Symptom Dimensions as Outcomes in Interventions for Patients With Cancer: A Systematic Review
Problem Identification: Symptom experience in patients with cancer consists of several dimensions, often measured descriptively within various populations but seldom used as intervention outcomes. This review aims at describing symptom dimensions as outcomes of interventions designed to alleviate symptoms in patients with cancer and to describe these interventions’ effects on at least two symptom dimensions.
Literature Search: The PRISMA statement for reporting systematic reviews was used. Searches were undertaken in various indexing sites.
Data Evaluation: Extracted data included design, participants, intervention and control group treatment, targeted symptom dimension, and summary of results.
Synthesis: 2,041 articles were identified and 15 were included. The symptom dimensions were intensity, distress, prevalence, frequency, consequences, and quality. Eleven interventions had significant effect on symptom dimensions, mostly on intensity and distress.
Implications for Practice: Oncology nurses need clinical skills to be able to understand patients’ experiences through their narratives. Various interventions are targeted at symptoms, and these need to be implemented to provide evidence-based symptom management.
Jump to a section
Patients affected by cancer often experience multiple symptoms, in both the short- and long-term perspective, as a result of the disease and its treatment. Symptoms negatively affect patients’ and families’ well-being and quality of life (Lang, France, Williams, Humphris, & Wells, 2013). Symptoms are defined as a subjective experience of altered functioning, which cannot be objectively observed (Dodd et al., 2001; Harver & Mahler, 1990). Patient-reported measurements are, therefore, used to assess symptoms, both in clinical practice and in research. The literature identifies an increasing focus on symptom clusters. However, the relation and interaction between symptoms within clusters and between clusters are not well investigated (Miaskowski, 2006; Miaskowski, Aouizerat, Dodd, & Cooper, 2007), nor is the interplay between the different dimensions within a singular symptom. One critical area of concern in cancer care is symptom relief before, during, and after treatment (Oksholm et al., 2015). In addition, how each symptom dimension determines symptom burden remains to be clarified (Wong et al., 2017).
Methods
In this systematic review, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement for reporting systematic reviews was used as a methodologic tool (Liberati et al., 2009). Systematic searches were undertaken in August 2016 in PubMed, CINAHL®, Web of Science, PsycINFO®, and Scopus using the following search terms: (“Symptom dimension*” OR “Symptom prevalence” OR “Symptom intensity” OR “Symptom distress” OR “Symptom frequency” OR “Symptom burden” OR “Symptom experience” OR “Symptom quality”) AND (RCT [in title or abstract] OR Randomized controlled trial [in title or abstract] OR Intervention [in title or abstract] OR Nursing [in title or abstract]) AND (Cancer [in title or abstract] OR neoplasm* [in title or abstract]). Limitations were set, where possible, that each paper should be a journal article, a clinical trial, written in English, and peer-reviewed. No time limitation was set to capture the full body of knowledge about symptom dimensions. These searches yielded 2,041 articles, and, after the removal of duplicates, 1,104 remained.
Study Selection
The inclusion criteria were that the article was an original article describing an intervention targeted toward symptoms in adult patients (aged 18 years or older) affected by cancer. The outcome should include at least two symptom dimensions. In addition, the design should be an intervention study, either with an intervention group and a control group, or a before-and-after measurement. At least 20 patients should be included. Eligibility assessment was performed independently by two reviewers (half of the sample was reviewed by ML and CO, and half of the sample was reviewed by KA and IH). Disagreements were resolved by consensus among the whole group. After reading titles and abstracts, 1,008 articles were excluded. One reviewer read the full text of the remaining 96 articles and the other authors checked for eligibility. An additional 81 articles were then excluded. Disagreements were resolved by discussions between the authors. The reasons for excluding articles are presented in Figure 1. Articles were mostly excluded because they did not report two or more symptom dimensions as outcomes. A final 15 remained for data analysis.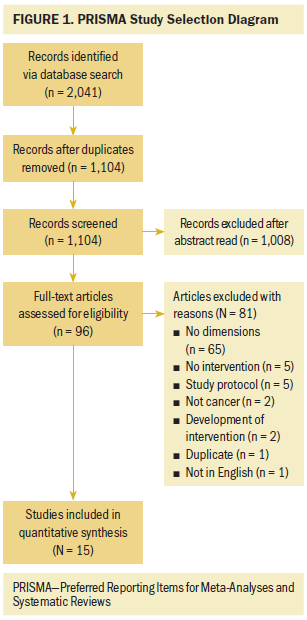
Data Collection Process
The authors developed a data extraction sheet, pilot tested it on 10 articles, and refined it accordingly. One reviewer (IH) extracted the following data, and a second author checked the extracted data: design, number of patients, diagnosis, intervention, care of control group patients, target symptom dimensions, results on symptom dimensions, and quality of evidence according to the Scottish Intercollegiate Guidelines Network (SIGN) (Harbour & Miller, 2001). The SIGN evidence statements provide guidelines related to the grading of studies eligible for the current study: 1++, indicating randomized, controlled trials (RCTs) with very low risk of bias; 1+, indicating an RCT with low risk of bias; 1–, indicating an RCT with high risk of bias; and 2++, indicating high-quality case control or cohort studies with low risk of confounding bias.
