of 2 or greater (Oken et al., 1982) or any medical or psychiatric conditions that would impair participation in physical activity (e.g., cardiac, pulmonary, or orthopedic history; psychotic disorders; dementia; inability to give informed consent).
Procedures
All potentially eligible participants were screened by a telephone interview, which included a brief description of the study and a systematic query of each inclusion and exclusion criterion. The treating oncologist for each potential eligible participant was contacted to evaluate whether the woman was medically fit to participate in a moderate-intensity aerobic exercise study. Following physician approval, eligible participants were scheduled for an in-person meeting at their homes with the study coordinator and the exercise trainer to discuss the goals, objectives, risks, and benefits of the study in detail. At that time, written informed consent was obtained.
All participants were provided with a walking program DVD (Sansone, 2014) and a physical activity tracker (Fitbit® Zip) at their first in-person session. The Fitbit Zip is a wireless physical activity tracker that syncs data to smartphones, as well as to a web-based data platform. The Fitbit was initialized to enable simultaneous viewing by participants and study personnel. In-person exercise sessions were provided weekly for the first six weeks and monthly for the remaining 20 weeks by a certified clinical exercise trainer from the University of Pennsylvania at the participants’ homes. Follow-up telephone calls were provided weekly by a research staff member based on an interview script for the entire 26 weeks. Participants were asked each week if they had any symptoms that prevented exercise and were encouraged to call study staff if they experienced any changes in their existing symptoms. If any changes in symptoms (e.g., fatigue from first several days after chemotherapy, aches, pains) were reported that required an alteration of exercise prescription, the exercise trainer would contact participants to develop a plan for maintaining optimal exercise adherence while preserving participant safety. Changes in symptoms were also reviewed with the gynecologic oncologists bimonthly to ensure appropriate medical oversight for the study activities. If, at any point, the treating physician felt exercise should be ceased, the woman was withdrawn from the study.
All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Measurements
Measurements were obtained from all participants by a trained staff member following a standardized protocol. At the first in-person session, height was measured without shoes, using a stadiometer to the nearest 0.1 cm. Weight was measured using a calibrated digital scale to the nearest 0.1 kg. Height and weight were used to calculate body mass index (BMI) in kg/m2. Demographic information (e.g., age, zip code, race) and tumor-related characteristics (e.g., date of diagnosis, treatment completion, stage, grade of histologic differentiation, adjuvant chemotherapy) were collected from the electronic health records at Penn Medicine.
Physical activity was measured using self-report and objective measures. The self-report exercise log queried activities, including warm-up, physical activity, stretches, date, and duration of exercise sessions, each week. An ActiGraph GT3X triaxial accelerometer was worn for one week at baseline and during the final week. Participants were provided with a diary to document accelerometer wear time. The ActiGraph is a valid and reliable objective measure of ambulatory activity (Bassett et al., 2000). The main outcomes obtained from the ActiGraph included (a) number of minutes of moderate-intensity physical activity (3–6 metabolic equivalents [METs]), (b) number of minutes of light-intensity physical activity (1–3 METs), (c) average minutes of moderate- to vigorous-intensity physical activity (3 METs or greater), and (d) ambulatory steps using validated cut-points appropriate for adults (Troiano et al., 2008). Participants were also asked to wear an activity tracker (Fitbit Zip) for the entire 26 weeks to objectively assess and track adherence to the exercise intervention. Fitbit data (daily steps) were recorded from the web-based database by research staff.
Participants were asked each week by research staff to identify any health events using a systemic interview script. All reported health events were graded by the Common Terminology Criteria for Adverse Events, version 4.02 (U.S. Department of Health and Human Services, 2009). Participants were asked whether there had been a change in symptoms during the past week (“yes” or “no”); if yes, participants were asked what symptoms changed (recorded verbatim) and whether they felt that the symptom change was related to study activities (“not at all,” “probably not,” “maybe,” “probably,” or “definitely”). All participants were asked about the barriers that impeded their participation in exercise. At the 26-week telephone follow-ups, participants were asked for comments and if they were satisfied with the exercise intervention.
