Patterns of Hand Grip Strength and Detection of Strength Loss in Patients Undergoing Bone Marrow Transplantation: A Feasibility Study
Purpose/Objectives: To determine the feasibility of measuring hand grip strength (HGS) daily in a population of recipients of bone marrow transplantation (BMT), to describe changes in strength measured by HGS, and to describe relationships between laboratory values (hematocrit, hemoglobin, and absolute neutrophil count) and HGS.
Design: Prospective, longitudinal, repeated measures, within subject.
Setting: Inpatient units at the University of Washington Medical Center in Seattle.
Sample: 33 patients admitted in preparation for BMT or for complications from BMT.
Methods: HGS measured on admission and daily.
Main Research Variables: HGS, absolute neutrophil count, hemoglobin, and hematocrit.
Findings: Participants found HGS testing to be relatively easy. Average time to complete testing was 7.2 minutes (SD = 1.95). Nineteen experienced 20% or greater decline in HGS during hospitalization, with nine experiencing decline during the conditioning phase. Age, gender, and hemoglobin correlated with HGS. Strength loss was more likely in those undergoing allogeneic compared to autologous BMT.
Conclusions: A majority of patients experienced strength decline during BMT, with a subgroup declining during conditioning. A positive relationship existed between HGS and hemoglobin and hematocrit in participants admitted for conditioning for BMT.
Implications for Nursing: Weakness increases risk for falls. Patients may experience as much as 50% strength loss during the course of hospitalization for BMT. Strength loss occurs in the conditioning phase for some patients.
Jump to a section
Patients hospitalized for oncologic diagnoses are at increased risk of sustaining a fall compared with other hospitalized patients on medical-surgical units (6.3 versus 3.1 per 1,000 patient days) and are more likely to be injured if they do fall (Fischer et al., 2005). The etiology for this increased risk of falling is not clearly understood, but may be related to generalized weakness, sarcopenia, sensory and balance impairments, and/or medications (Morishita et al., 2015). Lower-extremity muscle weakness is a well-known risk factor for falls (Oliver, Healey, & Haines, 2010) and, although new-onset weakness is often a consequence of hospitalization for treatment of acute and/or life-threatening illness, objective assessment of muscle strength is not standard of care in hospitals. This is despite the availability of validated, performance-based measures of strength, particularly dynamometry (Beseler et al., 2014; Bohannon, Magasi, Bubela, Wang, & Gershon, 2012).
Background
Weakness and Falls in Patients Undergoing Bone Marrow Transplantation
Evidence exists that patients may already have mild weakness when they present for bone marrow transplantation (BMT), but the change may be imperceptible to the patient (Mello, Tanaka, & Dulley, 2003). In a study of 56 patients receiving BMT (mean age = 43.9 years, SD = 11), White, Terrin, Miller, and Ryan (2005) found that hand grip strength (HGS) was reduced to less than 80% predicted in 39% of the participants and less than 60% predicted in 15% of the participants prior to transplantation. Similarly, 58% of participants in the study performed the six-minute walk test at less than 80% predicted distance.
Declines in strength, as measured by HGS, also occur during the course of BMT. Kramer et al. (2013) studied 40 patients who received BMT. When compared to baseline measurement (pretransplantation), a 10% decline in HGS was found one month post-transplantation (p = 0.02) with return to baseline at three months post-transplantation (p = 0.3), which suggests that weakness associated with BMT is time-limited, and patients might return to baseline strength within three months after transplantation.
Patients undergoing BMT are at risk for falls. Ueki et al. (2014) performed retrospective chart reviews on 77 patients who had received a BMT. The study found that 45% of the patients had sustained a fall while hospitalized, with about 70% of the falls occurring after engraftment, which was defined as more than three days of granulocyte counts of at least 5 x 108/L.
Limitations of Fall Risk Assessment in Patients Undergoing Bone Marrow Transplantation
The Joint Commission (2014) designated fall prevention as a National Patient Safety Goal and mandated use of a standardized, validated risk assessment for every hospitalized patient as one of the expected elements of performance needed to achieve accreditation. The subjective nature of some elements of fall risk screening tools can lead to an underestimation of risk. In a study of 25,000 falls in academic medical centers, the authors noted that 88% of patients who fell had been screened for risk prior to the fall. As many as 17% of those who fell had been deemed to be not at risk (Williams, Szekendi, & Thomas, 2014).