Results
Description of Included Studies
The selection process yielded 15 articles, with 7 published in the United States, 2 each in Australia and the Netherlands, and 1 each from the United Kingdom, Taiwan, Denmark, and Spain. The articles were published from 1999–2015. The patients in the studies included those who were affected by lung cancer, breast cancer (two studies), prostate cancer, ovarian cancer, leukemia, more than one type of cancer, an unspecified type of cancer (seven studies), and one with patients who were treated with stem cell transplantation. The designs in the studies included RCTs (11 studies), pre-/post-test design (three studies), and non-RCT (one study). The number of participants in the studies had a mean value of 135.1 (SD = 107) and a median of 115 (range = 22–380).
Target Symptoms Dimensions
Some studies focused on a single symptom and its dimensions. In these studies, the target symptom dimensions were:
• Psychological distress (Aranda et al., 2012)
• Breathlessness: distress and intensity (Bredin et al., 1999)
• Hot flash: severity (Carpenter, Neal, Payne, Kimmick, & Storniolo, 2007; Park et al., 2015), bother (Carpenter et al., 2007), and frequency (Park et al., 2015)
• Pain: intensity (Dalton, Keefe, Carlson, & Young-blood, 2004; Kutner et al., 2008; Kwekkeboom, Kneip, & Pearson, 2003), interference (Dalton et al., 2004; Kutner et al., 2008), worst pain (Kutner et al., 2008), distress (Kwekkeboom et al., 2003), and pain control (Kwekkeboom et al., 2003)
• Fatigue: quality dimensions (de Raaf et al., 2013; van Weert et al., 2006), intensity (Chang et al., 2008), and interference (Chang et al., 2008)
• Urinating: frequency, difficulty, and limiting activities (Serdà & Marcos-Gragera, 2014)
Other studies targeted several symptoms and symptom dimensions concurrently:
• Chemotherapy symptoms: prevalence, severity, and bother (Aranda et al., 2012)
• Symptom burden (an aggregated score of all intensity scorings) (de Raaf et al., 2013), severity (Donovan et al., 2014; Jarden, Nelausen, Hovgaard, Boesen, & Adamsen, 2009; Lewis et al., 2015) and distress (Donovan et al., 2014; Jarden et al., 2009); consequences of symptoms (Donovan et al., 2014), controllability of symptoms (Donovan et al., 2014), prevalence (Lewis et al., 2015), and bother (Lewis et al., 2015)
Complex Interventions
The interventions were characterized as complex if they were interventions consisting of more than one component. Nine studies were characterized as complex and, of those, seven showed significant differences in at least one dimension. Additional information on the studies is presented in Table 1.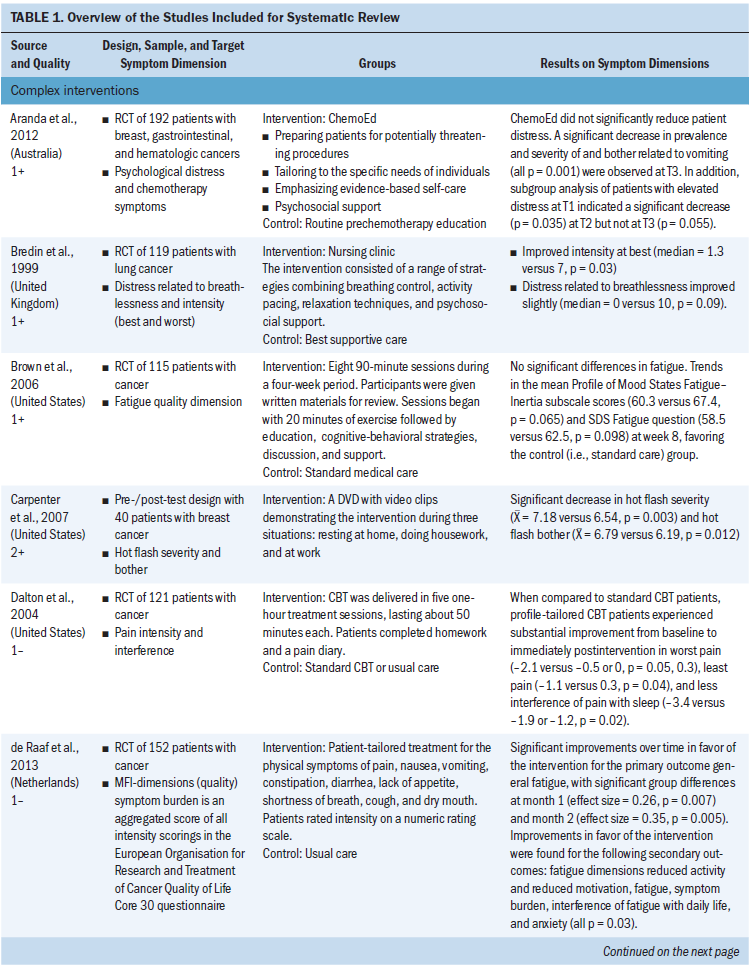
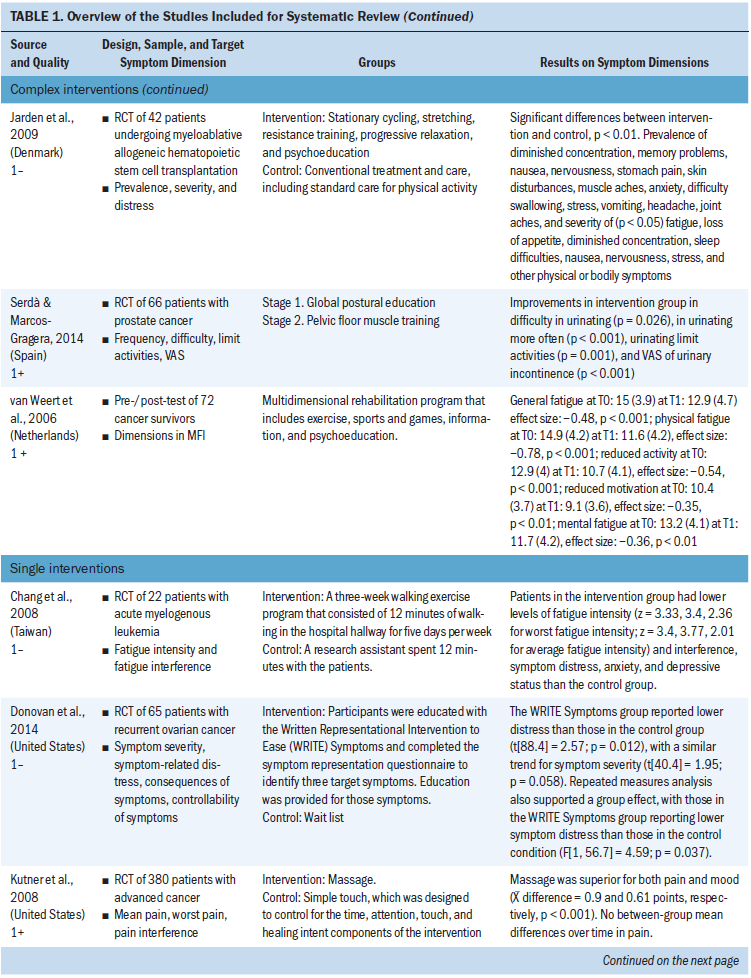
