Perceived Stress and the Fatigue Symptom Cluster in Childhood Brain Tumor Survivors
Objectives: To explore and estimate relationships among the elements of the symptom cluster in survivors of brain tumors aged 8–12 years during early survivorship.
Sample & Setting: Child participants completed treatment at least six months and less than six years prior to enrollment at Children’s Hospital of Alabama in Birmingham or Cook Children’s Medical Center in Fort Worth, Texas.
Methods & Variables: With cross-sectional methods, the authors measured child-perceived stress, sleep–wake disturbance (SWD) (parent report), and fatigue. Children also provided saliva samples for cortisol measurement (stress response) and completed actigraphy sleep monitoring.
Results: Mild to moderate stress, SWD, and fatigue were reported, and a wide range of sleep times and cortisol levels were noted. Meaningful effect sizes in relationships between variables were found.
Implications for Nursing: The stress, SWD, and fatigue symptom cluster in survivorship necessitates routine nursing assessment.
Jump to a section
In 2015, 21,894 children aged 0–19 years were living with primary brain and other central nervous system (CNS) tumors in the United States, with an estimated 49.4 per million diagnosed from 2011–2015. During this time, survival rates have increased to about 75% (National Cancer Institute [NCI], 2017b). This emerging population has potentially serious or burdensome long-term health effects from cancer and cancer treatment (Hobbie et al., 2016; Meeske, Patel, Palmer, Nelson, & Parow, 2007). Common symptoms and symptom clusters, such as psychological stress, sleep–wake disturbance (SWD), and fatigue, have been noted in children and adolescents on and off treatment for a variety of malignancies, including CNS, leukemia, and solid tumors (Gordijn et al., 2013; Olson, 2014; Tomlinson et al., 2016). Similar findings have been seen in adolescent and young adult survivors of non-CNS malignancies (Daniel et al., 2016; Desaulniers et al., 2015). Few studies explore biobehavioral interactions within the stress, SWD, and fatigue symptom cluster, particularly in childhood brain tumor survivors (Gordijn et al., 2012). Findings from the large longitudinal Childhood Cancer Survivor Study indicate that psychological distress predicts fatigue and SWD and that there is a 30% greater chance of childhood brain tumor survivors having comorbid distress (both affective and somatic) in adulthood compared to survivors of other childhood cancers (D’Agostino et al., 2016). In childhood, fatigue and related symptoms of SWD and stress also have been seen in survivors of acquired and traumatic brain injury and are correlated with lowered quality of life and functioning (Crichton et al., 2018; Wilkinson et al., 2018).
The study of psychological and physiologic stress is important in the exploration of related symptom clusters, aligns with the goals of symptom science research, and can improve care after treatment for childhood brain tumors (Miaskowski et al., 2017). A biobehavioral perspective provides a multidimensional view of symptoms (Marques, Silverman, & Sternberg, 2010) and contributes to the body of knowledge of the child survivorship experience across critical years for growth and development. In addition, exploring components of stress in survivors is beneficial because perception of stress and response to stress may be different after the cancer experience (Bower, Ganz, & Aziz, 2005; Costanzo, Stawski, Ryff, Coe, & Almeida, 2012).
Data were collected and provided insight into the experience of stress in young survivors of childhood brain tumors. Specific aims included estimations of the magnitude (effect size) of the relationships between child-perceived psychological stress, physiologic stress response (salivary cortisol measurement), SWD, and cancer-related fatigue (CRF). A secondary objective was to describe biobehavioral characteristics of the population during early survivorship.
Methods
An exploratory, correlational, and cross-sectional method was used to address the study aims. Instruments were chosen based on reliability and validity when used in the study population and the conceptual framework, which posited perceived stress and SWD as independent variables, physiologic stress response (cortisol change) as a mediator, and CRF as the outcome (see Figure 1). The feasibility of school-age children and their parents completing the study protocol was determined based on findings from a feasibility study involving five parent and child dyads (Johnson, Rice, & Avis, 2014). In addition to the study variables, selected demographic variables included time (months) since treatment, and diagnostic and treatment information (chemotherapy, radiation therapy, and/or surgical resection). 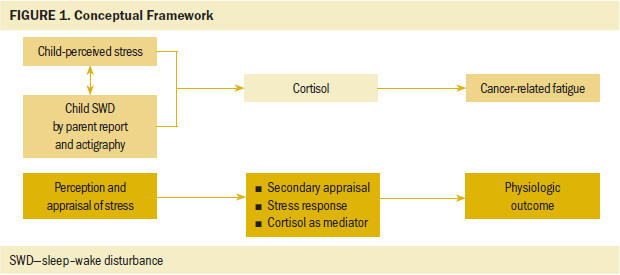
Sample and Setting
The convenience sample consisted of 21 children aged 8–12 years who received follow-up care in Children’s Hospital of Alabama in Birmingham or Cook Children’s Medical Center in Fort Worth, Texas. Inclusion criteria were as follows: (a) children aged 8–12 years diagnosed with a brain tumor; (b) completion of treatment at least six months but less than six years prior to recruitment; (c) children tumor-free or with a stable residual tumor not requiring treatment; (d) one consenting parent; (e) child and parent able to understand, speak, and respond in English; and (f) child and parent able to respond to instruments and follow instructions. Potential child participants with recurrent or active cancer were excluded.
The timing of recruitment in relation to completion of treatment was based on the NCI (2017a) guidelines for post-treatment CRF. The age group was selected because these children may experience the disease process differently than children in other age groups, particularly in areas of academic achievement, social development, stress, and coping (Aldwin, 2007; Davies, 2004; Dixon & Stein, 2006; Stam, Grootenhuis, Brons, Caron, & Last, 2006). Also, an increased incidence of CNS cancer diagnosis exists in children from birth to school age compared to adolescents (Howlader et al., 2014).