Fall risk screening tools that weigh confusion and age as risk factors for falls might serve to confound fall risk assessment in patients undergoing BMT (Tzeng & Yin, 2013). These patients tend to be younger than the general hospitalized population and are rarely confused.
Hand Grip Strength
HGS assessed by dynamometry is a well-established physiologic measure of muscle strength and correlates with tests of mobility and functional strength, so it may represent a noninvasive, inexpensive, and objective measure that can serve as a proxy for global functional strength (Bohannon et al., 2012; Cantarero-Villanueva et al., 2012; Kramer et al., 2013). Evidence exists of positive correlations between HGS and tests of mobility, such as the two- and six-minute walk tests (p = 0.002) and with functional strength as determined by the sit-to-stand test (p = 0.003) and measures of manual muscle (lower extremity) strength, such as knee extension (p < 0.001) (Bohannon et al., 2012; Cantarero-Villanueva et al., 2012).
Research established the minimum clinically important difference (MCID) for HGS. In a study of 50 patients who sustained a distal radius fracture and subsequent volar locking plate fixation, the MCID was found to be a decrease of 6.25 kg (19%) (Kim, Park, & Shin, 2014). This was calculated by correlating HGS changes with patient subjective report of grip, in addition to ensuring that expected standard error in measurement was accounted for.
Research Gap
Previous studies in participants undergoing BMT have assessed HGS pretransplantation (Kramer et al., 2013; Morishita et al., 2012; Pidala et al., 2013), at one and three months post-transplantation (Kramer et al., 2013), after discharge (de Souza et al., 2012), and after the diagnosis of graft-versus-host disease (median = 7.3 months post-transplantation) (Pidala et al., 2013). The pattern and timing of HGS changes during the course of hospitalization for participants undergoing BMT is not known. This is important to establish because declines in HGS may be a marker of new physical functional impairment, which in turn may increase the risk of in-hospital falls.
Patients receive different types of BMTs: allogeneic from a related or unrelated donor or autologous when patient’s cells are treated and reinfused. These two types of BMT introduce another potentially important factor to consider. The determination of which type of transplantation a patient receives is based on the specific disease that is being treated, with autologous transplantations generally being reserved for nonhematologic diseases (Majhail et al., 2015). Those receiving allogeneic BMT often have more aggressive disease and receive stronger doses of chemotherapy and radiation therapy in their conditioning regimen. A study by de Souza et al. (2012) compared HGS between participants receiving allogeneic versus autologous BMT at the time of hospital admission and found no significant difference (p = 0.61). No study has been reported that compares these two groups throughout hospitalization.
Limited study of laboratory values related to HGS in patients receiving BMT has been done; if values were found to be related, this might provide important clinical biomarkers for assessing risk. Pidala et al. (2013) included platelet counts at the time of enrollment, de Souza et al. (2012) included engraftment of neutrophils and platelets as an outcome measure, and Morishita et al. (2012) collected baseline data on hemoglobin. To date, no studies in this population have examined the relationship between HGS and absolute neutrophil count, hemoglobin, or hematocrit.
Finally, the relationship between HGS and falls has not been determined. Prior to launching such a study, it is important to understand how HGS changes throughout the course of hospitalization for BMT as a potential indicator for strength loss that could then be explored in relation to falls.
Objectives
The objectives of the study in a sample of participants undergoing myeloablative BMT in an inpatient setting were to (a) determine feasibility of daily HGS measurement, (b) describe changes in muscle strength as measured by daily HGS measurements, and (c) describe the relationships between selected laboratory values (hemoglobin, hematocrit, and absolute neutrophil count) and HGS during the course of hospitalization.
Methods
Design and Setting
This prospective, longitudinal, repeated measures study used a within-subject design in two inpatient units specializing in the care of patients undergoing BMT. These units are part of University of Washington Medical Center in Seattle, a large quaternary academic medical center within a National Comprehensive Cancer Network Member Institution.
Participant Recruitment
The Fred Hutchinson Cancer Research Center Institutional Review Board approved all procedures. Patients presenting to an academic medical center from October 2014 to May 2015 for their first myeloablative BMT (peritransplantation) or being admitted within 20 days of BMT for complications of transplantation, such as neutropenic fever, were approached for enrollment. Participants were aged 18 years or older and able to speak, understand, and read English. Participants were excluded if they required droplet isolation or intensive care treatment. The principal investigator (PI) identified potential participants by reviewing admissions to the BMT units daily. A convenience sample was used with no more than five participants enrolled at any one time based on available resources for daily collection of measures.