Two studies used education or information DVDs. Aranda et al. (2012) designed an intervention to prepare patients with various cancers for chemotherapy by providing a DVD with information, a question prompt list, self-care information, and education consultation. The patients also received a telephone and face-to-face follow-up. Carpenter et al. (2007) distributed a DVD with video clips to patients experiencing hot flashes after breast cancer. The video clips showed three situations: resting at home, doing housework, and at work in an environment where the intervention could be used. The proposed physiologic mechanism of action on core body temperature was demonstrated. Voice and text instructed the women to “stop, breathe, and focus” to help them remember how to use the behavioral intervention. The intervention should be used as soon as a hot flash is perceived (Carpenter et al., 2007).
Two studies included nursing clinic visits. In a study by Bredin et al. (1999), patients affected by lung cancer visited a nursing clinic, where a range of strategies were adopted, including breathing control, activity pacing, relaxation techniques, and psychosocial support (Bredin et al., 1999). de Raaf et al. (2013) designed a patient-tailored treatment for patients affected by various cancers. A nurse specialist coordinated an intervention targeting the physical symptoms of pain, nausea, vomiting, constipation, diarrhea, lack of appetite, shortness of breath, cough, and dry mouth. The nurse specialist asked patients to rate symptom intensity in the past week on a numeric rating scale, ranging from 0 (no suffering) to 10 (unbearable suffering) (de Raaf et al., 2013).
Three studies included education or therapy sessions. Brown et al.’s (2006) intervention included eight 90-minute sessions during four weeks for patients affected by various cancers. The patients received written materials covering the eight sessions. Each session focused on at least one of five quality-of-life domains. A psychiatrist or a psychologist led the sessions, depending on the topic. An advanced practice nurse, a chaplain, or a social worker cofacilitated each session. Sessions began with 20 minutes of exercises conducted by a physical therapist, followed by educational information, cognitive-behavioral strategies, discussion, and support (Brown et al., 2006). Dalton et al. (2004) adopted cognitive-behavioral therapy (CBT) techniques and delivered these to patients affected by various cancer diagnoses in five one-hour treatment sessions, lasting about 50 minutes each. Patients completed homework and a pain diary (Dalton et al., 2004). Jarden et al. (2009) combined stationary cycling, stretching, resistance training, progressive relaxation, and psychoeducation for patients undergoing stem cell transplantation.
Serdà and Marcos-Gragera (2014) designed an intervention for patients affected by prostate cancer in two stages, where stage 1 related to global postural re-education and stage 2 related to pelvic floor muscle training. The training aimed at controlling urinary incontinence by improving the pelvic floor muscle strength and, therefore, compensating for the insufficiencies in the damaged sphincters and decreasing urine retention. van Weert et al. (2006) used a multidimensional rehabilitation program, including exercise, sports and games, information, and psychoeducation for cancer survivors.
