Improving Functional Status in African Americans With Cancer Pain: A Randomized Clinical Trial
Objectives: To determine the efficacy of the Power Over Pain–Coaching (POP-C) intervention to improve functional status among African American outpatients with cancer pain.
Sample & Setting: 310 African American patients were recruited from an urban comprehensive cancer center. The study took place in the patients’ homes.
Methods & Variables: A two-group randomized design with repeated measures was used. Data were analyzed with linear mixed effects regression analysis and structural equation change score models. Variables were pain, pain-related distress, functional status, perceived control over pain, and the following antecedents to control: medication management, pain advocacy, and living with pain.
Results: Functional status was improved in POP-C participants relative to control group participants (p < 0.05). Distress also was differentially decreased (p < 0.05). Pain intensity ratings decreased significantly in all patients (p < 0.05). The largest intervention effects were observed in the living with pain component.
Implications for Nursing: Perceived control over pain was strongly related to functional status and is amenable to interventions using the POP-C intervention components described in this article.
Jump to a section
Despite the options of using analgesics and other modalities, pain continues to be moderate to severe in more than 50% of patients with cancer (Beuken-van Everdingen et al., 2007; Hammer et al., 2016). The multidimensional experience of pain involves many factors, including pain-related distress and perceived control over pain, which affect a patient’s functional status (Leung, Pachana, & McLaughlin, 2014; Vallerand, Templin, Hasenau, & Riley-Doucet, 2007; Wells & Sandlin, 2012).
Behavioral interventions to decrease cancer pain have focused on distress (Jacobsen, Møldrup, Christrup, Sjøgren, & Hansen, 2010; Wells & Sandlin, 2012). Although distress is important and should be assessed in all patients with cancer (National Comprehensive Cancer Network [NCCN], 2017), when general symptom distress and pain-related distress were compared in patients with cancer pain, distress from pain was found to be more upsetting than all other symptoms (Vallerand, Templin, et al., 2007). Assessing pain-related distress is essential in patients with cancer-related pain to develop interventions and effectively care for these patients. However, designing interventions to decrease distress is challenging because of the affective nature of the concept. The factors that lead to pain-related distress are more amenable to intervention strategies. Perceived control over pain, a factor that had not been previously considered, was found to have a direct effect on pain-related distress and mediated the effect of beliefs about pain and pain level on distress in ambulatory patients with cancer-related pain (Vallerand, Templin, et al., 2007).
African American patients with cancer have been shown to bear an excess burden of pain because of disparities in pain care (Anderson et al., 2015; Fisch et al., 2012; Meghani, Thompson, Chittams, Bruner, & Riegel, 2015; White-Means, Rice, Dapremont, Davis, & Martin, 2015). In a study of 281 patients from a Midwestern comprehensive cancer center, African American patients were found to have significantly higher pain levels, more distress, and poorer functional status than Caucasian patients (Vallerand, Hasenau, Templin, & Collins-Bohler, 2005). Perceived control over pain, “the perception that one has a way of gaining and/or maintaining control over an aversive event, such as pain” (Pellino & Ward, 1998, p. 111), was the only factor found to predict disparities in these groups. When perceived control over pain was statistically controlled for, the effects of disparities were significantly reduced in the outcomes of distress and functional status (Vallerand et al., 2005). Perceived control over pain may play an even greater role in minorities and patients with low socioeconomic status (McNeill, Reynolds, & Ney, 2007; Vallerand, Crawley, Pieper, & Templin, 2016; Vallerand et al., 2005).
Objectives
To help African American patients with cancer and caregivers to effectively manage pain, the authors developed and tested the Power Over Pain–Coaching (POP-C) intervention, a multicomponent, five-week, nurse-delivered home and telephone intervention. The intervention built on prior work by Vallerand, Hasenau, and Templin (2010) and Vallerand, Templin, et al., 2007. The current version included three potentially modifiable and measurable antecedents to perceived control over pain: medication management, pain advocacy, and living with pain.
The purpose of the current study was to determine the efficacy of the POP-C intervention to improve functional status among African American outpatients with cancer pain. The authors hypothesized that the mechanism for this intervention was improvements in perceived control over pain mediated by an aggregate improvement in the proposed antecedents to control: medication management, pain advocacy, and living with pain. The current study contributes significantly to the advancement of pain research by addressing a challenge of treatment that has remained unsolved for a traditionally underrepresented minority.
Methods
Sample and Setting
Based on effect sizes obtained in prior studies, the required complete-data sample size (after attrition) was 110 participants per treatment arm. This assumed an effect size for the treatment x time interaction of 0.35, a correlation among repeated measures of 0.6, and a two-tail alpha of 0.05. Recruitment continued until both treatment arms achieved the minimum sample size at week 7. This sample size also is adequate for structural equation modeling (SEM). The Satorra and Saris (1985) method was used to estimate the magnitude of standard path coefficients in the proposed structural equation model, which ranged from 0.15–0.17.