The study sites were outpatient clinics of the participants’ treatment center. Recruitment began after approval from University of Alabama at Birmingham and Cook Children’s Medical Center, and targeted patients were screened with inclusion criteria by staff members at the study site. The goal for recruitment was 30 participants, based on several feasibility factors: (a) the overall incidence of CNS cancer in children at birth through 19 years is 2,503 annually in the United States (NCI, n.d.; Ostrom et al., 2013), (b) the average annual incidence in the recruitment locations (NCI, n.d.; Ostrom et al., 2013), (c) the number of study participants at the two study sites, and (d) the expected medium to large effect sizes based on previous studies of CNS and non-CNS childhood cancer survivor SWD, stress, and fatigue (Gordijn et al., 2012, 2013; Verberne, Maurice-Stam, Grootenhuis, Van Santen, & Schouten-Van Meeteren, 2012).
Child-Perceived Stress
Perceived stress was measured using the Feel Bad Scale (FBS), a 40-item self-report instrument using a Likert-type scale ranging from 1 to 5 for questions related to the magnitude and frequency of stressful events (Lewis, Siegel, & Lewis, 1984). The FBS is aimed at exploring common sources of stress in a child’s life (Lewis et al., 1984). This tool has validity and reliability in diverse school-age populations (Jenkins, Rew, & Sternglanz, 2005; Lewis et al., 1984; Taxis, Rew, Jackson, & Kouzekanani, 2004). Reliability in the current study was 0.635, which may have been influenced by the difficulty for some participants to understand or complete a relatively lengthy questionnaire. These scores reflect recall of stress during the past year but are not meant to indicate a clinical diagnosis of anxiety or post-traumatic stress. Rather, this recall of stress reflects child feelings regarding issues such as experiencing family or school-related stress, feeling sick, being bigger or smaller than peers, and not being good enough at sports (Lewis et al., 1984).
Child Physiologic Stress
Stress includes subjective (perceived) and objective (physiologic) responses to a stressor (Aldwin, 2007). These responses are the body’s nonspecific reaction to life events, physical events, or adversity, and involve the hypothalamic pituitary adrenal (HPA) axis stress hormones, including cortisol (Selye, 1950). Salivary cortisol is highly correlated (0.9, p < 0.001) with plasma cortisol levels (Dorn, Lucke, Loucks, & Berga, 2007; Kirschbaum & Hellhammer, 1989; Woodside, Winter, & Fisman, 1991). Salivary cortisol was analyzed using an established assay technique (Beko et al., 2010; Salimetrics, 2016). The two saliva collection time frames (i.e., 10–11 am and 1–4 pm) were based on the normal expected diurnal pattern of cortisol (Salimetrics, 2016; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012), where the typical pattern includes a decrease from early morning to midafternoon. This type of pattern is described in a study of salivary cortisol from twins that revealed findings of genetic and environmental influences on morning to evening cortisol changes (Van Hulle et al., 2012). The pattern was also described in a large meta-analysis of diurnal salivary cortisol levels, which established reference ranges (Miller et al., 2016). In the meta-analysis, mean morning cortisol levels of children aged 8–12 years ranged from 0.08–0.14 mcg/dl (at 3.5 hours postawakening) and afternoon levels ranged from 0.05–0.08 mcg/dl (6 hours after awakening) and were age- and sex-dependent (Miller et al., 2016).
Sleep–Wake Disturbance
SWD was measured objectively with child actigraphy monitoring. Actigraphy is correlated (85%) with the gold standard polysomnography and validated in adults and children using an electrophysiologic wristwatch-like movement-monitoring device that measures one-minute epochs to determine total sleep time (TST), wake after sleep onset (WASO), and a sleep efficiency (SE) ratio (de Souza et al., 2003; Hyde et al., 2007; Sadeh, Lavie, Scher, Tirosh, & Epstein, 1991; So, Adamson, & Horne, 2007).
In addition, parents completed a daily sleep log that served as a method to check actigraphy software output for missing or incorrect data. Actigraphy parameters can be compared to estimated norms. For example, mean parent-reported TST for children aged 8–12 years was 9–10.5 hours per 24-hour time period in a longitudinal study of 493 participants aged six months to 16 years (Iglowstein, Jenni, Molinari, & Largo, 2003). Actigraphy TST means for this age group can also be used for comparison and were reported to be about eight hours in a cohort study by Kuula et al. (2016).
Exploration of subjective SWD was measured with the Children’s Sleep Habits Questionnaire (CSHQ), a 33-item parent report for child sleep disturbances, which asks for recall of child sleep during the most recent typical week (Owens, Spirito, & McGuinn, 2000). Original psychometric analysis of the CSHQ showed adequate internal consistency and reliability for a community sample of healthy children aged 4–10 years (alpha = 0.68) and a clinical sample (alpha = 0.78) of patients (mean age = 6.8 years) referred to a sleep clinic (Owens et al., 2000). Subsequent analysis for construct validity through correlation with actigraphy by Markovich, Gendron, and Corkum (2015) revealed low correlation between CSHQ subscales and corresponding sleep variables. This instrument demonstrated high reliability in the current sample (alpha = 0.83).
Cancer-Related Fatigue
CRF was measured with the PedsQL Multidimensional Fatigue Scale (MDFS), an 18-item (8–12 years version) self-report of recall during the past month, with three subscales: General Fatigue, Sleep/Rest Fatigue, and Cognitive Fatigue (Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). Internal consistency of 0.88 (total fatigue) and 0.74–0.79 (subscales) was noted in a study of patients with leukemia, brain tumor, and Wilms tumor (n = 220), and in a sample (n = 52) of healthy children (Varni et al., 2002). Construct validity was evidenced by significant differences between healthy and cancer populations in total fatigue, general fatigue, sleep/rest fatigue, and cognitive fatigue (Varni et al., 2002). Reliability in the current study was high (alpha = 0.85).