Measures
Demographic information included age, gender, hand dominance, transplantation type (allogeneic versus autologous), diagnosis, and date of diagnosis.
HGS was tested using a Jamar 200 KG Hydraulic Hand Dynamometer that was calibrated prior to the study by the Scientific Instruments department at the University of Washington in Seattle. After demonstrating inter-rater reliability on a volunteer test patient, the PI and two research assistants collected the data. Participants were tested at the time of enrollment. Testing was then conducted each day during hospitalization, provided participants were awake at the time of testing and agreed to proceed. HGS testing occurred at various times of the day depending on participant and researcher availability. Participants were positioned with their legs and feet on the bed and the head of the bed in the most upright position possible. The elbow was flexed at a 90-degree angle, and the shoulder was adducted. Each hand was tested three times, with the dominant hand tested first. Participants were asked to hold the maximum torque for four seconds. A one-minute rest period was given between tests (see Figure 1).
Clinical Data
Abstracted laboratory data included hemoglobin, hematocrit, absolute neutrophil count, and any blood transfusions that occurred. These data were abstracted for each day the HGS was measured. Use of benzodiazepines and opioids within 12 hours of the testing time was also recorded on each day of HGS measurement.
Feasibility Participant Survey
A simple five-point, Likert-type survey tool was administered at the conclusion of each HGS testing session. The survey was administered verbally, with the researcher asking how difficult it was to participate in the HGS test on a scale from 1 (not difficult) to 5 (very difficult).
Statistical Analysis
Stata 12.1 software was used for all statistical analyses. Descriptive statistics were used to evaluate each participant’s pattern of strength during hospitalization. Given the different number of data points in each group, subgroup analysis of the peritransplantation and complication groups was conducted. Participants were assigned to the peritransplantation group if they were admitted to the medical center before the transplantation occurred and stayed through day 0 of the transplantation. The complication group was assigned if participants were admitted after the transplantation for a complication (e.g., neutropenic fever, nausea/vomiting). A threshold for clinically important change in HGS was set a priori at 20%, per recommendations by Kim et al. (2014).
Univariate, multilevel analyses were conducted to examine the relationship between HGS and each of the following independent variables: transplantation day (with 0 being the day of transplantation), age, gender, transplantation type (allogeneic versus autologous), hematocrit, hemoglobin, and absolute neutrophil count, controlling for transfusion, in the peritransplantation group for days –7 to 0 and for days 1–8. Absolute neutrophil count readings were transformed to better analyze effects on HGS. The same analyses were completed on the complication group data. Multilevel modeling was used to account for each participant having multiple data points; therefore, each observation was nested within the individual, and a simple linear regression would have been inadequate (Snijders, 2014).
Variables found to be statistically significant (p < 0.05) were then included in a multivariate, multilevel regression analysis. Hematocrit was not included in the multivariate model as it was highly correlated with hemoglobin. In the complication group, the multivariate analysis included transplantation day, age, gender, hemoglobin, and absolute neutrophil count. Chi-square analyses were also conducted to examine differences between the strength loss (20% or less from baseline) and no strength loss groups in the peritransplantation subgroup.