Patients were enrolled from the waiting room of the Karmanos Cancer Institute in Detroit, Michigan. Inclusion criteria were the following:
• Being aged 18 years or older
• Self-identifying as African American
• Having cancer-related pain of 4 or greater on a 0–10 Likert-type scale, with higher scores indicating greater pain, during the past two weeks
As shown in Figure 1, initial screening included 472 patients, with 162 patients being excluded for not meeting the inclusion criteria, declining to participate, or being unavailable at the time of consent. Consent was obtained from 310 patients. The study was approved by the institutional review board of Wayne State University in Detroit, Michigan. The data were collected in the patients’ homes and from telephone calls.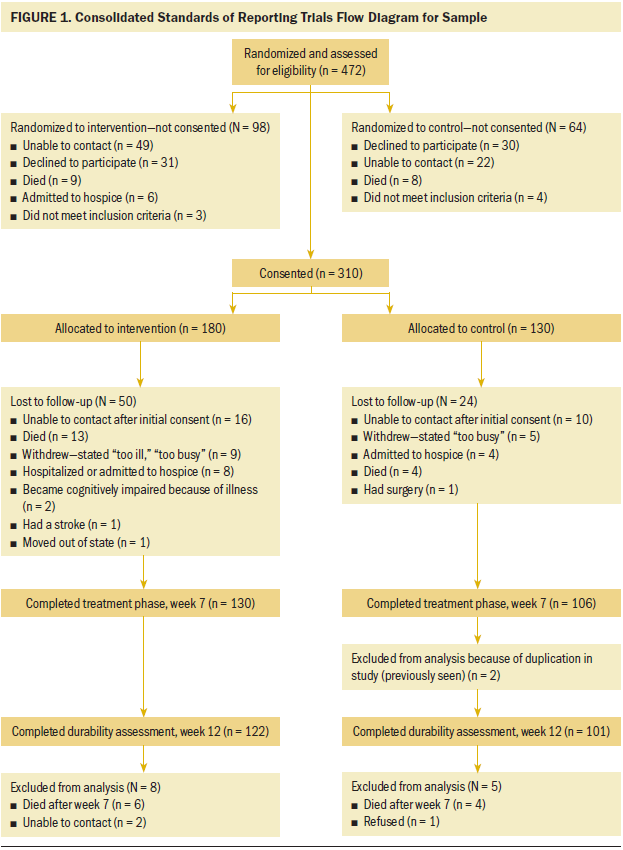
Design
A longitudinal randomized, controlled trial with repeated measures was used to test the efficacy of the POP-C intervention to improve pain, pain-related distress, and functional status by improving perceived control over pain among adult African American patients with cancer. Random assignment was achieved using a permuted block randomization schedule with a random assignment list generated in advance. The initial allocation ratio was 1:1. Interim monitoring revealed differential attrition with greater loss in the intervention condition. Consequently, the allocation ratio was adjusted to favor the intervention group. The adjustment overcompensated so that, at week 12, the numbers retained were in the ratio of 122:101.
Procedure
Nurses were trained as intervention nurses (INs) or data collection nurses (DCNs). INs were master’s-prepared RNs with prior experience in oncology or pain management, and they received additional training and mentoring by the principal investigator (PI). DCNs were RNs who received training by the co-investigators on obtaining informed consent and making home visits but received no additional training in pain management. The INs and DCNs received training on the study protocols and education for making home visits in the urban African American community, cultural sensitivity, and data collection. DCNs also were advised not to intervene but to suggest that the patients contact their clinician for any concerns. In addition to obtaining data from the control group, DCNs collected data from the intervention group at week 1, 7, and 12. Patients took 45–60 minutes to complete these measures. The separation of the INs from data collection responsibilities lowered threats to internal validity from patient and responder biases. Patients were followed by the same IN and DCN throughout the 12 weeks of the study.
Patients and caregivers in the intervention group received the POP-C intervention from an IN. The intervention consisted of a home visit in weeks 2, 4, and 6, covering the three POP-C components: medication management, pain advocacy, and living with pain (see Figure 2). Each visit focused on one component, but INs customized the specific coaching to individual participant needs. Examples of individualization included types of analgesics for specific types of pain, such as neuropathic or bone pain; strategies for communicating concerns about pain to clinicians; and clarifying misconceptions of what can be realistically achieved in pain control. A telephone call to the patient and caregiver was made during weeks 3 and 5 to reinforce and further adapt the intervention as needed. Data for primary measures (pain, pain-related distress, functional status) were collected by the IN during the five weekly intervention contacts. These weekly measurements took 10–15 minutes to complete and provided a way of responding to expected dynamic changes in pain, pain-related distress, and function. Patients in both groups received $25 gift cards at the beginning and end of the study. Patients in the intervention group also received a pill organizer, study-related coffee mug, and a hot/cold pack during the intervention weeks related to the intervention components. Intermediate measures (beliefs, perceived control over pain, intervention measures) were collected by a DCN at week 1 (enrollment), 7 (postintervention), and 12 (durability).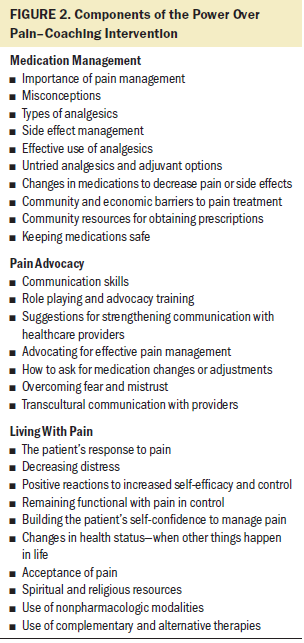
Main Research Variables
The main research variables are described in Table 1. Each of the variables is a composite measure consisting of a weighted sum of items or scales. The variables are classified in three groups: outcomes (pain, pain-related distress, functional status), mediator: perceived control over pain (pharmacologic control, cognitive control, pain catastrophizing, feelings of control), and intervention components (medication management, pain advocacy, living with pain). The outcomes each were analyzed separately; perceived control and the intervention components were latent constructs, so variables in these groups were used as indicators in the SEM. 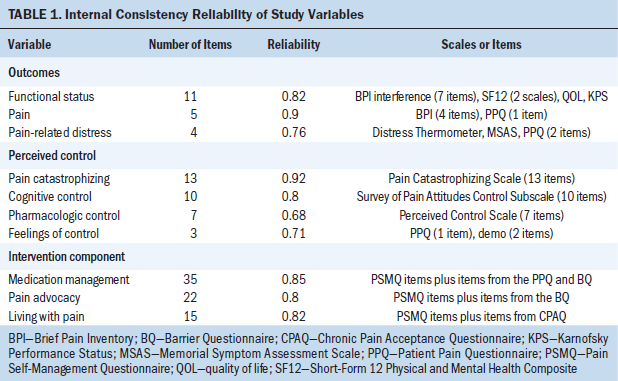