Procedures
On the day of the study visit, assent and consent were obtained in a quiet area of the clinic (e.g., waiting room, examination room), working around scheduled appointments. The morning salivary sample was collected using a passive drool technique, about 10 minutes after rinsing the mouth, and then the child and parent completed pen-and-paper study questionnaires. The investigator read questionnaires to the child participants to ensure understanding of the items and to enhance validity (Johnson et al., 2014). The child and parent were then free to attend other clinic visits or eat a light lunch. They returned in the afternoon for a second saliva collection. Children and parents were each given an incentive gift card and instructions regarding the seven-day home actigraphy monitoring period and how to return the monitor by insured mail.
The saliva samples were immediately placed in portable cold storage and transported within four hours to –4°F (–20°C) or lower storage for batching until all study samples were collected. After laboratory analysis using the ELISA method, stress response (cortisol change) was calculated for each participant by subtracting the afternoon cortisol level from the morning level to get a difference in micrograms per deciliter (mcg/dl), the standard unit of measurement of salivary cortisol (Beko et al., 2010). Intra-assay validity was improved by duplicating the laboratory analysis on each saliva sample and reporting the mean.
Data Management and Analysis
Data were managed and analyzed using IBM SPSS Statistics, version 21.0. Bivariate correlation was used to estimate effect sizes and confidence intervals (CIs) for these coefficients. The magnitude of the correlations was interpreted using Cohen’s (1988, 1992) general guidelines, which specify 0.1, 0.3, and 0.5 as small, medium, and large, respectively. CIs helped to describe the plausibility of the effect sizes given the sample size, using 0.25 as the clinical relevance threshold value. Actigraphy data were scored using the default medium sensitivity threshold (40 counts per epoch) with Actiware, version 6.0.0. 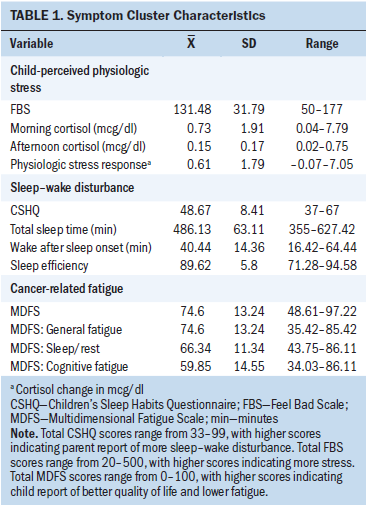
Results
Descriptive statistics included frequencies and means, standard deviations and ranges for ethnicity, tumor types, treatment, perceived stress scores, cortisol levels, SWD, and CRF scores (see Table 1). Most participants were White, and men and women were equally represented. Tumor locations varied, with four tumors in the sellar and suprasellar (above the sella turcica) region. Tumor pathology varied as well. The participants were an average of 52 months postdiagnosis and 39.6 months post-treatment at recruitment (SD = 21.1, range = 6–80), with no outliers detected using box plot graphs (see Table 2). 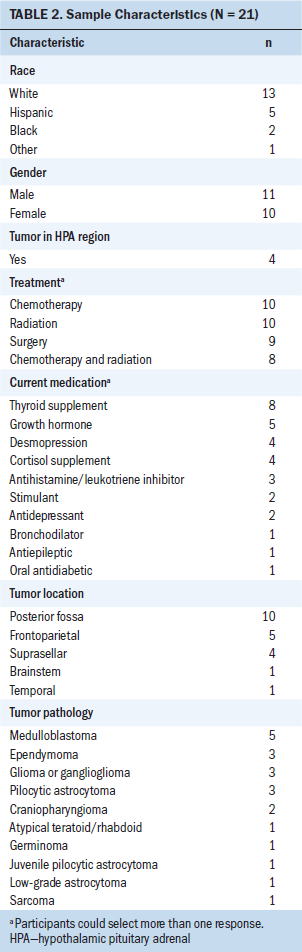
Child-Perceived and Physiologic Stress
FBS scores ranged from 50–177 (out of a possible score of 20–500), with a mean score of 131.48 (higher scores reflected more stress). The physiologic stress response mean was 0.610625, with a standard deviation of 1.7997694. Cortisol levels in the morning ranged from 0.04–7.8 mcg/dl and fell outside the expected range of values for children aged 8–12 years, even after participants on cortisol supplement were excluded (Miller et al., 2016). The same was true for afternoon levels, which ranged from 0.02–0.75 mcg/dl.
Five participants were excluded from the physiologic stress response calculations because of mistiming or missed saliva sampling; as a result, cortisol calculations were reported for 16 of 21 participants. Four participants were on cortisol supplements, three of whom had an elevated stress response (cortisol change from morning to afternoon) compared to the rest of the sample. Of participants not on cortisol supplement, four had higher afternoon versus morning cortisol and nine had lower afternoon versus morning cortisol. None remained the same. On a case-by-case review, participants with the altered stress response (higher afternoon versus morning) had the following characteristics:
• None on cortisol supplement
• None with tumors in HPA region
• None with recurrence
• 20–54 months since treatment
• Two with no chemotherapy or radiation therapy
• One with chemotherapy and radiation therapy
• One with chemotherapy only
• Perceived stress (FBS) scores of 107–177
Sleep–Wake Disturbance
CSHQ scores ranged from 37–67 out of a possible 33–99, with higher scores indicating parent report of more SWD. The mean score was 48.67, with a standard deviation of 8.41. No official cutoff points or normal scores have been established for the CSHQ; therefore, these scores are most useful for comparisons and correlations (Owens et al., 2000). Actigraphy measurement of sleep parameters mirrored the parent report in that there was a wide range of scores for TST (355–642 minutes), WASO (10–70 minutes), and SE (71.28%–95%).