Results
Participants
Fifty participants met inclusion criteria and were approached for enrollment. Five of those approached declined enrollment, with two giving specific reasons (not wanting sleep interrupted and enrolled in too many studies). Of the 45 who gave informed consent, 33 (20 peritransplantation; 13 complication) completed the study (see Table 1). Reasons for not completing the study included declining to continue participation because of feeling unwell or overwhelmed (n = 5), early discharge (n = 2), and being placed in droplet precautions (n = 1). In addition, participants were not woken up for daily testing. Participants who missed data collection for four days for any reason were removed from the study. Participants in the peritransplantation group who dropped out of the study prior to day 0 of the transplantation and any participant with less than three data points were excluded from data analysis. 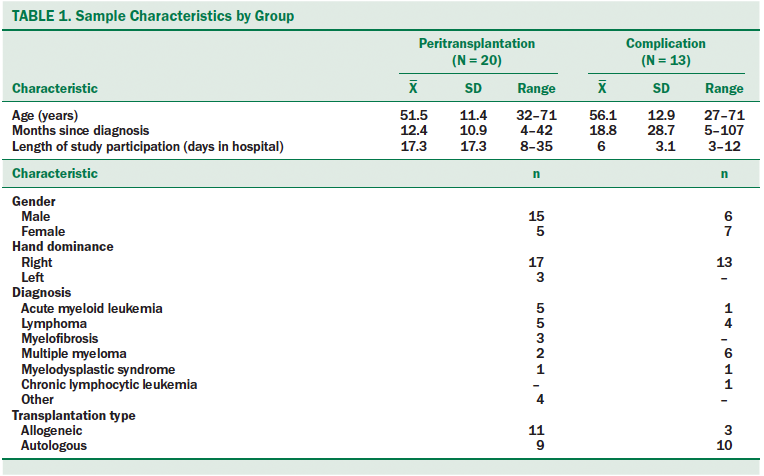
Feasibility and Acceptability
The mean time required to perform HGS testing was 7.12 minutes (SD = 1.95). Participants in the peritransplantation group rated the difficulty of performing HGS at 1.4 (SD = 0.73) on a scale of 1–5, with 5 being most difficult. Similarly, the complication group rated the difficulty at 1.8 (SD = 1.3). In addition to providing a score, many patients commented that they felt tired or weak at the time of testing.
Dependent Variable
Peritransplantation group: A total of 756 HGS readings were collected on the right hand of participants and 759 readings on the left hand during the course of the study. For each session, the maximum grip from both hands were recorded and included in the analysis. Thirteen of 20 participants in the peritransplantation group experienced a 20% or greater strength loss during the course of hospitalization (threshold of clinically important change). Participants experienced the strength loss from day 6 pretransplantation to day 11 post-transplantation (mean = 1.46 days before transplantation, SD = 4.3). Nine of the participants in the strength loss group reached the 20% decline during the conditioning phase (time period prior to transplantation when patients receive chemotherapy and/or radiation) of the transplantation. Seven participants experienced greater than 40% strength loss, with one of those participants declining by 52% (see Table 2). The nadir of HGS in the strength loss group appeared from day 6 pretransplantation to day 19 post-transplantation (mean = day 6.3 post-transplantation, SD = 7.2). 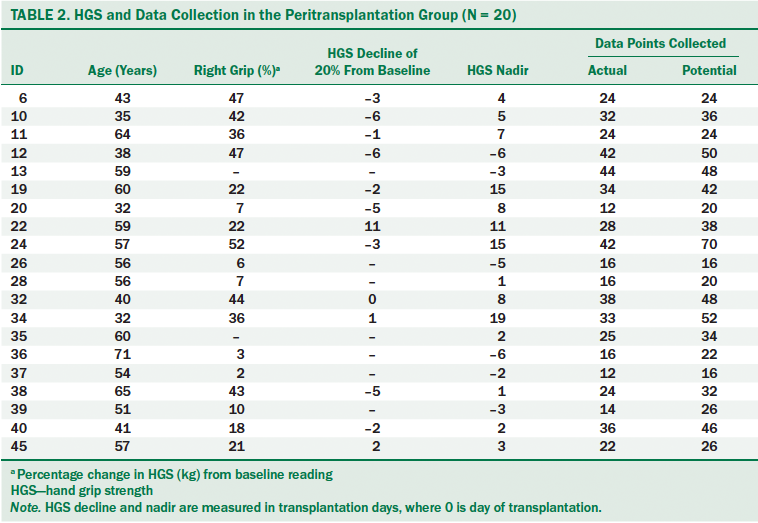
Chi-square analyses were performed to examine differences in selected characteristics between participants in the strength loss group and those with no strength loss. Age, gender, and mean time since diagnosis were not significantly different between those with and without strength loss (see Table 3). Those receiving allogeneic transplantations were more likely to be in the strength loss group than participants who received autologous transplantations (p = 0.02).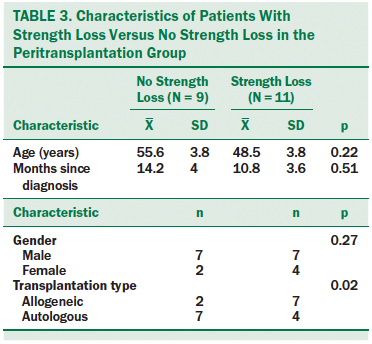
Complication group: A total of 204 HGS readings were collected from the right hand of the participants and 204 from the left hand. As in the peritransplantation group, the maximum reading from each session was included in the analysis. Six of 13 participants of the complication group experienced a 20% or greater loss of HGS during hospitalization, with five participants declining by greater than 40%. Participants experienced this loss from days 5–11 post-transplantation (mean = day 7.3 post-transplantation, SD = 2.8), with the HGS nadir occurring on day 9.8 post-transplantation (SD = 4.1).