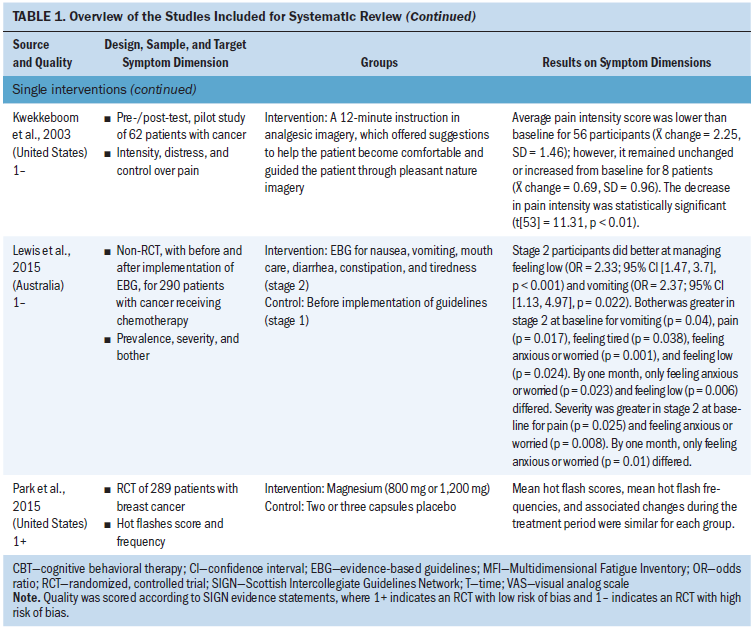
Single Interventions
Six studies described single interventions and, of these, four showed significant differences in symptom dimensions. The single-intervention studies related to physical exercise programs, writing exercises, massage, guided imagery, implementation of evidence-based care, or complementary medicine, such as magnesium. Chang et al. (2008) applied a walking exercise program (WEP) based on the principles of frequency, duration, and intensity of activity suggested by the American College of Sports Medicine and a literature review of patients affected by leukemia. The three-week WEP consisted of 12 minutes of walking in the hospital hallway five days per week (Chang et al., 2008). Donovan et al. (2014) designed a Written Representational Intervention to Ease (WRITE) Symptoms for patients affected by recurrent ovarian cancer. Participants completed the Symptom Representation Questionnaire to identify their three target symptoms. Self-care guides for each symptom were mailed or emailed to reinforce the education provided by the research nurse. The guides were based on the format used by Yarbro, Frogge, and Goodman (2004), providing a description of the symptom, strategies to prevent and/or manage the symptom, and a summary of important information to communicate with healthcare providers. Symptom management recommendations in the guide included two broad categories: symptom management that requires intervention by a healthcare provider (e.g., medication, procedures, referrals) and self-management strategies (Donovan et al., 2014). Kutner et al. (2008) applied a massage intervention to patients affected by advanced cancer. The massage included light/gentle effleurage, petrissage, and myofascial trigger point release. Effleurage is a smooth, gliding stroke; petrissage is squeezing, rolling, and kneading the muscles (Kutner et al., 2008). Kwekkeboom et al. (2003) used a 12-minute instruction in analgesic imagery, offering suggestions to help patients with various cancer diagnoses to become comfortable, and guided them through pleasant nature imagery, including a walk along a river, sitting in a field among wildflowers, and viewing a sunset (Kwekkeboom et al., 2003). Lewis et al. (2015) introduced evidence-based self-care guidelines to patients receiving chemotherapy and measured outcomes before implementation of guidelines and after (Lewis et al., 2015). The intervention designed by Park et al. (2015) included prescribing magnesium in different doses to patients affected by breast cancer to improve hot flashes.
Effects on Symptom Dimensions
The interventions had different effects on different dimensions (see Table 2). The symptom dimensions that were alleviated by the interventions were for single symptoms, such as breathlessness intensity but not distress, although there was a nonsignificant trend that these were improved (Bredin et al., 1999), and hot flash severity and bother were alleviated in Carpenter et al. (2007) but not in Park et al. (2015). Pain intensity was alleviated in two studies (Dalton et al., 2004; Kwekkeboom et al., 2003) and pain interference was alleviated in one study (Dalton et al., 2004), but, in another study, no differences were noted between the intervention and control group in either interference or worst pain (Kutner et al., 2008). In Brown et al. (2006), no significant differences were noted in the fatigue quality dimensions, but there were improvements in two other studies (de Raaf et al., 2013; van Weert et al., 2006) in physical fatigue, increased activity, improved motivation, and mental fatigue. There also were improvements in fatigue intensity and interference (Chang et al., 2008). Urinating frequency, difficulty urinating, and limiting activities were all alleviated by the intervention in Serdà and Marcos-Gragera (2014). 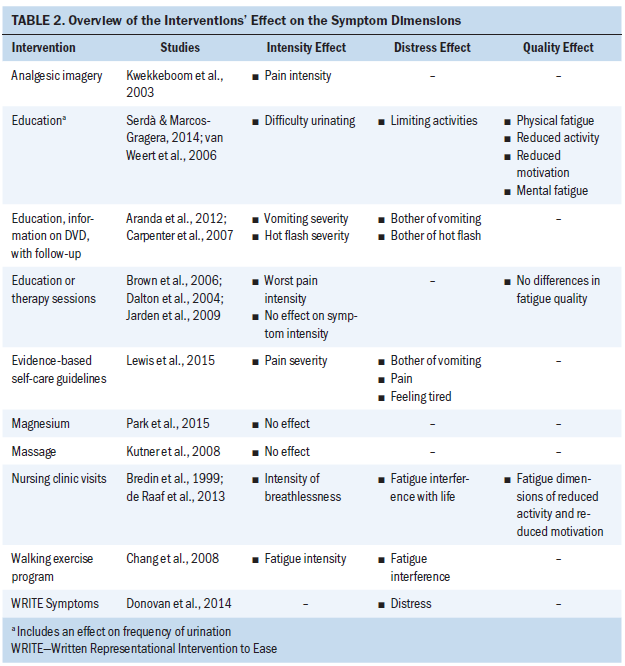
Significant decreases were noted in prevalence, severity, and bother of vomiting as a chemotherapy-induced symptom (Aranda et al., 2012). Symptom severity was alleviated in one study (Jarden et al., 2009). Symptom distress, but not intensity, was alleviated in one study (Donovan et al., 2014).