Cancer-Related Fatigue Symptom Cluster
The total score for CRF (MDFS) was 49–97 out of a possible 0–100, with higher scores representing better quality of life and lower fatigue. Overall, these scores indicate at most a moderate degree of CRF from the child’s perspective and had high reliability (Cronbach alpha = 0.85). The overall score can be broken down into three subscales (see Table 3). 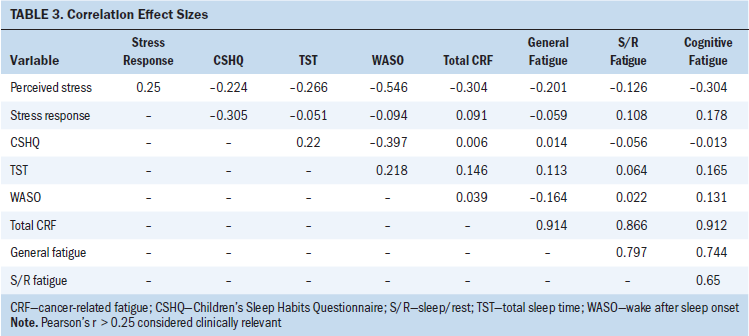
Among the study variables were meaningful effect sizes of child perceived stress with total CRF and cognitive fatigue (r = –0.304; 95%% CI [–0.65, 0.146]), WASO (r = –0.546; 95% CI [–0.834, –0.022]), and TST (r = 0.266; 95% CI [–0.187, 0.625]). The relationship between perceived stress and stress response had a small to medium effect size (r = 0.25; 95% CI [–0.28, 0.663]). There was also a meaningful effect size between subjective (parent report) SWD and stress response (r = –0.305; 95% CI [–0.719, 0.269]).
Discussion
The study sample reflects characteristics of the population of childhood brain tumor survivors, including ethnicity and tumor type (Siegel, Li, & Singh, 2017), and findings indicate the presence of perceived stress, SWD, altered cortisol pattern, and fatigue during early survivorship. Interestingly, suprasellar tumors were overrepresented compared to usual incidence (Müller, 2014). This tumor location is significant not only because of the effect on the crucial hypothalamic and pituitary region, but also because tumors in this location are usually not amenable to complete resection and require adjuvant treatment (Frim & Gupta, 2006).
Based on perceived stress scores, child participants had small to moderate amounts of perceived stress during the past year after being five years off treatment. These findings are congruent with previous findings by Haase and Rostad (1994) of continued fears and concerns that children express within the first year after completing cancer therapy, and is relevant to potential effects that this stress may have on psychological health and quality of life 5–10 years after diagnosis (Chen, Chen, & Haase, 2008; Marsland, Ewing, & Thompson, 2006). There is a paucity of findings for comparison of childhood survivor stress using the FBS (Bruce, Gumley, Isham, Fearon, & Phipps, 2011).
The physiologic stress indicator cortisol had overall higher than expected levels, indicative of prolonged stress during the course of the day. Four of the 16 participants included in cortisol analysis had higher afternoon versus morning salivary cortisol levels. None of them were receiving cortisol supplementation. This rise in afternoon cortisol differs from a typical cortisol pattern in children, which decreases from morning to afternoon (Dorn et al., 2007; Kiess et al., 1995). However, it is similar to a change seen in school-age children (without cancer) exposed to early life trauma or recurrent stress (Bevans, Cerbone, & Overstreet, 2008), in infants and young children with difficult temperaments in daycare (Geoffroy, Côté, Parent, & Séguin, 2006), and in children during a hospital specialty clinic (non-oncology) visit that included an IV line placement (McCarthy et al., 2009). In addition, a similar cortisol pattern was seen in children who attended long hours at preschool (Lumian, Dmitrieva, Mendoza, Badanes, & Watamura, 2016). In the study by Lumian et al. (2016), the altered cortisol pattern continued even on days when the longer-attending preschoolers were at home.
The role individual factors played in the altered cortisol pattern is unknown. Inter-individual differences have been seen in a large community cohort study of children aged 10–12 years (Rosmalen et al., 2005). In addition, the role cortisol played in the symptom relationships in the current study is unknown because the sample size limited more in-depth analysis.
Notable sequelae from chemotherapy or craniospinal radiation treatment of brain tumors include ototoxicity, peripheral neuropathy, endocrine dysfunction, and neurocognitive changes (Children’s Oncology Group, 2013). This creates a symptom burden and may be related to the sample’s perceived stress and physiologic stress response. This relationship fits with the assumption that stress is a physiologic response to a stressor, that stress activates the HPA axis (Selye, 1950), and that cancer and cancer treatment are stressors (Hockenberry-Eaton, Kemp, & Dilorio, 1994). The small positive effect size in the relationship between perceived stress and stress response could be interpreted using the Transactional Theory of Stress and Coping, which incorporates individual cognitive appraisal of stressors (Lazarus & Folkman, 1984).
The only study variable with a meaningful relationship with CRF was child-perceived stress. Children with higher perceived stress scores had higher fatigue. Negative effect sizes with CRF based on the PedsQL MDFS need to be interpreted with the knowledge that decreased CRF scores indicate increased fatigue, and that the CRF and perceived stress scores were both based on child report. A possible link between stress and CRF was also reported by Ruccione, Lu, and Meeske (2013); adolescents in the first six months after completion of cancer treatment who reported fatigue and post-traumatic stress symptoms were more likely to have lower psychosocial function (Ruccione et al., 2013).
The associations between stress and SWD are also of interest. Relevant effect sizes were observed between child perceived stress and objective SWD, specifically TST and WASO; the higher the perceived stress, the lower the TST and WASO. There was also a lower magnitude but a positive effect size of the relationship between perceived stress and subjective (parent report) SWD. Perhaps objective measurement of sleep captures symptoms of SWD better than parent observation. These findings can be compared to those of Ly, McGrath, and Gouin (2015), who noted a negative relationship between perceived stress and child report of sleep duration and SWD in healthy children aged 8–18 years. It is also noteworthy that there was a clinically relevant effect size between subjective (parent report) SWD and stress response, indicating that despite a lack of perceived stress related to SWD, there may be a physiologic response affecting sleep.