Clinical Data
Peritransplantation group: In the univariate analysis, statistically significant relationships were found between HGS and age, hemoglobin, and hematocrit (see Table 4). In addition, male gender was strongly correlated with HGS (p < 0.001). Receiving an allogeneic BMT was strongly negatively correlated with HGS. Those receiving allogeneic BMT had HGS that was about 11–12 kg lower than those receiving autologous BMT (p < 0.001). Absolute neutrophil count was not significantly associated with HGS.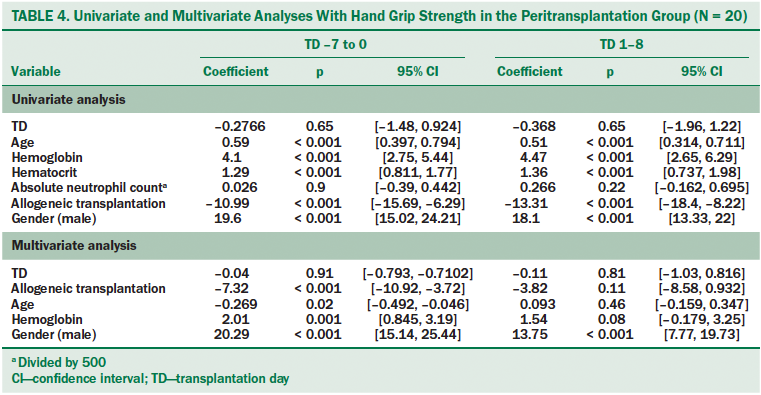
Variables that were found to be statistically significant (p < 0.05) in the univariate analyses were included in the multivariate analysis. One exception was hematocrit, which was not included because of collinearity between hemoglobin and hematocrit. Age was negatively correlated with HGS from days –7 to 0 but was not significantly related after day 0. Gender and hemoglobin remained positively correlated with HGS when adjusted for transplantation day and transfusions received. Allogeneic transplantations were negatively associated with HGS on transplantation days –7 to 0 (p < 0.001), but this association was not statistically significant on transplantation days 1–8 (p = 0.11).
Complication group: In the complication group, a very small but statistically significant negative correlation was found between HGS and absolute neutrophil count, hemoglobin, hematocrit, and age (see Table 5). As in the peritransplantation group, male gender was strongly associated with HGS (p < 0.001). When these variables were added to the multivariate analysis, only age and gender remained statistically significant.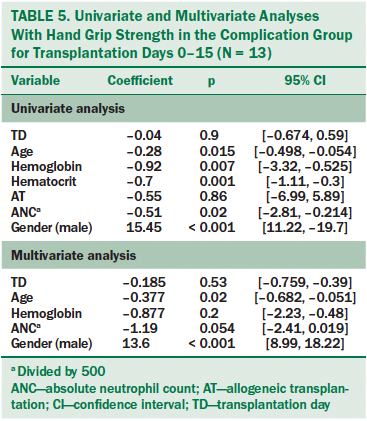
Discussion
Although studies have examined changes in strength before and after BMT (de Souza et al., 2012; Kramer et al., 2013; Mello et al., 2003; Pidala et al., 2013), this is the first study to measure HGS daily in participants undergoing BMT. The authors found that clinically important changes in HGS (greater than 20% decline from baseline) occurred in the majority of participants (n = 19, 57%), with a subgroup experiencing strength loss early in the BMT process during the conditioning phase. A high degree of variability was found for the day of nadir of HGS. In addition, six patients (46%) who were admitted for complications of BMT experienced continued strength loss after admission.
Because weakness is a risk factor for falls, the degree of progressive weakness found in this study during the course of BMT might explain, in part, the finding by Ueki et al. (2014) that patients undergoing allogeneic BMT had an increased risk of falling.