Intervention
All participants were asked to complete a 26-week high-dose, home-based exercise intervention of 225 minutes per week. The primary mode of exercise was walking; however, bicycling and exercise on cardiovascular training equipment were also allowed. The exercise trainer introduced the exercise prescription and familiarized participants with the Borg rating of perceived exertion scale. The Borg scale was used to track intensity because heart rate response to exercise may vary among women undergoing chemotherapy. The goal intensity level was 12–15, consistent with moderate intensity on the Borg scale that ranges from 6–20 (Borg, 1970). The exercise trainer also explained the completion of exercise logs, use of the walking program DVD and Fitbit Zip, appropriate warm-up and cool-down exercises, stretches, and proper footwear for aerobic exercise. For the first six weeks, participants met with the exercise trainer in their homes each week for the in-person session. After the first six weeks, in-person sessions were conducted on an as-needed basis as often as one time monthly for the remaining 20 weeks.
All participants received a telephone call each week during the 26-week exercise intervention. Individualized behavioral support was provided to all participants, including benefits of exercise for women with ovarian cancer, strategies to increase to the required exercise dose of 225 minutes per week, and methods to identify and overcome barriers to exercise. The total number of minutes of exercise was increased by at least 30 minutes per week over the participant’s baseline activity level until the prescribed target dose was reached.
Statistical Analysis
The feasibility of exercise was quantified by the proportion of participants who achieved 80% or greater of the prescribed exercise dose. The acceptability of exercise was defined by safety and ability to continue exercise when barriers occurred. The safety of exercise was quantified by the proportion of participants who experienced adverse events. Frequency, modified dose (“yes” or “no”), and the occurrence of participants who experienced exercise barriers were reported to better understand exercise acceptability. For each of the primary and secondary physiologic outcomes, a linear regression model was used to compare the change in exercise levels while adjusting for the baseline value of the dependent variable to improve efficiency. In exploratory analyses, average ambient temperature was used to examine the relationship between exercise adherence and climate. Weekly ambient temperatures were collected according to Philadelphia weather history data from Weather Underground (www.wunderground.com). Stata/MP, version 14.0, was used for all statistical analyses. A two-sided p value of 0.05 was considered statistically significant.
[[{"type":"media","view_mode":"media_original","fid":"32116","attributes":{"alt":"","class":"media-image","height":"463","typeof":"foaf:Image","width":"377"}}]]
Results