One of the study’s primary limitations was the small sample size. The goal of recruiting 30 participants for enrollment was not achieved after 18 months of recruitment. Although not statistically powered, results indicate the effect that perceived and physiologic stress may have on each other, as well as on SWD and CRF. Findings also reflect the presence of these symptoms in the post-treatment, early survivorship period.
Implications for Nursing
Childhood brain tumor survivors need comprehensive survivorship care, not just for the expected treatment effects, but also for factors such as stress-related SWD and fatigue problems during the early years after treatment. As found in this study, children report the presence of fatigue and perceived stress, and their parents report child SWD. Child salivary analysis reveals abnormal cortisol patterns in some participants; the cause of this abnormal stress response is unclear. These problems may be ameliorated through assessment and anticipatory guidance beginning during the tumor treatment phase. Guidance could include tools for parents and providers to help recognize and manage symptoms and may involve integrative therapy such as relaxation techniques and sleep hygiene to promote coping.
Given the small sample size, it is difficult to draw definitive conclusions from the study; therefore, this study should be repeated with a larger sample. Future research should test interventions for fatigue in brain tumor survivors, such as has been done with physical activity in children on maintenance treatment for leukemia (Hooke, Gilchrist, Tanner, Hart, & Withycombe, 2016) and in cancer survivors aged 8–12 years engaged in group physical activity (Ruble, Scarvalone, Gallicchio, Davis, & Wells, 2016). Interventions related to physical activity may be particularly important given evidence for inadequate activity in childhood brain tumor survivors (Valentino, 2018). Comprehensive survivorship care that addresses biobehavioral issues may also aid in primary prevention of disease sequelae.
Measurement of perceived psychological stress and HPA function to ascertain physiologic stress response was feasible in this study and fills a gap in symptom science research in young survivors of childhood brain tumors. Child-perceived stress scores in the current study provide insight into a variable elusive to measurement in a specific population. The current study also provides preliminary evidence that altered cortisol patterns may exist in childhood brain tumor survivors, apart from the effects of cortisol supplementation, tumor type, or treatment.
The child experiencing stress and SWD may also experience a negative effect on normal development. High levels of perceived stress and altered physiologic stress response in a developing child may affect neurocognitive development (Demir-Lira et al., 2016; Finegood, Wyman, O’Connor, & Blair, 2017) and be related to depression in adulthood (Björkenstam et al., 2015; Weber et al., 2008). Similarly, the effects of SWD on child development are deleterious (Dewald, Meijer, Oort, Kerkhof, & Bögels, 2010; Kurth et al., 2016), leading to neurocognitive or behavioral problems in adulthood (Cheung et al., 2017). The results of this study add evidence to earlier studies and illuminate the importance of exploring symptoms in a special population. 
Conclusion
This study demonstrates a biobehavioral approach to describe the stress experience through the lens of an individual age group, focusing on a specific cancer survivorship phase. Child brain tumor survivors are at a crossroads of their disease experience during early survivorship. This population may be dealing not only with residual treatment effects or the emergence of late effects of treatment, but also the hard-to-define symptoms of stress, SWD, and fatigue. Preliminary evidence (effect sizes) suggests a link between perceived stress and CRF, as well as links between perceived stress and SWD, and perceived stress and stress response. These links support future inquiry into the nature of this symptom cluster and provide insight into the young survivor experience. This knowledge could lead to improved awareness of the potential effects of stress on the child and prolonged fatigue during the cancer experience during and after treatment.
The authors would like to acknowledge the instrumental assistance of the study site champions, Dr. Lisa Bashore and Ashleigh Hines at Cook Children's, and Dr. Alyssa Reddy, Dr. Kristin Avis, and the Sleep Lab at Children's of Alabama.
About the Author(s)
Ann Hammack Johnson, PhD, APRN, CPNP-PC, is an assistant professor in the Harris College of Nursing and Health Sciences at Texas Christian University in Fort Worth; Marti Rice, PhD, RN, FAAN, is a professor and Anne Turner-Henson, PhD, RN, FAAN, is a retired professor, both in the School of Nursing at the University of Alabama in Birmingham; Joan E. Haase, PhD, RN, FAAN, is a Holmquist Professor of Pediatric Oncology Nursing in the School of Nursing at Indiana University in Indianapolis; and Andres Azuero, PhD, is an associate professor in the School of Nursing at the University of Alabama. This research was funded by the HRSA Maternal Child Health Bureau funded Leadership Education in Child Health Nursing Fellowship (T80MC09653) awarded by the University of Alabama School of Nursing, and by a small grant award from Texas Woman’s University and a Corrine Barnes Nursing Research Grant from the Society of Pediatric Nurses. Johnson, Rice, Turner-Henson, and Haase contributed to the conceptualization and design and the manuscript preparation. Johnson completed the data collection. Rice, Turner-Henson, and Azuero provided statistical support. Johnson, Rice, Turner-Henson, and Azuero provided the analysis. Johnson can be reached at ann.h.johnson@tcu.edu, with copy to ONFEditor@ons.org. (Submitted March 2018. Accepted June 25, 2018.)
References
Aldwin, C.M. (2007). Stress, coping, and development (2nd ed.). New York, NY: Guilford Press.