Because the variables primarily were based on self-report, the study protocol named, in advance, multiple scales or items for several of the variables to improve the reliability of the assessments. All scales and items except for the investigator-developed scale, the Pain Self-Management Questionnaire (PSMQ), were used in prior studies. Psychometric procedures were used to omit items that did not contribute to reliability as expected. Items and scales were standardized prior to summation by weights, which gave all items the same scale range.
Outcomes
Outcomes were comprised of measures of pain, pain-related distress, and functional status.
Pain: Four items rating pain intensity (worst, least, and average in the past two weeks and now) from the Brief Pain Inventory–Short Form (BPI) (Cleeland & Syrjala, 2001) and one item from the Patient Pain Questionnaire (PPQ) (Ferrell, Eberts, McCaffery, & Grant, 1991) were used to measure pain intensity.
Pain-related distress: The construct of pain-related distress was measured using the Distress Thermometer (Ransom, Jacobsen, & Booth-Jones, 2006), the Memorial Symptom Assessment Scale (Chang, Hwang, Feuerman, Kasimis, & Thaler, 2000), and two items from the PPQ.
Functional status: For the purposes of the current study, functional status was defined as the ability to participate in activities that are meaningful or important to the patient. Functional status was measured by the seven-item interference subscale of the BPI, the Short-Form 12 Physical and Mental Health Composite (Ware, Kosinski, & Keller, 1996), the Quality of Life scale of the American Chronic Pain Association (2003), and the Karnofsky Performance Status scale (Schag, Heinrich, & Ganz, 1984).
Mediator
Perceived control over pain was a mediating concept defined by three scales from existing instruments and three questions about feelings of control. The three scales were the Survey of Pain Attitudes Control Subscale (Jensen, Turner, Romano, & Lawler, 1994), Pain Catastrophizing Scale (Sullivan, Bishop, & Pivik, 1995), and Perceived Control Scale (Pellino & Ward, 1998). Perceived control over pain has been identified as a factor that influences self-efficacy (Vancleef & Peters, 2011), quality of care for cancer pain (McNeill et al., 2007), and functional status (Vallerand et al., 2016).
Three questions, measured on a 0–10 Likert-type scale, with higher scores indicating greater feelings of control, were used for individual feelings of control. One question from the PPQ asking to what extent the patients believed they could control their pain, and two questions from the demographic survey asking how controllable the patients believed their pain was and how controlled they believed their pain was currently, also were used.
Intervention Components
The intervention focused on medication management, pain advocacy, and living with pain. These components were informed by various theoretical perspectives, such as self-efficacy and acceptance and commitment therapy, but based primarily on the authors’ clinical experience. Although the medication management and pain advocacy components have been used in other studies (Vallerand et al., 2010), the addition of the living with pain component makes the intervention more comprehensive and relevant. Living with pain acknowledges the importance of maintaining function despite pain, particularly for activities that are meaningful for the patient. Acceptance does not negate the importance of pain control but rather emphasizes a realistic attitude and the recognition that, although pain relief is a goal, living with some pain is a reality. The use of all three components of POP-C is an innovative and promising approach to cancer pain management.
Medication management: Items considered for this component included topics such as the importance of pain management, misconceptions regarding medications, management of side effects, and community resources for prescriptions. This component was measured by items on the PSMQ, PPQ, and Barrier Questionnaire (BQ) (Gunnarsdottir, Donovan, Serlin, Voge, & Ward, 2002).
The PSMQ is a 35-question tool developed by the PI to measure aspects of the intervention not captured with other tools. It has three subscales matching the POP-C components. Six items from this questionnaire were used to measure medication management and were answered on an 11-point Likert-type scale. Higher scores on these items represented a better understanding of how and when to use medication and what medication to use in managing pain more effectively. Eight items from the PPQ were used to measure concepts such as when to take prescribed medication and effective dosages. The BQ is a 27-item self-report tool to assess the extent of patient concerns about reporting pain and using analgesics. Twenty-one questions were used for medication management that addressed issues of potential addiction and adverse side effects.