From February 26 to March 4, 2015, 76 letters were mailed to potentially eligible participants who were diagnosed with stages III–IV ovarian cancer and treated at Penn Medicine (see Figure 1). For each woman who responded to one letter of invitation, seven letters were mailed. A total of 21 potential eligible participants inquired about participating and were screened. Characteristics of eligible participants are presented in Table 1. Among the 21 screened potential eligible participants, 17 were eligible for participation. Among the 17 eligible participants, 10 were enrolled in a first-contacted, first-served manner. The remaining seven eligible participants were put on a waiting list because the study reached maximum accrual. Among the 10 enrolled participants, the median age was 62.5 years (interquartile range [IQR] = 59–72). Seven participants were aged younger than 70 years. All had stage III disease. Five were currently being treated with chemotherapy, and four had recurrent disease. The median BMI was 23.5 kg/m2 (IQR = 22.7–29.9).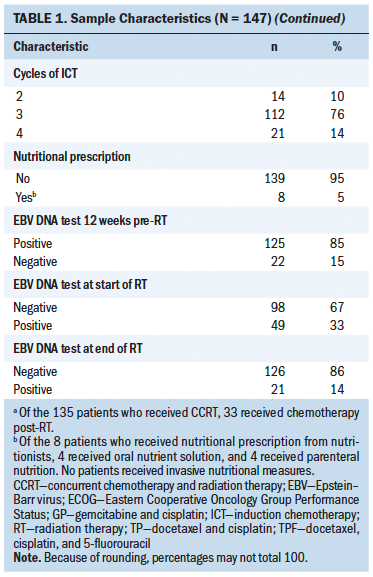
Feasibility and Acceptability
Weekly adherence rates are presented in Table 2. The average duration of exercise (minutes) each week is illustrated in Figure 2. The lowest average weekly exercise time was 193 minutes (86% of prescribed dose), and the highest average weekly exercise time was 311 minutes (138% of prescribed dose). On average, participants received 83% of in-person sessions with the trainer (86% completion of weekly in-person session during the first six weeks and 75% completion of the monthly in-person sessions during the remaining 20 weeks). 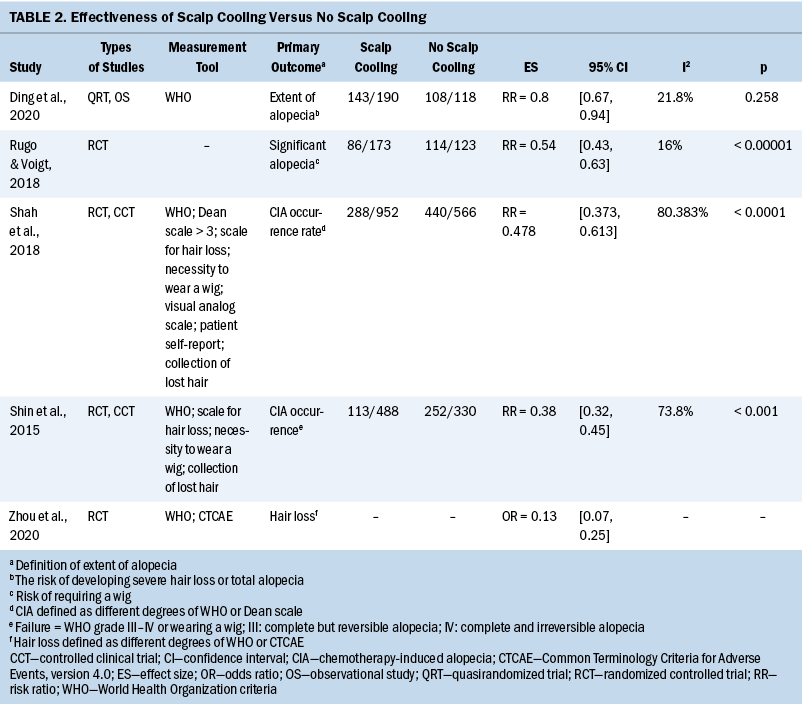
All exercise barriers are presented in Table 3. Participants who experienced aches and pains were asked to continue exercise with a reduced dose. Exercise barriers occurred during all months of the study without any identifiable patterns (data not shown). Six of 10 participants experienced recurrent barriers. However, no one experienced exercise barriers every week of any given month. Only two participants experienced exercise barriers during three weeks of any given month in the six-month exercise intervention. There were no major adverse events during the entire 26 weeks of exercise intervention.
[[{"type":"media","view_mode":"media_original","fid":"32121","attributes":{"alt":"","class":"media-image","height":"423","typeof":"foaf:Image","width":"374"}}]]
Two of 10 participants withdrew from the study. One was not interested in continuing the study and withdrew at week 10, and the other was withdrawn by the oncologist because of disease progression at week 13. Eight women completed all 26 weeks of the exercise intervention. All eight participants who completed the study reported the intervention to be “very helpful.” The majority reported improved function, reflected by comments that they were “feeling better” or “more energetic.” Two participants reported exchanging automobile transportation for walking. One said, “Instead of driving, I walk.” Participants also demonstrated understanding exercise progression. One participant said, “I ask myself to walk longer, to reach the end of block, touching the tree, and come back.” Participants were motivated by use of the Fitbit. One said, “When I saw I walked more than 8,000 steps on the Fitbit, I tried to walk more to reach 10,000 steps for the day.” All eight participants who completed the study said they will miss the time spent with the exercise trainer.
