Administration of Subcutaneous Monoclonal Antibodies in Patients With Cancer
Problem Identification: Subcutaneous (SC) formulations for monoclonal antibodies (mAbs) must be evaluated for efficacy and safety in comparison with preexisting IV formulations to identify potential benefits and risks.
Literature Search: This is a systematic review of clinical trials. MEDLINE®/PubMed, EMBASE, Cochrane Library, LILACS (Latin American and Caribbean Health Sciences Literature), and reference lists were searched for relevant studies.
Data Evaluation: Data regarding efficacy and safety were registered in a form designed for this review. Risk of bias was assessed using the Jadad scale.
Synthesis: SC administration of alemtuzumab, trastuzumab, and rituximab presented therapeutic efficacy with similar safety profiles compared to their respective IV formulations, except for the higher prevalence of local adverse events following SC administration.
Implications for Practice: SC mAbs require slow administration (no less than five minutes), and the injection site should be changed at each cycle. Patient guidelines should include information about expected adverse effects, signs or symptoms of side effects requiring emergency care, and how to reduce potential discomfort caused by the injection.
Jump to a section
Monoclonal antibodies (mAbs) represent major advances in the treatment of several types of cancer, and they have significantly improved patient survival with fewer side effects. Traditionally administered by the IV route, mAbs used in cancer treatment until 2013 were administered by infusion for 30 minutes to four hours at doses based on body surface area. However, the treatment of other chronic diseases has demonstrated the possibility of subcutaneous (SC) administration of mAbs (Jackisch, Müller, Maintz, Hell, & Ataseven, 2014; Leveque, 2014).
This route of administration has become attractive for use in cancer treatment because of its potential to eliminate the risks of venipuncture and reduce treatment time and costs (Jackisch et al., 2014). However, when changing the route of administration, the limitations of the SC tissue, particularly those related to volume, need to be considered. The SC tissue is composed of an extracellular matrix that maintains the structure of the skin and regulates the flow of fluids. Volumes exceeding 3 ml increase local pressure, distort the matrix, and cause pain (Arthur, 2015). To overcome the volume limits for bolus injection, SC formulations should contain hyaluronidase as an excipient.
Hyaluronidase is an enzyme that naturally occurs in the body; its function is to hydrolyze hyaluronic acid, one of the components responsible for the structure of the SC tissue. This process reduces extracellular matrix resistance and facilitates the infusion of fluids (Arthur, 2015). Hyaluronidase has been successfully used to facilitate SC delivery of drug volumes exceeding 3 ml (Arthur, 2015; Dychter et al., 2014).
The SC route represents a reduced risk of infection, allows self-administration by patients trained by healthcare providers, is more convenient for patients and nurses, shortens administration time, and can reduce treatment costs (Jackisch et al., 2014). Faced with this new possibility, healthcare providers should assess the risks and benefits of SC administration of mAbs. In this regard, nurses who administer these substances should know the safety precautions required when carrying out the procedure and provide the necessary guidelines to patients. Given that, the primary objective of this review was to identify and analyze the available scientific evidence on the SC administration of mAbs in cancer treatment regarding its therapeutic efficacy and local and systemic tolerance compared with IV administration. Secondarily, the authors described the implications for dose and volume calculation and recommendations for administration.
Methods
This systematic review was conducted by evaluating studies found in MEDLINE®/PubMed, EMBASE, Cochrane Library, and LILACS (Latin American and Caribbean Health Sciences Literature). The research question was formulated based on the PICOs (Patient/Problem, Intervention, Comparison/Control, Outcome and study type) strategy: “What is the scientific evidence on SC administration of mAbs in patients with cancer regarding therapeutic efficacy and local and systemic tolerance compared with IV administration?”
Controlled and noncontrolled descriptors were used in the following basic strategy: (Antibodies, Monoclonal OR “monoclonal antibodies”) AND (Infusions, Subcutaneous OR “subcutaneous infusion” OR “subcutaneous administration” OR Injections, Subcutaneous OR “subcutaneous injections”) AND (neoplasms OR neoplas* OR cancer* OR onco* OR tumor* OR tumour*). The filter for clinical studies was used when available in the search options in the databases. Detailed strategy for each database is available in the review protocol. The complete systematic review protocol was registered on PROSPERO and can be consulted through the following identification number: CRD42017067831.