Pain advocacy: This concept included items such as communication skills, role playing advocacy training, overcoming fear and mistrust, and transcultural communication. Measurement of this concept used the PSMQ and BQ. Ten items from the PSMQ were used, such as self-awareness of and importance of talking about pain with families and clinicians. Six items from the BQ were used, including “It’s important to be strong by not talking about pain,” answered on a six-point Likert-type scale, ranging from 0 (do not agree at all) to 5 (agree very much).
Living with pain: This concept included issues such as acceptance of pain, modifying patients’ and caregivers’ responses to pain, controlling pain and remaining functional, building confidence to manage pain, religious resources, and the use of nonpharmacologic therapies. The concept was measured by the PSMQ and the Chronic Pain Acceptance Questionnaire (McCracken, Vowles, & Eccleston, 2004). Six items from the PSMQ measured this concept. They included keeping pain in control to allow doing things important to the patients and keeping busy when in pain to keep their mind off pain. Nine items from the Chronic Pain Acceptance Questionnaire were used for this measure. They included such statements as, “I lead a full life even though I have chronic pain.”
Data Analysis Strategy
Two types of analyses were performed to address the two study aims:
• Confirmatory hypotheses test of the effect of the intervention on primary outcomes: pain, distress, and function
• Structural equation modeling to test the mediation of the intervention effects by perceived control
Primary outcome evaluation: Each outcome (pain, pain-related distress, and functional status) was evaluated separately using repeated measures linear mixed effects models (Fitzmaurice, Laird, & Ware, 2004; Laird & Ware, 1982). The analysis was conducted in two ways: by using all cases with baseline data (N = 310) and by using only cases that had complete data following the intervention. The sample size at the seven-week postbaseline assessment was 236; at the 12-week postbaseline assessment, it was 223. The first analysis is similar to intent-to-treat with the missing outcome data accounted for statistically with maximum likelihood estimation under the assumption of data missing at random. The second analysis is termed per protocol and did not make assumptions about the missing data mechanism. For each of these analyses, the authors aggregated week 1 and 2 data to provide a more stable baseline for estimation of change. This was not planned but deemed necessary because of the highly variable scores on some outcomes between the first two assessment weeks and appropriate because the intervention was first delivered at the week 2 visit; early intervention effects on self-report would work against finding a positive change. Prior to testing effects, the covariance matrix of repeated measures was modeled. All analyses were conducted with and without covariates.
Structural equation modeling: This analysis used the complete case data (n = 236 obtained from week 1 and 7). The use of complete cases facilitated the computation of individual change scores for each of the variables in the model. The POP-C intervention model was operationalized with latent constructs measuring intervention components, perceived control, and outcomes. According to the model, the effects of the intervention on outcomes are mediated by changes in perceived control. A latent change score SEM model was used to test the proposed mediation pathways. Latent change score indicators were created by subtracting week 1 from week 7 scores for each variable. The latent outcome constructs were corrected for measurement error using the reliability of the change score variables and the formula provided by Hayduk (1987). Covariates with nonsignificant pathways were trimmed from the model.
Results
Sample
At baseline (N = 310), participants were 63% (n = 196) female, and had a mean age of 56.28 years (SD = 11.49). Average pain in the past 24 hours was 5.81 (SD = 2.4) on a 0–10 Likert-type scale, with higher scores indicating greater pain. As shown in Table 2, no baseline differences were seen in gender, marital status, education, or presence of metastases. Primary sites of cancer included breast (n = 68, 22%), lung (n = 39, 13%), oral (n = 34, 11%), genitourinary (n = 30, 10%), and gastrointestinal (n = 24, 8%), with the remaining 110 (36%) reporting various other sites and 5 not reporting a site. Site of cancer did not differ significantly between groups. Patients who were randomized to the intervention arm were significantly older and reported longer time since diagnosis. No differences were seen in the study variables by treatment group at baseline (see Table 3).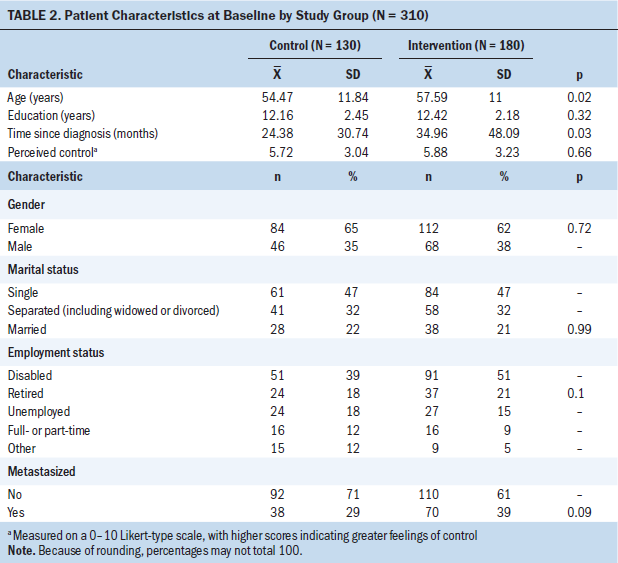
Of the 310 patients consented and enrolled, the total number completing the intervention (seven-week postbaseline) was 236, a loss of 74 participants. An additional 13 participants were lost to follow-up at the 12-week postbaseline durability assessment (N = 223). Because of the small percentage loss from week 7 to 12, attrition analysis was conducted only for loss during the intervention interval (week 1 to 7). Attrition was random across treatment arms, except for one variable; a greater number of patients with metastases were lost from the intervention group (n = 27) than the control group (n = 6) (p = 0.023). Attrition was primarily because of death (n = 25), illness severity (n = 17), and inability to contact (n = 26).