Beko, G., Varga, I., Glaz, E., Sereg, M., Feldman, K., Toth, M., . . . Patocs, A. (2010). Cutoff values of midnight salivary cortisol for the diagnosis of overt hypercortisolism are highly influenced by methods. Clinica Chimica Acta, 411, 364–367. https://doi.org/10.1016/j.cca.2009.11.033
Bevans, K., Cerbone, A., & Overstreet, S. (2008). Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Development and Psychopathology, 20, 257–272. https://doi.org/10.1017/s0954579408000126
Björkenstam, E., Burström, B., Brännström, L., Vinnerljung, B., Björkenstam, C., & Pebley, A.R. (2015). Cumulative exposure to childhood stressors and subsequent psychological distress. An analysis of US panel data. Social Science and Medicine, 142, 109–117. https://doi.org/10.1016/j.socscimed.2015.08.006
Bower, J.E., Ganz, P.A., & Aziz, N. (2005). Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine, 67, 277–280. https://doi.org/10.1097/01.psy.0000155666.55034.c6
Bruce, M., Gumley, D., Isham, L., Fearon, P., & Phipps, K. (2011). Post-traumatic stress symptoms in childhood brain tumour survivors and their parents. Child: Care, Health and Development, 37, 244–251. https://doi.org/10.1111/j.1365-2214.2010.01164.x
Chen, C.-M., Chen, Y.-C., & Haase, J.E. (2008). Games of lives in surviving childhood brain tumors. Western Journal of Nursing Research, 30, 435–457. https://doi.org/10.1177/0193945907303050
Cheung, Y.T., Brinkman, T.M.. Mulrooney, D.A., Mzayek, Y, Liu, W., Banerjee, P., . . . Krull, K.R. (2017). Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer, 123, 3410–3419. https://doi.org/10.1002/cncr.30742
Children’s Oncology Group. (2013). Long-term follow-up gudielines for survivors of childhood, adolescent, and young adult cancer [v.4.0]. Retrieved from http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf
Cohen, J. (1988). The concept of power analysis. In Statistical power analysis for the behavioral sciences (2nd ed.). Mahwah, NJ: Lawrence Erlbaum Associates.
Cohen, J. (1992). Quantitative methods in psychology: A power primer. Psychological Bulletin, 112, 155–159.
Costanzo, E.S., Stawski, R.S., Ryff, C.D., Coe, C.L., & Almeida, D.M. (2012). Cancer survivors’ responses to daily stressors: Implications for quality of life. Health Psychology, 31, 360–370. https://doi.org/10.1037/a0027018
Crichton, A., Oakley, E., Babl, F.E., Greenham, M., Hearps, S., Delzoppo, C., . . . Anderson, V. (2018). Predicting fatigue 12 months after child traumatic brain injury: Child factors and postinjury symptoms. Journal of the International Neuropsychological Society, 24, 224–236. https://doi.org/10.1017/S1355617717000893
D’Agostino, N.M., Edelstein, K., Zhang, N., Recklitis, C.J., Brinkman, T.M., Srivastava, D., . . . Krull, K.R. (2016). Comorbid symptoms of emotional distress in adult survivors of childhood cancer. Cancer, 122, 3215–3224. https://doi.org/10.1002/cncr.30171
Daniel, L., Kazak, A.E., Li, Y., Hobbie, W., Ginsberg, J., Butler, E., & Schwartz, L. (2016). Relationship between sleep problems and psychological outcomes in adolescent and young adult cancer survivors and controls. Supportive Care in Cancer, 24, 539–546. https://doi.org/10.1007/s00520-015-2798-2
Davies, D. (2004). Child development: A practitioner’s guide (2nd ed.). New York, NY: Guilford Press.
de Souza, L., Benedito-Silva, A.A., Pires, M.L., Poyares, D., Tufik, S., & Calil, H.M. (2003). Further validation of actigraphy for sleep studies. Sleep, 26, 81–85.
Demir-Lira, Ö.E., Voss, J.L., O’Neil, J.T., Briggs-Gowan, M.J., Wakschlag, L.S., & Booth, J.R. (2016). Early-life stress exposure associated with altered prefrontal resting-state fMRI connectivity in young children. Developmental Cognitive Neuroscience, 19, 107–114. https://doi.org/10.1016/j.dcn.2016.02.003
Dewald, J.F., Meijer, A.M., Oort, F.J., Kerkhof, G.A., & Bögels, S.M. (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews, 14, 179–189. https://doi.org/10.1016/j.smrv.2009.10.004
Desaulniers, G., Riley, L., Vangle, K., Gilleland, J., Higgins, M., & Wasilewski-Masker, K. (2015). Self-reported sleep problems in adolescent survivors of childhood cancer. Clinical Journal of Oncology Nursing, 19, 81–88. https://doi.org/10.1188/15.CJON.81-88
Dixon, S.D., & Stein, M.T. (2006). Seven to ten years: The world of middle childhood. In S. Dixon & M. Stein (Eds.), Encounters with children: Pediatric behavior and development (4th ed., pp. 504–534). Philadelphia, PA: Mosby.
Dorn, L.D., Lucke, J.F., Loucks, T.L., & Berga, S.L. (2007). Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Annals of Clinical Biochemistry, 44, 281–284. https://doi.org/10.1258/000456307780480954
Finegood, E.D., Wyman, C., O’Connor, T.G., & Blair, C.B. (2017). Salivary cortisol and cognitive development in infants from low-income communities. Stress, 20, 112–121. https://doi.org/10.1080/10253890.2017.1286325
Frim, D., & Gupta, N. (2006). Pediatric neurosurgery. Georgetown, TX: Landes Bioscience.
Geoffroy, M.-C., Côté, S.M., Parent, S., & Séguin, J.R. (2006). Daycare attendance, stress, and mental health. Canadian Journal of Psychiatry, 51, 607–615. https://doi.org/10.1177/070674370605100909
Gordijn, M.S., van Litsenburg, R.R., Gemke, R.J.B.J., Bierings, M.B., Hoogerbrugge, P.M., van de Ven, P.M., . . . Kaspers, G.J.L. (2012). Hypothalamic-pituitary-adrenal axis function in survivors of childhood acute lymphoblastic leukemia and healthy controls. Psychoneuroendocrinology, 37, 1448–1456. https://doi.org/10.1016/j.psyneuen.2012.01.014
Gordijn, M.S., van Litsenburg, R.R., Gemke, R.J., Huisman, J., Bierings, M.B., Hoogerbrugge, P.M., & Kaspers, G.J.L. (2013). Sleep, fatigue, depression, and quality of life in survivors of childhood acute lymphoblastic leukemia. Pediatric Blood and Cancer, 60, 479–485. https://doi.org/10.1002/pbc.24261
Haase, J.E., & Rostad, M. (1994). Experiences of completing cancer therapy: Children’s perspectives. Oncology Nursing Forum, 21, 1483–1492.