[[{"type":"media","view_mode":"media_original","fid":"32126","attributes":{"alt":"","class":"media-image","height":"397","typeof":"foaf:Image","width":"367"}}]]
Physical Activity
During the 26 weeks of exercise intervention, according to the accelerometer, moderate-intensity activity increased by 15 minutes per day (p = 0.05) (see Table 4). Light-intensity activity and sedentary activity did not significantly change. Step counts increased by 1,593 steps per day (p = 0.041). Moderate- to vigorous-intensity physical activity increased by 10.02 minutes per day on average (p = 0.078). Trends of exercise and temperature are presented in Figure 3. In the regression model, adjusted for baseline self-reported physical activity time, with each 10-degree increase in weekly average temperature, self-reported physical activity time increased by 15.5 minutes (p = 0.009), explaining 18% of the variability in exercise time.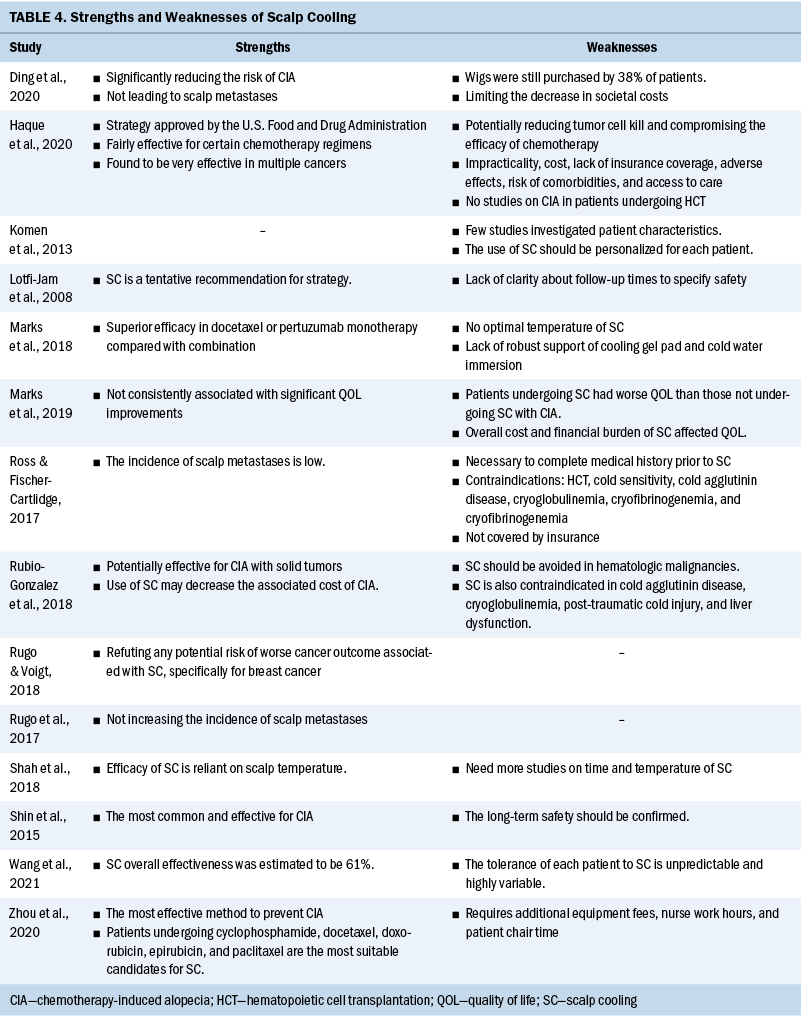
Discussion
This study demonstrates that it is feasible and safe for women with advanced ovarian cancer to participate in a 225-minutes-per-week exercise program for 26 weeks. Ten participants were recruited within six days. In addition, seven eligible patients were waitlisted after the recruitment goal of 10 participants was achieved. There were no serious adverse events during the 26-week intervention. The authors also established a favorable acceptability profile, supported by the high exercise adherence. All participants reached at least 80% of the prescribed exercise dose every week. During the intervention, time of moderate-intensity physical activity and step count significantly increased.