Summary of Symptom Dimensions
Symptom dimensions are sparsely used as outcomes in symptom intervention studies. Some studies seem to label the symptom dimensions differently. In the following list, symptom dimensions that are similar have been arbitrarily grouped together. The most frequently used dimensions relate to the following:
• Intensity (Bredin et al., 1999; Chang et al., 2008; Dalton et al., 2004; Kwekkeboom et al., 2003; Lewis et al., 2015), severity (Aranda et al., 2012; Carpenter et al., 2007; Donovan et al., 2014; Jarden et al., 2009; Park et al., 2015), and difficulty (Serdà & Marcos-Gragera, 2014)
• Distress (Bredin et al., 1999; Donovan et al., 2014; Jarden et al., 2009; Kwekkeboom et al., 2003), bother (Aranda et al., 2012; Carpenter et al., 2007; Lewis et al., 2015), burden (de Raaf et al., 2013), and interference (Chang et al., 2008; Dalton et al., 2004; de Raaf et al., 2013; Kutner et al., 2008)
• Prevalence (Aranda et al., 2012; Jarden et al., 2009; Lewis et al., 2015)
• Frequency (Park et al., 2015; Serdà & Marcos-Gragera, 2014)
• Consequences (Donovan et al., 2014), limiting activities (Serdà & Marcos-Gragera, 2014), and control (Donovan et al., 2014; Kwekkeboom et al., 2003)
• Quality (Brown et al., 2006; de Raaf et al., 2013; van Weert et al., 2006)
Differences were noted in which dimensions are alleviated by the interventions, and one intervention could alleviate one dimension but not another. More complex interventions seem to use more outcome dimensions and also have an effect on more dimensions.
Discussion
There is a growing body of evidence that symptom experience is multidimensional (Armstrong, 2003; Brant et al., 2010; Dodd et al., 2001; Humphrey et al., 2014; Lenz et al., 1997), but the dimensions were found to be sparsely used as outcomes in symptom interventions studies. One reason for this could be that studies are easier to design and implement with a single outcome measure, or that it is easier to use available instruments.
In some instruments, several dimensions are measured, but a composite measure is presented, as in the MSAS (Portenoy et al., 1994) or the Symptom Distress Scale (McCorkle & Young, 1978) and, accordingly, these studies were excluded. The reason for presenting composite measures might be that one wants to acknowledge the multidimensionality of the symptom experience, but it is easier to present only one measure as the outcome. Implicit in this way of presenting data is that each dimension is given a minor significance. In addition, the patients who complete a symptom assessment within several dimensions are given a larger workload of completing multiple questions about the same symptom, and this often involves the participation of severely diseased patients, sometimes with life-threatening illnesses, who need to focus on their own well-being rather than on completing lengthy questionnaires.
When symptoms are presented, there is an agreement of what is meant by the label of the symptom, but the current study shows that the terms for the dimensions differ between studies, making it difficult to compare the results of the interventions. The studies in the current review used at least two dimensions, but there is no evidence of which dimension is most important or if the dimensions are equally important. Tishelman et al. (2005) found that symptom intensity and symptom distress were not equivalent in that breathing, pain, and fatigue caused the most distress, but fatigue had the highest intensity. Symptom distress was also found to be most consistent over time, but symptom intensity varied. If that is a stable result, the dimension that might be most possible to be influenced by an intervention could be symptom intensity. In most of the studies included in the current review, symptom dimensions were not measured longitudinally. Healthcare providers should explore which dimensions affect patients’ well-being most. Although patients have been found to score symptom distress and symptom severity on different levels (Tishelman et al., 2005), how patients communicate their perceptions of separate symptoms is sometimes problematic. A study of women affected by ovarian cancer found that they experience multiple symptoms, but they did not discuss their symptoms with healthcare professionals and did not receive symptom management recommendations (Donovan, Hartenbach, & Method, 2005). It was concluded that women would benefit from more active symptom assessment, which also was acknowledged by Sarna (1998), who designed an intervention with symptom assessment and found less symptom distress in the intervention group. If communication about single symptoms is difficult, then it would be even more difficult to communicate about multiple symptom dimensions for patients.
In the current review, it was shown that complex interventions seem to have a better effect on more than one dimension. There is an agreement that complex interventions need to be developed to have an effect on patients’ suffering (Campbell et al., 2000; Craig et al., 2008). A complex intervention could be characterized as such by (a) the existence of several interacting components within the experimental and control interventions, (b) the complexity of behaviors required by those delivering or receiving the intervention, (c) the number of groups or organizational levels targeted by the intervention, (d) the variability of outcomes, and (e) the degree of flexibility or tailoring of the intervention permitted (Craig et al., 2008). In the current article, the authors labeled an intervention as complex if there were more than one component included in the intervention, which is in line with Craig et al. (2008). However, Craig et al. (2008) also included the variability of outcomes in their definition of complexity; with such a criterion, all the studies in the current article would be considered as complex. When designing a complex intervention, the outcomes need to be decided in advance, and, if symptom dimensions are considered to be an important part of the patients’ symptom experience, the outcomes in symptom studies should include one or more symptom dimensions.