Studies were included if they addressed SC administration of mAbs in patients with cancer; studies were excluded if they did not compare SC and IV administration of the same mAb. Studies were selected regardless of language or year of publication. The reference lists of the selected studies also were checked to find additional studies. Additional searches also were conducted to find recent phases of cited studies. The flowchart representing the search and selection of articles for review is depicted in Figure 1. 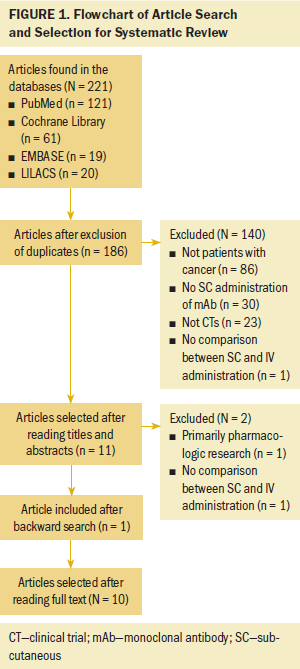
After reading the selected studies, the most relevant data were extracted using the instrument specifically designed for this review. The information recorded in the instrument referred to the identification of the study, the characteristics of the method used in each study, the main results found, and the authors’ recommendations.
The studies were classified according to their level of evidence, as proposed by Melnyk and Fineout-Overholt (2011). Methodologic quality was assessed using the Jadad scale, which assesses studies based on “yes” or “no” answers to five questions. The Jadad scale score ranges from 0 (worst quality) to 5 (best quality). The questions refer to the method of randomization and blinding of the primary study. The choice of this scale is based on the assumption that this is an instrument to assess the quality of clinical studies, with quality being defined as the “likelihood of the trial design to generate unbiased results” (Jadad et al., 1996, p. 2).
Results and Discussion
The search yielded 10 studies that investigated the use of three different mAbs: alemtuzumab, trastuzumab, and rituximab. The descriptions of the selected articles are presented in Table 1. Information on the methods of randomization used and scores on the Jadad scale are depicted in Table 2. Evidence on each mAb identified in this review is analyzed as follows. 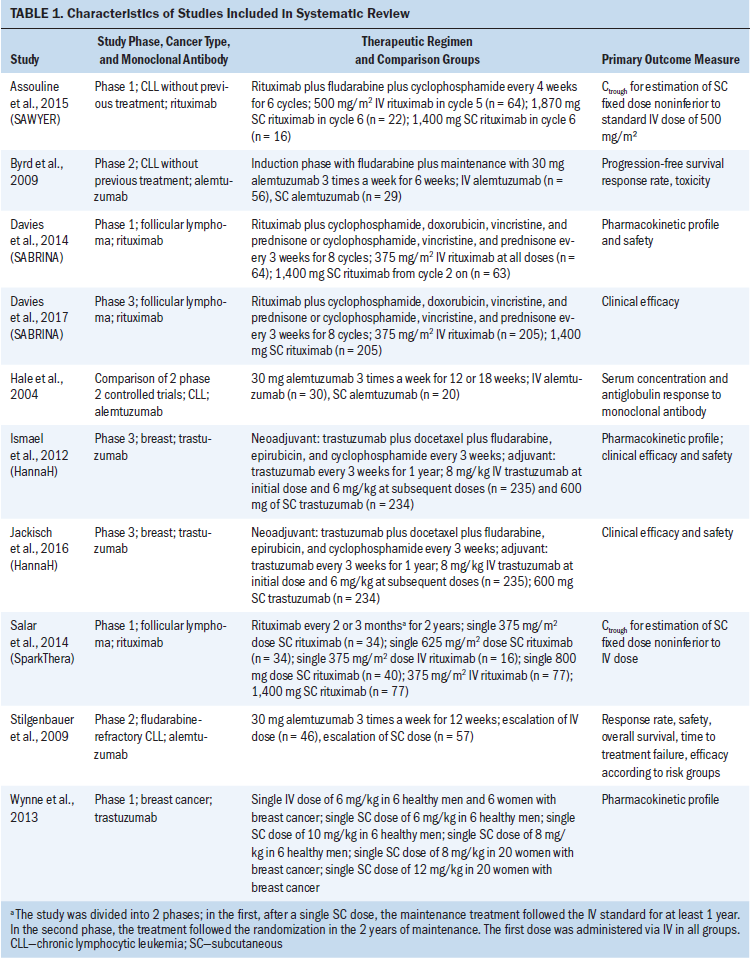
Alemtuzumab
Alemtuzumab is a mAb against CD52, a glycoprotein present in the B-cell membrane, and can be used in the treatment of B-cell chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL). The selected studies compared IV and SC administration of alemtuzumab without changing the standard therapeutic regimen (30 mg three times a week after dose escalation in the first week). Hale et al. (2004) compared blood concentrations of alemtuzumab in patients with CLL following the two routes of administration and found wide variation in alemtuzumab concentrations after IV and SC administration. The group treated with the SC formulation presented with pronounced injection site reactions (erythema, edema, and pain) and, therefore, received lower total doses because of the difficulty in completing dose escalation. The authors did not report significant differences in response rates between the groups. Of note, groups analyzed in the study are not fully comparable because the IV group had previously been treated with cytotoxic agents, whereas patients in the SC group had not received previous treatments. 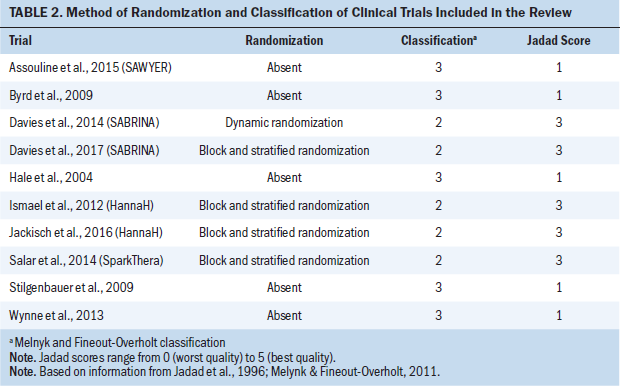