[[{"type":"media","view_mode":"media_original","fid":"32131","attributes":{"alt":"","class":"media-image","height":"378","typeof":"foaf:Image","width":"374"}}]]
The combination of three recruitment approaches led to the success of achieving the maximum accrual goal in six days at a single institution. Fifty percent of the participants were recruited through mailing recruitment letters. The high response rate suggests that using cancer registries to recruit women with ovarian cancer into a lifestyle intervention is feasible. Of note, five participants inquired about the study after seeing the distributed flyers, and three enrolled. This suggests that distributed flyers may be another effective recruitment method for ovarian cancer lifestyle interventions. Given the success of recruitment from the current study, the combination of mailing recruitment letters, physician referrals, and distributed flyers is a feasible method to use to recruit women with ovarian cancer to a lifestyle intervention.
The high adherence rate and significant improvement in physical activity may have been related to the home-based exercise setting. Currently, the majority of women with ovarian cancer do not meet general public recommendations for exercise (Mizrahi, Naumann, et al., 2015; Smits, Smits, et al., 2015). Evidence suggests that 87% of women with ovarian cancer are somewhat interested in physical activity, and 49% prefer a home-based exercise program (Stevinson et al., 2009). Prior studies also suggest that walking should be encouraged in women with ovarian cancer (Mizrahi, Broderick, et al., 2015; Mizrahi, Naumann, et al., 2015). The high adherence and increase of 105 minutes per week (15 minutes per day) in moderate-intensity physical activity demonstrates that a flexible home-based exercise intervention is feasible and acceptable for women with advanced ovarian cancer to participate in a moderate-intensity exercise program (225 minutes per week).
In addition, the close monitoring provided (weekly follow-up by telephone, weekly in-person training session with the exercise trainer in the first six weeks, and monthly in-person training sessions in the last 20 weeks) also have contributed to the intervention’s success. Each week, the exercise trainer asked all participants to gradually increase the total time of physical activity until the prescribed target dose was reached. The exercise trainer provided all participants with personalized feedback and behavioral support to increase physical activity. Participants who experienced symptoms, such as aches or pains, were asked to continue exercising at a lower dose to avoid becoming sedentary. For participants who experienced significant life events, such as family emergency or vacation, the exercise trainer provided strategies to find time to exercise. For each in-person training session, the exercise trainer supervised participants exercising (primarily walking) and provided professional counseling. All participants reported that the in-person sessions were helpful to them.
Exercise barriers were common among women with advanced ovarian cancer. In the current study, 7 of 10 participants experienced aches and pains, and 6 had life events. This study showed that it was necessary to have an exercise trainer to assist participants in overcoming barriers and encouraging them to keep active using a lifestyle intervention. Most exercise barriers occurred only during one or two weeks in a month. In the current study, the authors provided weekly check-in telephone calls. The pattern of exercise barriers suggests that biweekly telephone follow-ups might be sufficient. This would reduce the workload of the study coordinator and could still capture all symptom changes and exercise barriers without causing a negative effect on physical activity levels in women with advanced ovarian cancer.
Intervention Barriers
The authors provided a physical activity monitor to all participants. The Fitbit Zip was selected because of its small size that could be clipped on to a belt while remaining discrete, and had a battery life that could last as many as six months without needing a charge. Although Fitbit Zip could show steps and time of exercise and could sync the data to smartphones, which all participants liked, the malfunction rate was high. During the 26 weeks, the authors replaced five Fitbit Zip devices because of malfunction (e.g., blank screen, not recording). In addition, three participants lost their Fitbit Zip. Participants reported they liked to check their daily progress through the Fitbit Zip device, which could motivate them to do more exercise. Therefore, additional studies may consider using a different model of objective monitors. Based on this experience, monitors with a progress interface on the device would be desirable. In addition, investigators are urged to plan for as much as 50% replacement costs. The authors also provided a walking program DVD, but no participants used it on a daily basis. The participants reported that they did not like walking at home. With the finding that higher weekly outside temperature was associated with increased physical activity, alternative exercise modes should be provided for women with ovarian cancer to continue exercising during inclement weather.