[[{"type":"media","view_mode":"media_original","fid":"39961","attributes":{"alt":"","class":"media-image","height":"425","typeof":"foaf:Image","width":"617"}}]]
Linear Mixed Effects Analysis of Primary Outcomes
The primary analysis used all randomized cases (N = 310). A group x time (2 x 3) repeated measures analysis with two contrasts on time was used to test the intervention effects. The first contrast (C1) compared baseline assessments with those of week 7; the second contrast (C2) compared assessments from week 7 with those of week 12. The effect of the intervention was tested using the group by C1 contrast effect. The durability of the intervention was tested with the group x C2 contrast effect. The results are shown in Table 4 and Figure 3. As hypothesized, distress and function were improved following the intervention at week 7 to a greater degree in the intervention group than the control group (group x C1, p < 0.05). Contrary to expectations, pain was not significantly different between the two groups (p = 0.157). However, all outcomes, including pain, significantly improved from baseline to week 7 (p < 0.001). Effect sizes were in the small to medium-small range. Effects were not changed from week 7 to 12 (C2 main effect and C2 x group interaction effect, p > 0.05), indicating no further improvements or declines in the intervention effect.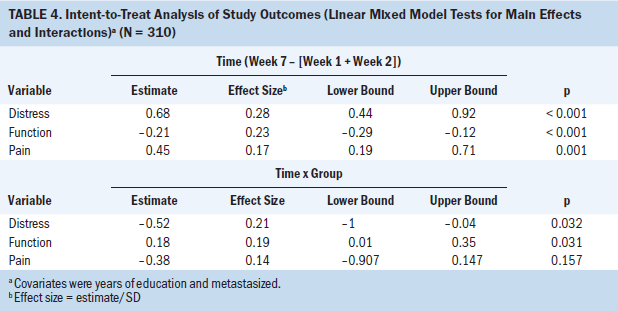
Additional analysis of primary outcomes: Using additional covariates as well as omitting all covariates that had little effect on the results had no effect on judgments of significance. An alternative analysis was conducted using only cases complete to week 7 per protocol (n = 236 and 223), and the results were unchanged.
[[{"type":"media","view_mode":"media_original","fid":"39966","attributes":{"alt":"","class":"media-image","height":"477","typeof":"foaf:Image","width":"618"}}]]
Structural Equation Model Testing
The POP-C SEM results are shown in Figure 4. The models were estimated using full information maximum likelihood and the bias-corrected bootstrap, the recommended method of testing mediation in SEM (Shrout & Bolger, 2002). In addition, all standardized coefficients were tested for significance. The authors attempted to fit the four covariates associated with treatment imbalance and attrition in the model, but none was significantly correlated across variables connected by a pathway and, therefore, was potentially confounding. The authors retained metastasis because it was differentially associated with attrition, although it was not significant.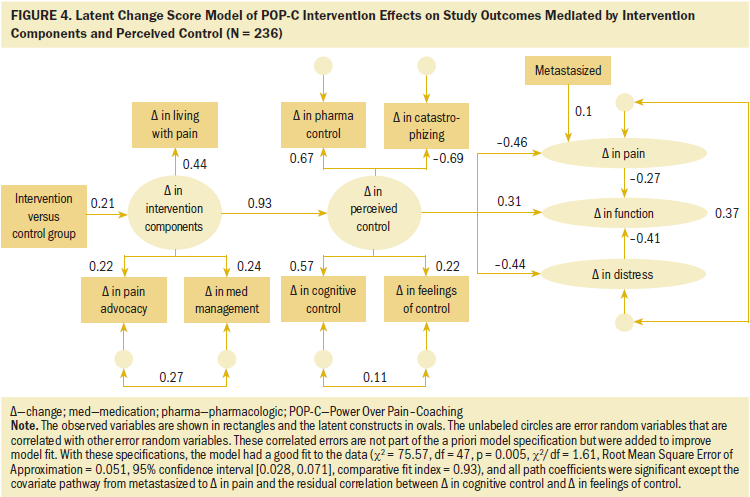
Effect of the intervention on the latent factor of change in intervention components: The two treatment groups were dummy coded (1 = intervention and 0 = control). The effect of the intervention on the latent change in the intervention components was positive and significant, as expected (standardized path = 0.21, p = 0.049). The variance in the latent change in intervention components came primarily from the change in living with pain component (standardized loading = 0.44, p = 0.018), with change in pain advocacy (standardized loading = 0.22, p = 0.028) and change in medication management (standardized loading = 0.24, p = 0.003) contributing equally.
Test of mediation effects: To determine whether intervention-related changes in perceived control mediated the intervention’s effect on changes in outcomes, the indirect effect of the intervention group on perception of control and on each of the outcomes was determined. As hypothesized, these indirect effects were significant. The indirect effect of the intervention group on perceived control was 0.197 (p = 0.027). The indirect effects of the intervention group on pain, distress, and functional status each were significant (p = –0.086, –0.09, and 0.121, respectively).