Byrd et al. (2009) conducted a study using alemtuzumab as consolidation therapy in patients with CLL. Response rates were similar in the IV and SC cohorts. The study findings demonstrated similar efficacy for both routes of administration. As for adverse events (AEs), the researchers reported that virtually all patients exhibited acute reactions during IV infusion. Such reactions were shivering, fever, and/or hypotension. There were no acute systemic reactions to the injection in the SC group, but almost all participants presented with local inflammatory reactions with erythema and pain. Acute and local systemic symptoms were more frequent in the initial weeks.
Stilgenbauer et al. (2009) compared the IV and SC administration of alemtuzumab in patients with CLL only in the dose escalation phase (i.e., after escalation, all patients followed SC treatment). The major contribution of the study was to report differences in safety profile between the routes of administration. More skin reactions and fewer episodes of shivering were reported during the dose escalation of the SC infusion.
The SC administration of alemtuzumab was mainly aimed at reducing the incidence of infusion-related reactions (Alinari et al., 2007). The studies by Byrd et al. (2009) and Stilgenbauer et al. (2009) support this hypothesis, with SC administration being associated with reduced prevalence of systemic acute reactions. Because of local injection-related reactions, the authors of both studies recommended rotation of injection sites. Importantly, one study (Hale et al., 2004) described the administration of a fractionated dose via two injections at different sites.
Nevertheless, the quality of evidence regarding SC administration of alemtuzumab is low. The absence of randomization and blinding increases the risk of bias in the presented results. Trials using alemtuzumab for cancer treatment were included only to present a complete literature review of the subject, because it is no longer marketed for cancer treatment and its use in patients with CLL or lymphoma is currently considered off-label.
Trastuzumab
Trastuzumab is a humanized mAb directed against the extracellular domain of the human epidermal growth factor receptor 2 (HER2). It is indicated for the treatment of HER2-overexpressing breast cancer and HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. Its IV administration should be carried out in 250 ml of saline solution. Its SC formulation has been developed for injection of a fixed dose using hyaluronidase as excipient to allow bolus administration (European Medicines Agency [EMA], 2013; Ismael et al., 2012).