Of note, those who received allogeneic transplantations were more likely to experience clinically important strength loss (greater than 20% decline from baseline) than those who received autologous transplantations (p = 0.02). Of the five studies of HGS in patients receiving BMT that were reviewed for this study, four included only patients receiving allogeneic transplantations (Kramer et al., 2013; Mello et al., 2003; Morishita et al., 2013; Pidala et al., 2013). The study by de Souza et al. (2012) examined patients receiving allogeneic and autologous BMT. The authors of that study found no significant difference in HGS between groups at baseline (p = 0.61) and did not compare the two groups after BMT.
Testing HGS in patients hospitalized for BMT was feasible and did not require extensive time. In general, participants found the HGS testing to be relatively easy to perform. The mean time to complete testing was 7.15 minutes (SD = 1.95). This time included instructions given to the patient and any positioning required. Many of the participants were interested in learning how their HGS was changing over time. No adverse events, such as injury or infection, related to the HGS testing occurred during this study.
Limitations
There were some limitations to this study. First, this feasibility study was conducted in a single medical center. Although more than 1,000 HGS measurements were recorded, the sample size was small, with 33 participants completing the study. Given that this was a feasibility study, no power analysis was conducted. Therefore, the results may not be generalizable to other inpatient settings.
The research team did not wake patients for testing and, as would be expected, participants were asleep at various times of the day. In an effort to ensure ongoing assent, participants were asked each day, “Are you ready for a grip strength test?” Many patients declined on various days for reasons, such as nausea, diarrhea, or generally feeling unwell. Participants were unenrolled from the study if they refused data collection or were sleeping at the time of data collection for four consecutive days. In addition, many missing data points occurred because of the decision to minimize burden and disruption to participants by not waking them for testing. As participants progressed in the BMT process, they tended to nap during the day, which limited their availability for testing. Given the acuity of illness and the dynamic care needs of the participants, the authors were not able to perform HGS measurements at the same time each day. This may have affected the results because strength may vary during the course of a day. In total, the authors collected data on 78% of the potential days for the peritransplantation group and 85% of the potential days for the complication group. Despite gaps in data collection, the authors were able to see trends of HGS during hospitalization; however, missing data points may have affected the results.
Another limitation is that the authors did not control for conditioning regimens. Although every participant received myeloablative BMT, some conditioning regimens were more intense (e.g., higher doses of chemotherapy and radiation) than others. There is a possibility that those with intense conditioning regimens had a higher degree of strength loss, but this study was not designed to examine that question. Other variables that might have affected the results but were not collected include comorbidities, body mass index, and baseline functional status. Although the impetus for this study was identification of an objective method of quantifying strength loss to potentially prevent falls, the authors did not measure falls, lower body strength, or gait. Therefore, the authors are not able to draw conclusions about the relationship between HGS and falls. However, the data collected during this study suggest that weakness may be a key factor in patient falls in this population.
Implications for Nursing
A majority of patients who present for BMT appear to experience a clinically important decline in strength during hospitalization, and those receiving allogeneic BMT are more likely to experience this decline than patients receiving autologous transplantation. This strength loss may account, in part, for the increased risk of falls in patients receiving BMT. In addition, this study provides important information on the potential timing of clinically important declines in strength that may occur early in the transplantation period during the conditioning phase. Although not directly assessed in the current study, it is likely that patients may not recognize their own strength decline. Therefore, nursing and patient decisions about the need for assistance with mobility may not be fully informed. Nurses should be aware that patients hospitalized for BMT are at risk for significant weakness and potential falls. This needs to be incorporated into care planning and patient education about fall prevention interventions.
Nurses should assess extremity strength carefully, including in the days prior to transplantation. Based on the findings of the current study, use of a dynamometer is feasible and acceptable to patients and, when used for daily repeated measures, provides an objective measure of changes in strength. Should weakness be detected, nurses can implement individualized fall prevention measures to protect patients from injury. 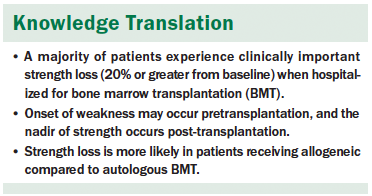
Conclusion
HGS may be a more sensitive, real-time indicator of declines in strength than subjective nursing assessment, but it requires further study to determine use in the prevention of falls. HGS by dynamometry provides a practical, objective measure of strength that will allow care providers to identify clinically important weakness as soon as it occurs. This identification may give healthcare providers critical information that will inform the design of an individualized fall prevention plan.