Hobbie, W.L., Ogle, S., Reilly, M., Barakat, L., Lucas, M.S., Ginsberg, J.P., . . . Deatrick, J.A. (2016). Adolescent and young adult survivors of childhood brain tumors: Life after treatment in their own words. Cancer Nursing, 39, 134–143. https://doi.org/10.1097/NCC.0000000000000266
Hockenberry-Eaton, M., Kemp, V., & Dilorio, C. (1994). Cancer stressors and protective factors: Predictors of stress experienced during treatment for childhood cancer. Research in Nursing and Health, 17, 351–361.
Hooke, M.C., Gilchrist, L., Tanner, L., Hart, N., & Withycombe, J.S. (2016). Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatric Blood and Cancer, 63, 684–689. https://doi.org/10.1002/pbc.25860
Howlader, N., Noone, A.M., Krapcho, M., Garshell, J., Miller, D., Altekruse, S.F., . . . Cronin, K.A. (Eds.). (2014). SEER Cancer Statistics Review, 1975–2011. Retrieved from http://seer.cancer.gov/csr/1975_2011
Hyde, M., O’Driscoll, D.M., Binette, S., Galang, C., Tan, S.K., Verginis, N., . . . Horne, R.S.C. (2007). Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. Journal of Sleep Research, 16, 213–216.
Iglowstein, I., Jenni, O.G., Molinari, L., & Largo, R.H. (2003). Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics, 111, 302–307.
Jenkins, S.K., Rew, L., & Sternglanz, R.W. (2005). Eating behaviors among school-age children associated with perceptions of stress. Issues in Comprehensive Pediatric Nursing, 28, 175–191. https://doi.org/10.1080/01460860500227580
Johnson, A., Rice, M., & Avis, K. (2014, February). The feasibility of biobehavioral research in childhood central nervous system (CNS) cancer survivors. Paper presented at the 28th Annual Southern Nursing Research Society Conference, San Antonio, TX.
Kiess, W., Meidert, A., Dressendörfer, R.A., Schriever, K., Kessler, U., Köunig, A., . . . Strasburger, C.J. (1995). Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatric Research, 37, 502–506. https://doi.org/10.1203/00006450-199504000-00020
Kirschbaum, C., & Hellhammer, D.H. (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22, 150–169. https://doi.org/10.1159/000118611
Kurth, S., Dean, D.C., III, Achermann, P., O’Muircheartaigh, J., Huber, R., Deoni, S.C.L., & LeBourgeois, M.K. (2016). Increased sleep depth in developing neural networks: New insights from sleep restriction in children. Frontiers in Human Neuroscience, 10, 456. https://doi.org/10.3389/fnhum.2016.00456
Kuula, L., Pesonen, A.-K., Kajantie, E., Lahti, J., Andersson, S., Strandberg, T., & Räikkönen, K. (2016). Sleep and lipid profile during transition from childhood to adolescence. Journal of Pediatrics, 177, 173–178.e1. https://doi.org/10.1016/j.jpeds.2016.06.026
Lazarus, R.S., & Folkman, S. (1984). Stress, appraisal, and coping. New York: Springer.
Lewis, C.E., Siegel, J.M., & Lewis, M.A. (1984). Feeling bad: Exploring sources of distress among pre-adolescent children. American Journal of Public Health, 74, 117–122.
Lumian, D.S., Dmitrieva, J., Mendoza, M.M., Badanes, L.S., & Watamura, S.E. (2016). The impact of program structure on cortisol patterning in children attending out-of-home child care. Early Childhood Research Quarterly, 34, 92–103. https://doi.org/10.1016/j.ecresq.2015.09.004
Ly, J., McGrath, J.J., & Gouin, J.-P. (2015). Poor sleep as a pathophysiological pathway underlying the association between stressful experiences and the diurnal cortisol profile among children and adolescents. Psychoneuroendocrinology, 57, 51–60. https://doi.org/10.1016/j.psyneuen.2015.03.006
Markovich, A.N., Gendron, M.A., & Corkum, P.V. (2015). Validating the Children’s Sleep Habits Questionnaire against polysomnography and actigraphy in school-aged children. Frontiers in Psychiatry, 5, 188.
Marques, A.H., Silverman, M.N., & Sternberg, E.M. (2010). Evaluation of stress systems by applying noninvasive methodologies: Measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation, 17, 205–208. https://doi.org/10.1159/000258725
Marsland, A., Ewing, L., & Thompson, A. (2006). Psychological and social effects of surviving childhood cancer. In R.T. Brown (Ed.), Comprehensive handbook of childhood cancer and sickle cell disease: A biopsychosocial approach. New York, NY: Oxford University Press.