Wynne et al. (2013) examined the pharmacokinetics of trastuzumab after SC administration in healthy men and in women with HER2-positive early-stage breast cancer. This was a phase 1 clinical trial to determine the pharmacokinetics of trastuzumab to propose a fixed dose for SC administration in larger studies. After the pharmacokinetic analyses, the fixed dose of 600 mg was chosen for the SC formulation as equivalent to the standard body surface area–based IV regimen. The researchers also assessed safety and tolerability and found a lower occurrence of AEs in the cohorts receiving the SC formulation. AEs reported after SC administration included headache, local reactions (erythema, pain, edema, and discoloration), upper respiratory tract infection, and diarrhea. More than 70% of the reported AEs were classified as mild.
The 600 mg fixed-dose SC formulation of trastuzumab was assessed in a phase 3 clinical trial known as the HannaH Study (Ismael et al., 2012). The pharmacokinetic profiles of the IV and SC formulations were found to be equivalent. Regarding the clinical responses, the study identified that 45% achieved a pathological complete response (pCR) in the SC group and 41% achieved pCR in the IV group. The difference in pCR between the cohorts was not statistically significant and demonstrated clinical efficacy of SC trastuzumab. As for AEs, the formulations presented a similar safety profile.
The most common events in both groups were alopecia, nausea, neutropenia, diarrhea, asthenia, and fatigue. The occurrence of grade 3 and 4 AEs was numerically higher in the IV group; however, the SC group presented a higher incidence of AEs classified as serious, mainly related to neutropenia and febrile neutropenia. Local reactions were more frequent in the SC group, with pain being the most common event. Most local reactions were classified as grade 1.
After two years of treatment-free follow-up, the authors of the HannaH Study published the long-term results (Jackisch et al., 2016). Event-free survival and overall survival rates after three years of randomization were comparable between the groups. The cardiac safety profile did not differ between the formulations.
All publications on the use SC trastuzumab are related to the HannaH study, which presents good methodologic quality, despite the absence of blinding. Changing the route of administration makes it impossible to blind the participants, which is an intrinsic limitation. Despite this limitation, the risk of bias cannot be disregarded, particularly in the assessment of AEs. The first results of the HannaH study suggest a possible increase in the number of serious AEs with the use of the SC route; however, the authors argued that this classification may have been a consequence of a more conservative approach used by the investigators toward patients receiving SC trastuzumab. The number of grade 3–4 AEs was higher in the IV group, but the clinical management was more cautious in the SC group, resulting in a higher rate of hospital admissions (Ismael et al., 2012). This difference in clinical management is possibly more related to the absence of blinding than to the severity of AEs.
Patients’ preference for the route of administration of trastuzumab was assessed by Pivot et al. (2014), who identified that SC was preferred by 89% of the patients. The main reason for such preference was the reduction of time spent on treatment. The authors assessed AEs during SC and IV administration and found that the highest number of AEs occurred during SC administration, mainly because of local reactions at the injection site.
This finding confirms the results of the HannaH study. EMA (2013) approved SC injection of trastuzumab for breast cancer treatment in 2013 based on several pharmacokinetic studies and on the clinical evidence of the HannaH study. Australia, New Zealand, and Brazil are examples of countries outside Europe that also approved the new formulation of trastuzumab. In the United States, IV trastuzumab has been approved for breast cancer treatment since 1998; however, its SC formulation has not yet been approved for clinical use.
Rituximab
Rituximab is targeted at the CD20 phosphoprotein expressed on the surface of B lymphocytes and is used in the treatment of NHL and CLL. Its standard IV administration is carried out at doses based on the body surface area and diluted in volumes of 500 ml or greater. The SC formulation of rituximab has been made possible with the use of hyaluronidase and the identification of fixed dose independent of body surface area.
The stage 1 analysis of the SABRINA study assessed pharmacologic parameters to investigate the noninferiority of the fixed dose of 1,400 mg of SC rituximab in comparison to the standard IV regimen for follicular lymphoma treatment (Davies et al., 2014). The researchers assessed the reduction in the number of B cells in peripheral blood and the tumor response at the end of the induction period. Both groups exhibited similar reduction in the number of tumorous cells and in response rates.