The HGS test by dynamometry was found to be feasible for use in the inpatient setting for participants receiving BMT. In the future, it would be useful to perform the HGS test when the participant is already awake for vital signs or some other routine care, which would increase the days when data could be collected and provide a standard time of measurement. Engaging participants in the process of measuring and trending HGS during hospitalization is an important factor. One participant stated that he would like to see a chart that would allow him to track his HGS over time.
The results of this study add to the body of knowledge that has emerged about the use of HGS in acute care settings. The authors have demonstrated that it is feasible to test HGS daily in hospitalized participants who are acutely ill. Given the success of this feasibility pilot, a study with a larger sample size sufficiently powered for statistical significance is indicated. Potential confounders, such as comorbidities, weight, and baseline physical activity, could be included in the analysis of a future study. The design of this pilot study could be used for such a future study with a few modifications to improve data capture and interpretation of results. For example, HGS measurements should be timed to coincide with vital signs to minimize missing data.
Engagement of the patient in the assessment of strength may enhance nurse–patient discussions about the safety of independent mobilization and may increase the likelihood that patients will ask for assistance with mobilization. This objective data could help patients to overcome resistance in asking for help, saving them from devastating injuries.
About the Author(s)
Sayre is a chief nursing officer and associate administrator of patient care services at the University of Washington Medical Center; Belza is the associate dean of academic affairs and the Aljoya Endowed Professor in Aging, Biobehavioral Nursing, and Health Informatics in the School of Nursing at the University of Washington; Shannon Dorcy is the director of clinical/nursing research, education, and practice in the Fred Hutchinson Cancer Research Center at Seattle Cancer Care Alliance; and Phelan is an associate professor in the School of Medicine and Division of Gerontology, and Whitney is a professor of biobehavioral nursing and health informatics in the School of Nursing, both at the University of Washington, all in Seattle. Phelan has previously consulted for Healthwise. No other financial relationships to disclose. Sayre and Shannon Dorcy completed the data collection. Sayre provided statistical support. All authors contributed to the conceptualization and design, analysis, and manuscript preparation. Sayre can be reached at casayre@uw.edu, with copy to editor at ONFEditor@ons.org. Submitted December 2016. Accepted for publication April 3, 2017.
References
Beseler, M.R., Rubio, C., Duarte, E., Hervás, D., Guevara, M.C., Giner-Pascual, M., & Viosca, E. (2014). Clinical effectiveness of grip strength in predicting ambulation of elderly inpatients. Clinical Interventions in Aging, 9, 1873–1877. doi:10.2147/cia.s62002
Bohannon, R.W., Magasi, S.R., Bubela, D.J., Wang, Y.C., & Gershon, R.C. (2012). Grip and knee extension muscle strength reflect a common construct among adults. Muscle and Nerve, 46, 555–558. doi:10.1002/mus.23350
Bohannon, R.W., Peolsson, A., Massy-Westropp, N., Desrosiers, J., & Bear-Lehman, J. (2006). Reference values for adult grip strength measured with a Jamar dynamometer: A descriptive meta-analysis. Physiotherapy, 92, 11–15. doi:10.1016/j.physio.2005.05.003
Cantarero-Villanueva, I., Fernández-Lao, C., Díaz-Rodríguez, L., Fernández-de-Las-Peñas, C., Ruiz, J.R., & Arroyo-Morales, M. (2012). The handgrip strength test as a measure of function in breast cancer survivors: Relationship to cancer-related symptoms and physical and physiological parameters. American Journal of Physical Medicine and Rehabilitation, 91, 774–782. doi:10.1097/PHM.0b013e31825f1538