Strengths and Limitations
To the best of the authors’ knowledge, this was the first study to prescribe 225 minutes per week of exercise in women with advanced-stage ovarian cancer. Although the sample size was small, given seven additional eligible participants on the waiting list and high adherence rate, this study suggested that it is feasible to recruit women with advanced ovarian cancer and that these women have the interest and ability to perform physical activity regardless of treatment or disease trajectory. The study provided 10 in-person sessions with an exercise trainer. The exercise trainer met all participants at their homes, which reduced the travel burden for participation. In addition, personalized feedback and behavioral support may have contributed to the success. The Fitbit Zip and exercise counseling served as incentives for participation.
The primary limitation to this study is the small sample size, which may limit interpretation of the study findings. The nonrandomized study design and lack of a control group may overestimate the findings. In addition, all participants were recruited through one institution, which may limit its generalizability. It is possible that women with ovarian cancer who were treated at the authors’ institution might be different compared with those treated elsewhere. Therefore, a large multicenter clinical trial is needed to extend to a wider ovarian cancer population.
Future Research
The next step is to propose a dose-response randomized study to assess therapeutic effects of exercise in women with ovarian cancer. A prior randomized clinical trial showed that 225 minutes per week of walking could increase natural killer cell activity by 57% compared with sedentary healthy women, which suggests this level of exercise may improve immune function (Nieman et al., 1990). One hundred and fifty minutes per week of walking during six months improved anti-inflammatory effects and downregulated T-cell and monocyte activation (Viana et al., 2014). In another study, 150 minutes per week of exercise decreased circulating vascular endothelial growth factor and angiogenic activation (DuttaRoy et al., 2015; Eleuteri et al., 2013). Based on this evidence, moderate exercise has effects on immune, inflammation, and angiogenesis pathways. However, the dose-response relationship of exercise to these outcomes or whether these effects would occur in women with ovarian cancer is not known. The preliminary finding from the current study supports that women with ovarian cancer are able to complete 225 minutes per week of moderate exercise. The authors will propose a larger-scale, dose-response trial to randomize women with ovarian cancer to groups with no exercise, 180 minutes per week, or 225 minutes per week to assess the therapeutic effects of an exercise intervention.
Implications for Nursing
These findings suggest that nursing professionals could recommend that women with ovarian cancer exercise for 225 minutes per week, including those receiving chemotherapy; however, exercise barriers may often occur. Nurses are well positioned to provide behavioral support and tailor exercise for participants who report aches and pains to enable the maintenance of a physically active lifestyle. The efficacy of exercise in ovarian cancer progression is unknown. Future large-scale, dose-response randomized trials are needed to examine the therapeutic effects of exercise in patients with ovarian cancer.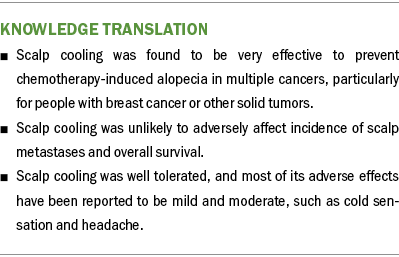
Conclusion
The findings suggest that it is feasible, safe, and acceptable to prescribe a 225-minutes-per-week exercise program to women with advanced-stage ovarian cancer. These findings contribute toward the goal of conducting a definitive dose-response clinical trial to assess therapeutic effects of exercise on symptoms or disease-related endpoints among women with ovarian cancer.