Davies et al. (2014) also assessed the safety profile of SC rituximab and reported AEs in 92% of the participants in the SC group (versus 88% in the IV group). AEs related to administration (erythema, pruritus, chills, and vomiting) were mostly grade 2–3 in both groups and occurred in greater numbers in the SC group. Erythema at the injection site occurred in 10% of the patients in the SC group, and 5% experienced a grade 3 reaction during SC injection (rash, dry mouth). The most common systemic toxicities were neutropenia, febrile neutropenia, and constipation in both groups. There was no significant difference in the incidence of serious AEs. The researchers concluded that the use of the SC route does not significantly alter the safety profile, because there was no increase in the occurrence of grade 3–4 events.
In 2017, Davies et al. published the results of phase 3 of the SABRINA study, and the conclusions regarding pharmacokinetics and clinical efficacy of SC rituximab were confirmed. The IV and SC formulations exhibited similar safety profiles; however, the incidence of reactions related to administration was higher in the SC group. The most common AEs reported were gastrointestinal disorders, infections, and local or systemic events related to administration. Reactions related to administration were mostly grade 1–2 and were restricted to the injection site in the SC group. The most common reactions were erythema, pruritus, rash, and pain. In the IV group, the most common reactions were chills and pruritus.
Stage 1 data from the SparkThera study (Salar et al., 2014) predicted that an SC fixed dose of rituximab would be noninferior to the IV dose during follicular lymphoma maintenance therapy. During the clinical trial, the pharmacokinetic equivalence of the 1,400 mg dose was confirmed. Secondarily, the researchers observed reported AEs and identified similar occurrences of severe events between the groups. Reactions related to administration were more frequent in the SC group, with grade 1–2 erythema being the most frequently observed reaction.
Assouline et al. (2015) investigated SC rituximab in the treatment of CLL. The standard dose for IV treatment with rituximab in CLL is higher than that used in NHL, thereby justifying a new pharmacokinetic study. The pharmacokinetic analysis identified a fixed dose of 1,600 mg. The patients received a single dose of SC rituximab in the last treatment cycle, and the primary outcome measure was pharmacokinetics. Secondarily, the authors described a slight increase in the number of AEs during the SC cycle. The majority of AEs were grade 1 and 2, and the most commonly reported were neutropenia and leucopenia. More patients experienced administration-related reactions during the SC cycle, and the most commonly reported local reactions were grade 1–2 pain and erythema at the injection site.
Although it was not possible to blind those involved in the administration of the therapy, the SABRINA study reduced the risk of bias in the assessment of the pathological response by including independent raters in the review of the diagnostic examinations. However, the classification of AEs remains at risk for bias in all studies because of the absence of blinding. It should be noted that the results of the SparkThera and SAWYER studies reported in the current article refer to the first phase of these clinical trials. This phase was carried out with a small sample and was primarily aimed at estimating the appropriate dose for the following phases.
The use of SC rituximab was assessed in at least two other clinical trials (Lugtenburg et al., 2017; Rummel et al., 2015), which were not included in this review because their texts were not fully published. The content of the trials was presented through published abstracts from poster presentations. Lugtenburg et al. (2017) assessed the use of SC rituximab in the treatment of diffuse large B-cell lymphoma compared with the standard IV formulation and demonstrated its therapeutic efficacy and safety. The authors also investigated patients’ satisfaction with the treatment and found improvement in overall satisfaction with SC rituximab. Rummel et al. (2015) investigated patients’ preference for IV or SC rituximab and identified greater preference for the SC administration because of reduction in clinic time and more comfort during administration.
Rituximab for SC use was first approved in Europe in 2014 with a fixed dose of 1,400 mg for the treatment of follicular lymphoma and CD20-positive diffuse large B-cell NHL. In 2016, EMA extended SC rituximab approval to include the 1,600 mg formulation for CLL treatment (EMA, 2016). A year later, the U.S. Food and Drug Administration approved SC rituximab for the same indications already used in Europe (U.S. Food and Drug Administration, 2017).