Alternative models: Additional models were tested that included the direct effects of the intervention on perceived control and functional status. These models did not fit better, and the direct-effect pathways were not significant. A third model examined the reciprocal relationship between pain and perceived control by adding a pathway from pain to perceived control. This pathway did not change the fit of the model, and the coefficient on the pathway was small and not significant.
Discussion
The current study determined the efficacy of the POP-C intervention to improve functional status among African Americans who bear an excess burden of cancer pain. The authors found greater improvements in distress and function in participants randomized to the POP-C intervention. Pain was decreased in both groups. The authors supported the hypothesis that the mechanism for this was improvement in perceived control over pain mediated by aggregate improvement in the intervention components: medication management, pain advocacy, and living with pain.
Beliefs about one’s ability to control daily symptoms, such as pain, have been found to be more influential on well-being than beliefs about one’s ability to control the progression of disease (Vallerand, Saunders, & Anthony, 2007; Wallhagen & Brod, 1997). In addition, control does not need to be exercised for it to be effective; it just needs to be perceived (Thompson, 1981). These results suggest that increasing patients’ perceived control over pain provides a means of decreasing pain-related disparities and improving functional status in patients with cancer-related pain.
An urgent need exists for effective pain management interventions tailored to African American patients with cancer. Numerous studies have shown disparities in pain experiences and in the management of pain when comparing African American and non-African American patients (Bonham, 2001; Ezenwa, Ameringer, Ward, & Serlin, 2006; Green et al., 2003; Lasch, 2000; Todd, Deaton, D’Adamo, & Goe, 2000; Vallerand et al., 2005). Distress and emotional response to pain are significantly higher among African American patients (Riley et al., 2002), who also report greater pain-related interference with daily living (Ruehlman, Karoly, & Newton, 2005; Vallerand et al., 2005). Pain-related beliefs and barriers reported by African Americans included concerns about addiction and tolerance, difficulties in communicating with physicians, and reluctance to complain of pain. The authors’ prior research showed that these disparities arise in part from a lower perception of control over pain (Vallerand et al., 2005).
As in most behavioral interventions, the effect sizes were small. However, the significance of the findings was encouraging, and increasing the intervention duration with additional booster sessions may increase the effect sizes. The current study was limited to African American patients with cancer pain. Additional studies with diverse populations and other types of chronic pain are needed.
Implications for Nursing
Most patients with cancer rank pain as their most distressing symptom (Clotfelter, 1999; Vallerand, Saunders, et al., 2007; Wells, Murphy, Wujcik, & Johnson, 2003). The NCCN (2017) guidelines recommend that distress be assessed in all patients with cancer. However, designing interventions to decrease distress is challenging because of the abstract nature of the concept. The antecedent factors that lead to pain-related distress are more amenable to intervention strategies. Based on the authors’ findings, improving patients’ perceived control over pain can decrease pain-related distress and improve function. Potential methods to improve perceived control over pain include the following (Vallerand, Saunders, et al., 2007):
• Educating patients about various pain management modalities
• Individualizing therapy to meet patients’ needs, including acceptable modifications of the pharmacologic regimen
• Providing options for patients to manage episodes of breakthrough pain
• Teaching patients how to use nonpharmacologic modalities
• Educating patients about when to call their healthcare providers if pain is not controlled
Conclusion
Development of a better approach to therapeutic intervention can, when demonstrated to be effective with at-risk populations, be translated into clinical practice to address the critical national health problems of pain and pain-related disparities. Lack of a proven intervention to address patients’ beliefs and enhance the effectiveness of pain management has been a barrier to progress in the field. The results of the current study contribute significantly to the advancement of pain research by addressing a challenge of treatment that has remained unsolved for a traditionally underrepresented minority. Understanding responses to pain as seen through the cultural lenses of specific groups will aid in more accurate interpretation of the experience and allow for more successful interventions (Edwards, Fillingim, & Keefe, 2001). This project represents the critical next step toward translating the POP-C intervention into practical clinical applications with diverse patient populations.
About the Author(s)
April Hazard Vallerand, PhD, RN, FAAN, is the associate dean for research, the director of the PhD program, and an alumni endowed professor, and Susan M. Hasenau, PhD, RN, NNP, CTN-A, is a research associate, both in the College of Nursing at Wayne State University in Detroit, MI; Sheria G. Robinson-Lane, PhD, RN, is an assistant professor in the School of Nursing at the University of Michigan in Ann Arbor; and Thomas N. Templin, PhD, is a professor in the College of Nursing at Wayne State University. This research was funded by a grant (1 RO1 CA149432-01A1) from the National Cancer Institute. Robinson-Lane has previously served on speakers bureaus for Lansing Community College and is currently an independent contractor for SGR Consulting in San Francisco, CA, and during the writing of this article, was supported by funding from the Michigan Center for Urban African American Aging Research. Vallerand, Hasenau, and Templin contributed to the conceptualization and design and provided statistical support and the analysis. Vallerand, Hasenau, and Robinson-Lane completed the data collection. All authors contributed to the manuscript preparation. Vallerand can be reached at april.vallerand@wayne.edu, with copy to ONFEditor@ons.org. (Submitted August 2017. Accepted October 18, 2017.)