McCarthy, A.M., Hanrahan, K., Kleiber, C., Zimmerman, M.B., Lutgendorf, S., & Tsalikian, E. (2009). Normative salivary cortisol values and responsivity in children. Applied Nursing Research, 22, 54–62. https://doi.org/10.1016/j.apnr.2007.04.009
Meeske, K.A., Patel, S.K., Palmer, S.N., Nelson, M.B., & Parow, A.M. (2007). Factors associated with health-related quality of life in pediatric cancer survivors. Pediatric Blood and Cancer, 49, 298–305. https://doi.org/10.1002/pbc.20923
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P.A., Jacobsen, P., . . . Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute, 109(4). https://doi.org/10.1093/jnci/djw253
Miller, R., Stalder, T., Jarczok, M., Almeida, D.M., Badrick, E., Bartels, M., . . . Kirschbaum, C. (2016). The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology, 73, 16–23. https://doi.org/10.1016/j.psyneuen.2016.07.201
Müller, H.L. (2014). Craniopharyngioma. Handbook of Clinical Neurology, 124, 235–253. https://doi.org/10.1016/b978-0-444-59602-4.00016-2
National Cancer Institute. (n.d.). Quick profiles for states. Retrieved from http://statecancerprofiles.cancer.gov/index.html
National Cancer Institute. (2017a). Fatigue (PDQ®)—Health professional version. Retrieved from https://www.cancer.gov/about-cancer/treatment/side-effects/fatigue/fati…
National Cancer Institute. (2017b). SEER Cancer Statistics Review, 1975–2015. Retrieved from https://seer.cancer.gov/csr/1975_2015
Olson, K. (2014). Sleep-related disturbances among adolescents with cancer: A systematic review. Sleep Medicine, 15, 496–501. https://doi.org/10.1016/j.sleep.2014.01.006
Ostrom, Q.T., Gittleman, H., Farah, P., Ondracek, A., Chen, Y., Wolinsky, Y., . . . Barnholtz-Sloan, J.S. (2013). CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncology, 15(Suppl. 2), ii1–ii56. https://doi.org/10.1093/neuonc/not151
Owens, J.A., Spirito, A., & McGuinn, M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23, 1043–1051.
Rosmalen, J.G.M., Oldehinkel, A.J., Ormel, J., de Winter, A.F., Buitelaar, J.K., & Verhulst, F.C. (2005). Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology, 30, 483–495. https://doi.org/10.1016/j.psyneuen.2004.12.007
Ruble, K., Scarvalone, S., Gallicchio, L., Davis, C., & Wells, D. (2016). Group physical activity intervention for childhood cancer survivors: A pilot study. Journal of Physical Activity and Health, 13, 352–359. https://doi.org/10.1123/jpah.2015-0050
Ruccione, K., Lu, Y., & Meeske, K. (2013). Adolescents’ psychosocial health-related quality of life within 6 months after cancer treatment completion. Cancer Nursing, 36, E61–E72. https://doi.org/10.1097/NCC.0b013e3182902119
Sadeh, A., Lavie, P., Scher, A., Tirosh, E., & Epstein, R. (1991). Actigraphic home-monitoring sleep-disturbed and control infants and young children: A new method for pediatric assessment of sleep-wake patterns. Pediatrics, 87, 494–499.
Salimetrics. (2016). Expanded range high sensitivity salivary cortisol enzyme immunoassay kit. Retrieved from https://www.salimetrics.com/wp-content/uploads/2018/03/salivary-cortiso…
Selye, H. (1950). Stress and the general adaptation syndrome. BMJ, 1, 1383–1392.
Siegel, D.A., Li, J., & Singh, S. (2017). Racial differences in survival of pediatric patients with brain and central nervous system cancer, United States, 2001–2012. Annals of Epidemiology, 27, 505. https://doi.org/10.1016/j.annepidem.2017.07.040
So, K., Adamson, T.M., & Horne, R.S.C. (2007). The use of actigraphy for assessment of the development of sleep/wake patterns in infants during the first 12 months of life. Journal of Sleep Research, 16, 181–187. https://doi.org/10.1111/j.1365-2869.2007.00582.x
Stam, H., Grootenhuis, M.A., Brons, P.P.T., Caron, H.N., & Last, B.F. (2006). Health-related quality of life in children and emotional reactions of parents following completion of cancer treatment. Pediatric Blood and Cancer, 47, 312–319. https://doi.org/10.1002/pbc.20661
Taxis, J.C., Rew, L., Jackson, K., & Kouzekanani, K. (2004). Protective resources and perceptions of stress in a multi-ethnic sample of school-age children. Pediatric Nursing, 30, 477–487.
Tomlinson, D., Zupanec, S., Jones, H., O’Sullivan, C., Hesser, T., & Sung, L. (2016). The lived experience of fatigue in children and adolescents with cancer: A systematic review. Supportive Care in Cancer, 24, 3623–3631. https://doi.org/10.1007/s00520-016-3253-8
Valentino, K. (2018). Physical activity in survivors of pediatric brain tumors: A review. Pediatric Nursing, 44, 76–80.
Van Hulle, C.A., Shirtcliff, E.A., Lemery-Chalfant, K., & Goldsmith, H.H. (2012). Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior, 62, 36–42.
Varni, J.W., Burwinkle, T.M., Katz, E.R., Meeske, K., & Dickinson, P. (2002). The PedsQL in pediatric cancer: Reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer, 94, 2090–2106. https://doi.org/10.1002/cncr.10428
Verberne, L.M., Maurice-Stam, H., Grootenhuis, M.A., Van Santen, H.M., & Schouten-Van Meeteren, A.Y.N. (2012). Sleep disorders in children after treatment for a CNS tumour. Journal of Sleep Research, 21, 461–469.
Weber, K., Rockstroh, B., Borgelt, J., Awiszus, B., Popov, T., Hoffmann, K., . . . Pröpster, K. (2008). Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry, 8, 63. https://doi.org/10.1186/1471-244X-8-63
Wilkinson, J., Marmol, N.L., Godfrey, C., Wills, H., van Eijndhoven, Q., Botchway, E.N., . . . Catroppa, C. (2018). Fatigue following paediatric acquired brain injury and its impact on functional outcomes: A systematic review. Neuropsychology Review, 28, 73–87. https://doi.org/10.1007/s11065-018-9370-z
Woodside, D.B., Winter, K., & Fisman, S. (1991). Salivary cortisol in children: Correlations with serum values and effect of psychotropic drug administration. Canadian Journal of Psychiatry, 36, 746–748.