de Souza, C.V., Miranda, E.C., Garcia, C., Jr., Aranha, F.J., de Souza, C.A., & Vigorito, A.C. (2012). Functional evaluation indicates physical losses after hematopoetic stem cell transplantation. Revista Brasileira de Hematologia e Hemoterapia, 34, 345–351. doi:10.5581/1516-8484.20120090
Fischer, I.D., Krauss, M.J., Dunagan, W.C., Birge, S., Hitcho, E., Johnson, S., . . . Fraser, V.J. (2005). Patterns and predictors of inpatient falls and fall-related injuries in a large academic hospital. Infection Control and Hospital Epidemiology, 26, 822–827. doi:10.1086/502500
Joint Commission. (2014). Summary data of sentinel events reviewed by the Joint Commission. Retrieved from http://www.jointcommission.org/assets/1/18/2004_to_2Q_2013_SE_Stats_-_S…
Kim, J.K., Park, M.G., & Shin, S.J. (2014). What is the minimum clinically important difference in grip strength? Clinical Orthopaedics and Related Research, 472, 2536–2541. doi:10.1007/s11999-014-3666-y
Kramer, M., Heussner, P., Herzberg, P.Y., Andree, H., Hilgendorf, I., Leithaeuser, M., . . . Wolff, D. (2013). Validation of the grip test and human activity profile for evaluation of physical performance during the intermediate phase after allogeneic hematopoietic stem cell transplantation. Supportive Care in Cancer, 21, 1121–1129. doi:10.1007/s00520-012-1634-1
Majhail, N.S., Farnia, S.H., Carpenter, P.A., Champlin, R.E., Crawford, S., Marks, D.I., . . . LeMaistre, C.F. (2015). Indications for autologous and allogeneic hematopoietic cell transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation, 21, 1863–1869. doi:10.1016/j.bbmt.2015.07.032
Mello, M., Tanaka, C., & Dulley, F.L. (2003). Effects of an exercise program on muscle performance in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplantation, 32, 723–728. doi:10.1038/sj.bmt.1704227
Morishita, S., Kaida, K., Aoki, O., Yamauchi, S., Wakasugi, T., Ikegame, K., . . . Domen, K. (2015). Balance function in patients who had undergone allogeneic hematopoietic stem cell trans-plantation. Gait and Posture, 42, 406–408. doi:10.1016/j.gaitpost.2015.07.011
Morishita, S., Kaida, K., Tanaka, T., Itani, Y., Ikegame, K., Okada, M., . . . Domen, K. (2012). Prevalence of sarcopenia and relevance of body composition, physiological function, fatigue, and health-related quality of life in patients before allogeneic hematopoietic stem cell transplantation. Supportive Care in Cancer, 20, 3161–3168. doi:10.1007/s00520-012-1460-5
Morishita, S., Kaida, K., Yamauchi, S., Sota, K., Ishii, S., Ikegame, K., . . . Domen, K. (2013). Relationship between corticosteroid dose and declines in physical function among allogeneic hematopoietic stem cell transplantation patients. Supportive Care in Cancer, 21, 2161–2169. doi:10.1007/s00520-013-1778-7
Oliver, D., Healey, F., & Haines, T.P. (2010). Preventing falls and fall-related injuries in hospitals. Clinics in Geriatric Medicine, 26, 645–692. doi:10.1016/j.cger.2010.06.005
Pidala, J., Chai, X., Martin, P., Inamoto, Y., Cutler, C., Palmer, J., . . . Lee, S.J. (2013). Hand grip strength and 2-minute walk test in chronic graft-versus-host disease assessment: Analysis from the Chronic GVHD Consortium. Biology of Blood and Marrow Transplantation, 19, 967–972. doi:10.1016/j.bbmt.2013.03.014
Snijders, T.B. (2014). Multilevel analysis. In M. Lovric (Ed.), International encyclopedia of statistical science (pp. 879–882): Heidelberg, Berlin: Springer.
Tzeng, H.M., & Yin, C.Y. (2013). Frequently observed risk factors for fall-related injuries and effective preventive interventions: A multihospital survey of nurses’ perceptions. Journal of Nursing Care Quality, 28, 130–138. doi:10.1097/NCQ.0b013e3182780037
Ueki, S., Ikegame, K., Kozawa, M., Miyamoto, J., Mori, R., & Ogawa, H. (2014). Risk analysis of falls in patients undergoing allogeneic hematopoietic stem cell transplantation. Clinical Journal of Oncology Nursing, 18, 396–399. doi:10.1188/14.CJON.396-399
White, A.C., Terrin, N., Miller, K.B., & Ryan, H.F. (2005). Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest, 128, 145–152. doi:10.1378/chest.128.1.145
Williams, T., Szekendi, M., & Thomas, S. (2014). An analysis of patient falls and fall prevention programs across academic medical centers. Journal of Nursing Care Quality, 29, 19–29. doi:10.1097/NCQ.0b013e3182a0cd19