References
American Chronic Pain Association. (2003). Quality of life scale: A measure of function for people with pain. Retrieved from https://www.theacpa.org/uploads/documents/Life_Scale_3.pdf
Anderson, K.O., Palos, G.R., Mendoza, T.R., Cleeland, C.S., Liao, K.P., Fisch, M.J., . . . Payne, R. (2015). Automated pain intervention for underserved minority women with breast cancer. Cancer, 121, 1882–1890. https://doi.org/10.1002/cncr.29204
Beuken-van Everdingen, M.H.J., de Pijke, J.M., Kessels, A.G., Schouten, H.C., Van Kleef, M., & Parijn, J. (2007). Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Annals of Oncology, 18, 1437–1449. https://doi.org/10.1093/annonc/mdm056
Bonham, V.L. (2001). Race, ethnicity, and pain treatment: Striving to understand the causes and solutions to the disparities in pain treatment. Journal of Law, Medicine, and Ethics, 29, 52–68. https://doi.org/10.1111/j.1748-720X.2001.tb00039.x
Chang, V.T., Hwang, S.S., Feuerman, M., Kasimis, B.S., & Thaler, H.T. (2000). The Memorial Symptom Assessment Scale Short Form (MSAS-SF). Cancer, 89, 1162–1171. https://doi.org/10.1002/1097-0142(20000901)89:5<1162::AID-CNCR26>3.0.CO…
Cleeland, C.S., & Syrjala, K.L. (2001). How to assess cancer pain. In D.C. Turk, & R. Melzack, Handbook of pain assessment (2nd ed., pp 362–387). New York, NY: Guilford Press.
Clotfelter, C.E. (1999). The effect of an educational intervention on decreasing pain intensity in elderly people with cancer. Oncology Nursing Forum, 26, 27–33.
Edwards, C.L., Fillingim, R.B., & Keefe, F. (2001). Race, ethnicity, and pain. Pain, 94, 133–137. https://doi.org/10.1016/S0304-3959(01)00408-0
Ezenwa, M.O., Ameringer, S., Ward, S.E., & Serlin, R.C. (2006). Racial and ethnic disparities in pain management in the United States. Journal of Nursing Scholarship, 38, 225–233. https://doi.org/10.1111/j.1547-5069.2006.00107.x
Ferrell, B.R., Eberts, M.T., McCaffery, M., & Grant, M. (1991). Clinical decision making and pain. Cancer Nursing, 14(6), 289–297. https://doi.org/10.1097/00002820-199112000-00002
Fisch, M.J., Lee, J.W., Weiss, M, Wagner, L.I., Chang, V.T., Cella, D., . . . Cleeland, C.S. (2012). Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. Journal of Clinical Oncology, 30, 1980–1988. https://doi.org/10.1200/JCO.2011.39.2381
Fitzmaurice, G.M., Laird, N.M, & Ware, J.H. (2004). Applied longitudinal analysis. Hoboken, NJ: John Wiley and Sons.
Green, C.R., Anderson, K.O., Baker, T.A., Campbell, L.C., Decker, S., Fillingim, R.B., . . . Vallerand, A.H. (2003). The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine, 4, 277–294. https://doi.org/10.1046/j.1526-4637.2003.03034.x
Gunnarsdottir, S., Donovan, H.S., Serlin, R.C., Voge, C., & Ward, S. (2002). Patient-related barriers to pain management: The Barriers Questionnaire-II (BQ-II). Pain, 99, 385–396. https://doi.org/10.1016/S0304-3959(02)00243-9
Hammer, K.J., Segal, E.M., Alwan, L., Li, S., Patel, A.M., Tran, M., & Marshal, H.M. (2016). Collaborative practice model for management of pain in patients with cancer. American Journal of Health-System Pharmacy, 73, 1434–1441. https://doi.org/10.2146/ajhp150770
Hayduk, L.A. (1987). Structural equation modeling with LISREL: Essentials and advances. Baltimore, MD: Johns Hopkins University Press.
Jacobsen, R., Møldrup, C. Christrup, L., Sjøgren, P., & Hansen, O.B. (2010). Psychological and behavioural predictors of pain management outcomes in patients with cancer. Scandinavian Journal of Caring Sciences, 24, 781–790. https://doi.org/10.1111/j.1471-6712.2010.00776.x
Jensen, M.P., Turner, J.A., Romano, J.M., & Lawler, B.K. (1994). Relationship of pain-specific beliefs to chronic pain adjustment. Pain, 57, 301–309. https://doi.org/10.1016/0304-3959(94)90005-1
Laird, N.M., & Ware, J.H. (1982). Random-effects models for longitudinal data. Biometrics, 38, 963–974. https://doi.org/10.2307/2529876
Lasch, K.E. (2000). Culture, pain, and culturally sensitive pain care. Pain Management Nursing, 1(Suppl. 1), 16–22. https://doi.org/10.1053/jpmn.2000.9761
Leung, J., Pachana, N.A., & McLaughlin, D. (2014). Social support and health-related quality of life in women with breast cancer: A longitudinal sutdy. Psycho-Oncology, 23, 1014–sd1020.