About the Author(s)
Zhang is a research data management specialist in the Department of Public Health Sciences in the Penn State College of Medicine at Penn State University in Hershey, PA; McClean is a research coordinator in the Department of Urogynecology, Ko is an assistant professor in the Department of Obstetrics and Gynecology, and Morgan is the chief of the Division of Gynecologic Oncology, all in the Perelman School of Medicine at the University of Pennsylvania in Philadelphia; and Schmitz is a professor and associate director of population sciences in the Department of Public Health Sciences in the Penn State College of Medicine at Penn State University in Hershey. The principal investigator (Schmitz) was funded by a grant (U54-CA155850) from the National Cancer Institute. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. All of the authors contributed to the conceptualization and design. Zhang and McClean completed the data collection. Zhang, McClean, and Schmitz provided statistical support. Zhang provided the analysis. Zhang, Ko, Morgan, and Schmitz contributed to the manuscript preparation. Zhang can be reached at zxchstar@gmail.com, with copy to editor at ONFEditor@ons.org. Submitted March 2016. Accepted for publication September 10, 2016.
References
Bassett, D.R., Jr., Ainsworth, B.E., Swartz, A.M., Strath, S.J., O’Brien, W.L., & King, G.A. (2000). Validity of four motion sensors in measuring moderate intensity physical activity. Medicine and Science in Sports and Exercise, 32(9, Suppl.), S471–S480.
Borg, G. (1970). Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine, 2, 92–98.
Brown, J.C., Troxel, A.B., Ky, B., Damjanov, N., Zemel, B.S., Rickels, M.R., . . . Schmitz, K.H. (2016). A randomized phase II dose-response exercise trial among colon cancer survivors: Purpose, study design, methods, and recruitment results. Contemporary Clinical Trials, 47, 366–375. doi:10.1016/j.cct.2016.03.001
DuttaRoy, S., Nilsson, J., Hammarsten, O., Cider, Å., Bäck, M., Karlsson, T., . . . Borjesson, M. (2015). High frequency home-based exercise decreases levels of vascular endothelial growth factor in patients with stable angina pectoris. European Journal of Preventive Cardiology, 22, 575–581. doi:10.1177/2047487314529349
Eleuteri, E., Mezzani, A., Di Stefano, A., Vallese, D., Gnemmi, I., Delle Donne, L., . . . Giannuzzi, P. (2013). Aerobic training and angiogenesis activation in patients with stable chronic heart failure: A preliminary report. Biomarkers, 18, 418–424. doi:10.3109/1354750X.2013.805342
Hwang, K.H., Cho, O.H., & Yoo, Y.S. (2016). The effect of comprehensive care program for ovarian cancer survivors. Clinical Nursing Research, 25, 192–208.
Mizrahi, D., Broderick, C., Friedlander, M., Ryan, M., Harrison, M., Pumpa, K., & Naumann, F. (2015). An exercise intervention during chemotherapy for women with recurrent ovarian cancer: A feasibility study. International Journal of Gynecological Cancer, 25, 985–992. doi:10.1097/IGC.0000000000000460
Mizrahi, D., Naumann, F., Broderick, C., Samara, J., Ryan, M., & Friedlander, M. (2015). Quantifying physical activity and the associated barriers for women with ovarian cancer. International Journal of Gynecological Cancer, 25, 577–583. doi:10.1097/IGC.0000000000000349
Moonsammy, S.H., Guglietti, C.L., Santa Mina, D., Ferguson, S., Kuk, J.L., Urowitz, S., . . . Ritvo, P. (2013). A pilot study of an exercise and cognitive behavioral therapy intervention for epithelial ovarian cancer patients. Journal of Ovarian Research, 6, 21. doi:10.1186/1757-2215-6-21
Moorman, P.G., Jones, L.W., Akushevich, L., & Schildkraut, J.M. (2011). Recreational physical activity and ovarian cancer risk and survival. Annals of Epidemiology, 21, 178–187. doi:10.1016/j.annepidem.2010.10.014
Newton, M.J., Hayes, S.C., Janda, M., Webb, P.M., Obermair, A., Eakin, E.G., . . . Beesley, V.L. (2011). Safety, feasibility and effects of an individualised walking intervention for women undergoing chemotherapy for ovarian cancer: A pilot study. BMC Cancer, 11, 389.