The frontier of symptom research was, in 2004, symptom clusters, and it was argued that researchers need to determine which dimensions of a symptom are critical for the assessment of a symptom within a symptom cluster (Miaskowski, Dodd, & Lee, 2004). In most studies, symptom clusters are measured in one dimension. It could be argued that symptom dimensions and symptom clusters are different and complementary understandings of symptoms. Symptom dimensions are aimed at dividing the symptom experience into several pieces, but symptom clusters are aimed at finding multiple symptoms that are linked to each other. More research is needed to determine which symptom dimensions should be included in a symptom cluster; it might be that different dimensions from each different symptom should be included. In some studies and in some instruments, such as the MSAS, multiple dimensions are clustered together to a composite measure, sometimes called symptom burden. There is a need to explore whether symptom cluster could be considered as a symptom burden measure.
Implications for Nursing
Symptom relief before, during, and after treatment is crucial in oncology nursing, and oncology nurses need to acknowledge patients’ experience of symptoms. So far, research has stated that symptoms need to be structurally assessed and managed, but, because the symptom experience is complex, to only measure intensity might be insufficient. As long as no consensus exists about how to assess the total symptom experience, oncology nurses need to develop clinical skills to be able to understand and extract and, thereby, assess the patients’ experiences through listening to their narratives. The current article also showed that various interventions are targeted at symptoms, and those interventions need to be implemented in practice to provide evidence-based symptom management.
Conclusion
Although symptom dimensions are included in nursing symptom theories, very few studies were found that used two or more symptom dimensions as outcome measures in symptom intervention studies. Intensity and distress were the most frequently used dimensions. Additional studies are needed to determine the appropriate dimensions in symptom studies. Various interventions were found in the included studies, but their robustness, their influence on the experience of single symptoms and on symptom clusters, and the most appropriate way to implement them, need to be explored.
About the Author(s)
Ingela Henoch, PhD, RN, is a senior lecturer and an associate professor in the Sahlgrenska Academy Institute of Health and Care Sciences at the University of Gothenburg in Sweden; Cecilia Olsson, PhD, RN, is a senior lecturer and an assistant professor, and Maria Larsson, PhD, RN, is a professor, both in the Department of Health Sciences at Karlstad University in Sweden; and Karin Ahlberg, PhD, RN, is a senior lecturer and an associate professor in the Sahlgrenska Academy Institute of Health and Care Sciences at the University of Gothenburg. No financial relationships to disclose. All authors completed the data collection, contributed to the conceptualization and design, provided the analysis, and contributed to the manuscript preparation. Henoch can be reached at ingela.henoch@gu.se, with copy to ONFEditor@ons.org. (Submitted August 2017. Accepted September 29, 2017.)
References
Aranda, S., Jefford, M., Yates, P., Gough, K., Seymour, J., Francis, P., . . . Schofield, P. (2012). Impact of a novel nurse-led prechemotherapy education intervention (ChemoEd) on patient distress, symptom burden, and treatment-related information and support needs: Results from a randomised, controlled trial. Annals of Oncology, 23, 222–231. https://doi.org/10.1093/annonc/mdr042
Armstrong, T.S. (2003). Symptoms experience: A concept analysis. Oncology Nursing Forum, 30, 601–606. https://doi.org/10.1188/03.ONF.601-606
Brant, J.M., Beck, S., & Miaskowski, C. (2010). Building dynamic models and theories to advance the science of symptom management research. Journal of Advanced Nursing, 66, 228–240. https://doi.org/10.1111/j.1365-2648.2009.05179.x
Bredin, M., Corner, J., Krishnasamy, M., Plant, H., Bailey, C., & A’Hern, R. (1999). Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ, 318, 901–904. https://doi.org/10.1136/bmj.318.7188.901
Browall, M., Kenne Sarenmalm, E., Nasic, S., Wengström, Y., & Gaston-Johansson, F. (2013). Validity and reliability of the Swedish version of the Memorial Symptom Assessment Scale (MSAS): An instrument for the evaluation of symptom prevalence, characteristics, and distress. Journal of Pain and Symptom Management, 46, 131–141. https://doi.org/10.1016/j.jpainsymman.2012.07.023
Brown, P., Clark, M.M., Atherton, P., Huschka, M., Sloan, J.A., Gamble, G., . . . Rummans, T.A. (2006). Will improvement in quality of life (QOL) impact fatigue in patients receiving radiation therapy for advanced cancer? American Journal of Clinical Oncology, 29, 52–58. https://doi.org/10.1097/01.coc.0000190459.14841.55
Campbell, M., Fitzpatrick, R., Haines, A., Kinmonth, A.L., Sandercock, P., Spiegelhalter, D., & Tyrer, P. (2000). Framework for design and evaluation of complex interventions to improve health. BMJ, 321, 694–696.