McCracken, L.M., Vowles, K.E., & Eccleston, C. (2004). Acceptance of chronic pain: Component analysis and a revised assessment method. Pain, 107, 159–166. https://doi.org/10.1016/j.pain.2003.10.012
McNeill, J.A., Reynolds, J.R., & Ney, M.L. (2007). Unequal quality of cancer pain management: Disparity in perceived control and proposed solutions. Oncology Nursing Forum, 34, 1121–1128. https://doi.org/10.1188/07.ONF.1121-1128
Meghani, S.H., Thompson, A.M.L., Chittams, J., Bruner, D.W., & Riegel, B. (2015). Adherence to analgesics for cancer pain: A comparative study of African Americans and Whites using an electronic monitoring device. Journal of Pain, 16, 825–835. https://doi.org/10.1016/j.jpain.2015.05.009
National Comprehensive Cancer Network. (2017). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Distress management [v.2.2017]. Retrieved from https://www.nccn.org/professionals/physician_gls/PDF/distress.pdf
Pellino, T.A., & Ward, S.E. (1998). Perceived control mediates the relationship between pain severity and patient satisfaction. Journal of Pain and Symptom Management, 15, 110–116. https://doi.org/10.1016/S0885-3924(97)00255-8
Ransom, S., Jacobsen, P.B., & Booth-Jones, M. (2006). Validation of the distress thermometer with bone marrow transplant patients. Psycho-Oncology, 15, 604–612. https://doi.org/10.1002/pon.993
Riley, J.L., 3rd., Wade, J.B., Myers, C.D., Sheffield, D., Papas, R.K., & Price, D.D. (2002). Racial/ethnic differences in the experience of chronic pain. Pain, 100, 291–298. https://doi.org/10.1016/S0304-3959(02)00306-8
Ruehlman, L.S., Karoly, P., & Newton, C. (2005). Comparing the experiential and psychosocial dimensions of chronic pain in African Americans and Caucasians: Findings from a national community sample. Pain Medicine, 6, 49–60. https://doi.org/10.1111/j.1526-4637.2005.05002.x
Satorra, A., & Saris, W.E. (1985). Power of the likelihood ratio test in covariance structure analysis. Psychometrika, 50, 83–90. https://doi.org/10.1007/BF02294150
Schag, C.C., Heinrich, R.L., & Ganz, P.A. (1984). Karnofsky Performance Status revisited: Reliability, validity, and guidelines. Journal of Clinical Oncology, 2, 187–193. https://doi.org/10.1200/JCO.1984.2.3.187
Shrout, P.E., & Bolger, N. (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7, 422–445. https://doi.org/10.1037/1082-989X.7.4.422
Sullivan, M.J.L., Bishop, S.R., & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7, 524–532. http://doi.org/10.1037/1040-3590.7.4.524
Thompson, S.C. (1981). Will it hurt less if I can control it? A complex answer to a simple question. Psychological Bulletin, 90, 89–101. https://doi.org/10.1037/0033-2909.90.1.89
Todd, K.H., Deaton, C., D’Adamo, A.P., & Goe, L. (2000). Ethnicity and analgesic practice. Annals of Emergency Medicine, 35, 11–16.
Vallerand, A.H., Crawley, J., Pieper, B., & Templin, T.N. (2016). The perceived control over pain construct and functional status. Pain Medicine, 17, 692–703. https://doi.org/10.1111/pme.12924
Vallerand, A.H., Hasenau, S.M., & Templin, T. (2010). Improving cancer pain management in the home. Journal of Pain Management, 3, 41–51.
Vallerand, A.H., Hasenau, S., Templin, T., & Collins-Bohler, D. (2005). Disparities between Black and White patients with cancer pain: The effect of perception of control over pain. Pain Medicine, 6, 242–250.
Vallerand, A.H., Saunders, M.M., & Anthony, M. (2007). Perceptions of control over pain by patients with cancer and their caregivers. Pain Management Nursing, 8, 55–63. https://doi.org/10.1016/j.pmn.2007.02.001
Vallerand, A.H., Templin, T., Hasenau, S.M., & Riley-Doucet, C. (2007). Factors that affect functional status in patients with cancer-related pain. Pain, 132, 82–90. https://doi.org/10.1016/j.pain.2007.01.029
Vancleef, L.M., & Peters, M.L. (2011). The influence of perceived control and self-efficacy on the sensory evaluation of experimentally induced pain. Journal of Behavior Therapy and Experimental Psychiatry, 42, 511–517. https://doi.org/10.1016/j.jbtep.2011.05.006
Wallhagen, M.I., & Brod, M. (1997). Perceived control and well-being in Parkinson’s disease. Western Journal of Nursing Research, 19, 11–25. https://doi.org/10.1177/019394599701900102
Ware, J., Jr., Kosinski, M., & Keller, S.D. (1996). A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. https://doi.org/10.1097/00005650-199603000-00003
Wells, N.L., & Sandlin, V. (2012). Expectations of pain and accompanying symptoms during cancer treatment. Current Pain and Headache Reports, 16, 292–299. https://doi.org/10.1007/s11916-012-0272-0
Wells, N., Murphy, B., Wujcik, D., & Johnson, R. (2003). Pain-related distress and interference with daily life of ambulatory patients with cancer with pain. Oncology Nursing Forum, 30, 977–986. https://doi.org/10.1188/03.ONF.977-986
White-Means, S., Rice, M., Dapremont, J., Davis, B., & Martin, J. (2015). African American women: Surviving breast cancer mortality against the highest odds. International Journal of Environmental Research and Public Health, 13, 6.