Nieman, D.C., Nehlsen-Cannarella, S.L., Markoff, P.A., Balk-Lamberton, A.J., Yang, H., Chritton, D.B., . . . Arabatzis, K. (1990). The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. International Journal of Sports Medicine, 11, 467–473. doi:10.1055/s-2007-1024839
Oken, M.M., Creech, R.H., Tormey, D.C., Horton, J., Davis, T.E., McFadden, E.T., & Carbone, P.P. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 5, 649–656.
Rogerino, A., Grant, L.L., Wilcox, H., III, & Schmitz, K.H. (2009). Geographic recruitment of breast cancer survivors into community-based exercise interventions. Medicine and Science in Sports and Exercise, 41, 1413–1420. doi:10.1249/MSS.0b013e31819af871
Sansone, L. (2014). Walk off fat fast [DVD]. United States: Anchor Bay.
Smits, A., Lopes, A., Das, N., Bekkers, R., Massuger, L., & Galaal, K. (2015). The effect of lifestyle interventions on the quality of life of gynaecological cancer survivors: A systematic review and meta-analysis. Gynecologic Oncology, 139, 546–552. doi:10.1016/j.ygyno.2015.10.002
Smits, A., Smits, E., Lopes, A., Das, N., Hughes, G., Talaat, A., . . . Galaal, K. (2015). Body mass index, physical activity and quality of life of ovarian cancer survivors: Time to get moving? Gynecologic Oncology, 139, 148–154. doi:10.1016/j.ygyno.2015.08.005
Stevinson, C., Capstick, V., Schepansky, A., Tonkin, K., Vallance, J.K., Ladha, A.B., . . . Courneya, K.S. (2009). Physical activity preferences of ovarian cancer survivors. Psycho-Oncology, 18, 422–428. doi:10.1002/pon.1396
Thigpen, J.T., Alberts, D., Birrer, M., Copeland, L., Coleman, R.L., Markman, M., . . . Ranganathan, A. (2010). Current challenges and future directions in the management of ovarian cancer: Proceedings of the first global workshop on ovarian cancer. Clinical Ovarian Cancer, 3, 81–97. doi:10.3816/COC.2010.n.015
Troiano, R.P., Berrigan, D., Dodd, K.W., Masse, L.C., Tilert, T., & McDowell, M. (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40, 181–188. doi:10.1249/mss.0b013e31815a51b3
U.S. Department of Health and Human Services. (2009). Common terminology criteria for adverse events, version 4.02. Retrieved from https://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02_2009-09-15_QuickR…
Viana, J.L., Kosmadakis, G.C., Watson, E.L., Bevington, A., Feehally, J., Bishop, N.C., & Smith, A.C. (2014). Evidence for anti-inflammatory effects of exercise in CKD. Journal of the American Society of Nephrology, 25, 2121–2130. doi:10.1681/ASN.2013070702
Zhou, Y., Chlebowski, R., LaMonte, M.J., Bea, J.W., Qi, L., Wallace, R., . . . Irwin, M.L. (2014). Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: Results from the women’s health initiative. Gynecologic Oncology, 133, 4–10. doi:10.1016/j.ygyno.2014.01.033
Zhou, Y., Gottlieb, L., Cartmel, B., Li, F., Ercolano, E., Harrigan, M., . . . Irwin M.L. (2015). Randomized trial of exercise on quality of life and fatigue in women diagnosed with ovarian cancer: The Women’s Activity and Lifestyle Study in Connecticut (WALC) [Abstract 9505]. Journal of Clinical Oncology, 33(Suppl.), 9505.