Carpenter, J.S., Neal, J.G., Payne, J., Kimmick, G., & Storniolo, A.M. (2007). Cognitive-behavioral intervention for hot flashes [Online exclusive]. Oncology Nursing Forum, 34, E1–E8. https://doi.org/10.1188/07.ONF.E1-E8
Chang, P.H., Lai, Y.H., Shun, S.C., Lin, L.Y., Chen, M.L., Yang, Y., . . . Cheng, S.Y. (2008). Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: A randomized controlled trial. Journal of Pain and Symptom Management, 35, 524–534. https://doi.org/10.1016/j.jpainsymman.2007.06.013
Craig, P., Dieppe, P., Macintyre, S., Michie, S., Nazareth, I., & Petticrew, M. (2008). Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ, 337, a1655. https://doi.org/10.1136/bmj.a1655
Dalton, J.A., Keefe, F.J., Carlson, J., & Youngblood, R. (2004). Tailoring cognitive-behavioral treatment for cancer pain. Pain Management Nursing, 5, 3–18.
de Raaf, P.J., de Klerk, C., Timman, R., Busschbach, J.J., Oldenmenger, W.H., & van der Rijt, C.C. (2013). Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: A randomized controlled trial. Journal of Clinical Oncology, 31, 716–723. https://doi.org/10.1200/JCO.2012.44.4216
Dodd, M., Janson, S., Facione, N., Faucett, J., Froelicher, E.S., Humphreys, J., . . . Taylor, D. (2001). Advancing the science of symptom management. Journal of Advanced Nursing, 33, 668–676.
Donovan, H.S., Hartenbach, E.M., & Method, M.W. (2005). Patient- provider communication and perceived control for women experiencing multiple symptoms associated with ovarian cancer. Gynecologic Oncology, 99, 404–411. https://doi.org/10.1016/j.ygyno.2005.06.062
Donovan, H.S., Ward, S.E., Sereika, S.M., Knapp, J.E., Sherwood, P.R., Bender, C.M., . . . Ingel, R. (2014). Web-based symptom management for women with recurrent ovarian cancer: A pilot randomized controlled trial of the WRITE symptoms intervention. Journal of Pain and Symptom Management, 47, 218–230. https://doi.org/10.1016/j.jpainsymman.2013.04.005
Fawcett, J. (2005). The structure of contemporary nursing knowledge. In J. Fawcett (Ed.), Contemporary nursing knowledge: Analysis and evaluation of nursing models and theories (2nd ed., pp. 3–30). Philadelphia, PA: F.A. Davis.
Fu, M.R., McDaniel, R.W., & Rhodes, V.A. (2007). Measuring symptom occurrence and symptom distress: Development of the symptom experience index. Journal of Advanced Nursing, 59, 623–634. https://doi.org/10.1111/j.1365-2648.2007.04335.x
Harbour, R., & Miller, J. (2001). A new system for grading recommendations in evidence based guidelines. BMJ, 323, 334–336.
Harver, A., & Mahler, D.A. (1990). The symptom of dyspnea. In D.A. Mahler (Ed.), Dyspnea (pp. 1–53). New York, NY: Futura Publishing.
Haworth, S.K., & Dluhy, N.M. (2001). Holistic symptom management: Modelling the interaction phase. Journal of Advanced Nursing, 36, 302–310.
Henoch, I., Bergman, B., Gustafsson, M., Gaston-Johansson, F., & Danielson, E. (2008). Dyspnea experience in patients with lung cancer in palliative care. European Journal of Oncology Nursing, 12, 86–96. https://doi.org/10.1016/j.ejon.2007.09.006
Hui, D., Titus, A., Curtis, T., Ho-Nguyen, V.T., Frederickson, D., Wray, C., . . . Rieber, A. (2017). Implementation of the Edmonton Symptom Assessment System for symtpom distress screening at a community cancer center: A pilot program. Oncologist, 22, 995–1001.
Humphreys, J., Janson, S., Donesky, D., Dracup, K., Lee, K.A., Puntillo, K., & Kennedy, C. (2014). Theory of symptom management. In M.J. Smith, & P. Liehr (Eds.), Middle range theory for nursing (pp. 141–164). New York, NY: Springer.
Jarden, M., Nelausen, K., Hovgaard, D., Boesen, E., & Adamsen, L. (2009). The effect of a multimodal intervention on treatment-related symptoms in patients undergoing hematopoietic stem cell transplantation: A randomized controlled trial. Journal of Pain and Symptom Management, 38, 174–190. https://doi.org/10.1016/j.jpainsymman.2008.09.005
Kirkova, J., Davis, M.P., Walsh, D., Tiernan, E., O’Leary, N., LeGrand, S.B., . . . Russell, K.M. (2006). Cancer symptom assessment instruments: A systematic review. Journal of Clinical Oncology, 24, 1459–1473. https://doi.org/10.1200/JCO.2005.02.8332
Körner, P., Ehrmann, K., Hartmannsgruber, J., Metz, M., Steigerwald, S., Flentje, M., & van Oorschot, B. (2017). Patient-reported symptoms during radiotherapy: Clinically relevant symptom burden in patients treated with palliative and curative intent. Strahlentherapie und Onkologie, 193, 570–577. https://doi.org/10.1007/s00066-017-1146-5
Kutner, J.S., Smith, M.C., Corbin, L., Hemphill, L., Benton, K., Mellis, B.K., . . . Fairclough, D.L. (2008). Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: A randomized trial. Annals of Internal Medicine, 149, 369–379.
Kwekkeboom, K.L., Kneip, J., & Pearson, L. (2003). A pilot study to predict success with guided imagery for cancer pain. Pain Management Nursing, 4, 112–123. https://doi.org/10.1016/S1524-9042(02)54213-2
Lang, H., France, E., Williams, B., Humphris, G., & Wells, M. (2013). The psychological experience of living with head and neck cancer: A systematic review and meta-synthesis. Psycho-Oncology, 22, 2648–2663. https://doi.org/10.1002/pon.3343
Lenz, E.R., Pugh, L.C., Milligan, R.A., Gift, A., & Suppe, F. (1997). The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science, 19(3), 14–27.
Lewis, L., Williams, A.M., Athifa, M., Brown, D., Budgeon, C.A., & Bremner, A.P. (2015). Evidence-based self-care guidelines for people receiving chemotherapy: Do they reduce symptom burden and psychological distress? Cancer Nursing, 38, E1–E8. https://doi.org/10.1097/NCC.0000000000000154
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLOS Medicine, 6(7), e1000100. https://doi.org/10.1371/journal.pmed.1000100
McCorkle, R., & Young, K. (1978). Development of a symptom distress scale. Cancer Nursing, 1, 373–378.
Miaskowski, C. (2006). Symptom clusters: Establishing the link between clinical practice and symptom management research. Supportive Care in Cancer, 14, 792–794.
Miaskowski, C., Aouizerat, B.E., Dodd, M., & Cooper, B. (2007). Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. Journal of the National Cancer Institute. Monographs, 37, 39–46. https://doi.org/10.1093/jncimonographs/lgm003
Miaskowski, C., Dodd, M., & Lee, K. (2004). Symptom clusters: The new frontier in symptom management research. Journal of the National Cancer Institute. Monographs, 32, 17–21. https://doi.org/10.1093/jncimonographs/lgh023
Oksholm, T., Rustoen, T., Cooper, B., Paul, S.M., Solberg, S., Henriksen, K., . . . Miaskowski, C. (2015). Trajectories of symptom occurrence and severity from before through five months after lung cancer surgery. Journal of Pain and Symptom Management, 49, 995–1015. https://doi.org/10.1016/j.jpainsymman.2014.11.297
Park, H., Qin, R., Smith, T.J., Atherton, P.J., Barton, D.L., Sturtz, K., . . . Loprinzi, C.L. (2015). North Central Cancer Treatment Group N10C2 (Alliance): A double-blind placebo-controlled study of magnesium supplements to reduce menopausal hot flashes. Menopause, 22, 627–632. https://doi.org/10.1097/GME.0000000000000374
Parshall, M.B., Schwartzstein, R.M., Adams, L., Banzett, R.B., Manning, H.L., Bourbeau, J., . . . O’Donnell, D.E. (2012). An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine, 185, 435–452. https://doi.org/10.1164/rccm.201111-2042ST
Penrod, J.D., Garrido, M.M., McKendrick, K., May, P., Aldridge, M.D., Meier, D.E., . . . Morrison, R.S. (2017). Characteristics of hospitalized cancer patients referred for inpatient palliative care consultation. Journal of Palliative Medicine. Advance online publication. https://doi.org/10.1089/jpm.2017.0111
Portenoy, R.K., Thaler, H.T., Kornblith, A.B., Lepore, J.M., Friedlander-Klar, H., Kiyasu, E., . . . Scher, H. (1994). The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer, 30A, 1326–1336.
Rhodes, V.A., McDaniel, R.W., Homan, S.S., Johnson, M., & Madsen, R. (2000). An instrument to measure symptom experience. Symptom occurrence and symptom distress. Cancer Nursing, 23, 49–54.
Rhodes, V.A., & Watson, P.M. (1987). Symptom distress—The concept: Past and present. Seminars in Oncology Nursing, 3, 242–247.
Sarna, L. (1998). Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncology Nursing Forum, 25, 1041–1048.
Serdà, B.C., & Marcos-Gragera, R. (2014). Urinary incontinence and prostate cancer: A progressive rehabilitation program design. Rehabilitation Nursing, 39, 271–280.
Tantoy, I.Y., Dhruva, A., Cataldo, J., Venook, A., Cooper, B.A., Paul, S.M., . . . Miaskowski, C. (2017). Differences in symptom occurrence, severity, and distress ratings between patients with gastrointestinal cancers who received chemotherapy alone or chemotherapy with targeted therapy. Journal of Gastrointestinal Oncology, 8, 109–126. https://doi.org/10.21037/jgo.2017.01.09
Tishelman, C., Degner, L.F., Rudman, A., Bertilsson, K., Bond, R., Broberger, E., . . . Levealahti, H. (2005). Symptoms in patients with lung carcinoma: Distinguishing distress from intensity. Cancer, 104, 2013–2021. https://doi.org/10.1002/cncr.21398
Tishelman, C., Petersson, L.M., Degner, L.F., & Sprangers, M.A. (2007). Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. Journal of Clinical Oncology, 25, 5381–5389.
van Weert, E., Hoekstra-Weebers, J., Otter, R., Postema, K., Sanderman, R., & van der Schans, C. (2006). Cancer-related fatigue: Predictors and effects of rehabilitation. Oncologist, 11, 184–196.
Wilkie, D.J., Huang, H.Y., Reilly, N., & Cain, K.C. (2001). Nociceptive and neuropathic pain in patients with lung cancer: A comparison of pain quality descriptors. Journal of Pain and Symptom Management, 22, 899–910.
Wong, M.L., Cooper, B.A., Paul, S.M., Levine, J.D., Conley, Y.P., Wright, F., . . . Miaskowski, C. (2017). Differences in symptom clusters identified using ratings of symptom occurrence vs. severity in lung cancer patients receiving chemotherapy. Journal of Pain and Symptom Management, 54, 194–203.
Yarbro, C.H., Frogge, M.H., & Goodman, M. (Eds.). (2004). Cancer symptom management. Sudbury, MA: Jones and Bartlett.