Like trastuzumab, the SC formulation of rituximab is intended to be a more convenient option for patients and health services by reducing treatment time and potentially reducing costs (Ponzetti, Canciani, Farina, Era, & Walzer, 2016b). In addition to reducing administration time by using the SC route, the use of fixed doses reduces the risks related to medication errors and increases the possibility of cost savings by avoiding waste of doses in the vials and eliminating the need for discarding prepared doses in case of patient cancellation (Ponzetti, Canciani, Farina, Era, & Walzer, 2016a).
Considering that rituximab can trigger serious immunogenic reactions, particularly in the first administration, the first dose should be administered via IV and the SC route should only be used in subsequent cycles if there are no serious reactions (Carlson, Cox, Bedwell, & Ku, 2015). None of the clinical trials analyzed in this review administered the first dose of SC rituximab; therefore, its safety for initiation of treatment is unknown. Table 3 summarizes the recommendations for SC administration of rituximab and trastuzumab. 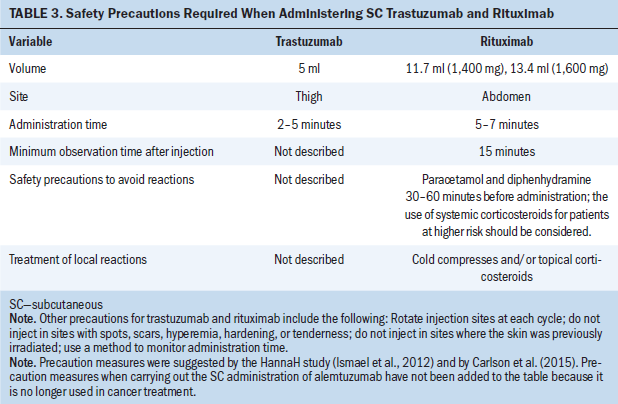
Implications for Nursing
The SC administration of mAbs is a possibility for patients whose treatment includes trastuzumab or rituximab. This route of administration may be more convenient because it demands less time in the clinic. However, there is increased risk of local AEs, which are usually mild. The risk must be addressed with patients when the SC route is suggested.
While administering mAbs subcutaneously, nurses must be attentive to the administration time of each drug. Using handheld syringes, the only way to control flow rate is by observing a watch, a timer, or a chronometer while applying constant pressure to the syringe plunger. Single-use injection devices are presented as more practical to ensure correct time of injection. Such devices were tested in the PrefHer study (Pivot et al., 2014) and showed similar results regarding safety and patient preference when compared to handheld syringes. Devices with automated injection flow control may be used in the future to ensure adequate time of injection of mAbs, but they are not currently available.
Rotating locale of administration at each cycle also is important to attempt to reduce injection-related reactions. Current recommendations narrow the possibilities to the internal area of the thighs when administering SC trastuzumab and the abdominal area when SC rituximab is prescribed. This limitation occurs because these were the only body areas tested for each drug in clinical trials. Safety and tolerability data about SC injection of these drugs in different areas are not available and could be addressed in future research.
Self-administration is the next possible scenario for SC mAbs treatment. Pivot et al. (2014) foresaw this possibility with the single-use injection device. There was a cohort of patients in the PrefHer study who performed self-administration in the hospital or clinic setting using the single-use injection device. Self-administration in the home setting is still to be evaluated. The BELIS study (Cocquyt et al., 2017) assessed the safety and tolerability of SC trastuzumab administrated at home by a healthcare provider; the findings from this study and the use of single-use injection devices may help develop protocols to teach patients self-administration of trastuzumab. To the current authors’ knowledge, self-administration of SC rituximab has not been assessed. Rituximab is a more immunogenic mAb when compared to trastuzumab and presents a higher risk of serious infusion-related reactions. Therefore, its administration requires more precautions, such as premedication and a healthcare setting with professionals able to intervene if necessary. For more detailed information about immunotherapy administration in general, nurses may consult the Oncology Nursing Society recommendations (Wiley et al., 2017).
Not enough evidence exists about how to prevent and treat local reactions because this is a new route of administration. The recommendations from the original clinical trials with SC trastuzumab and SC rituximab have been reported in this article; however, it is still an open opportunity for nursing research. 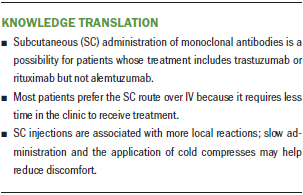
Conclusion
The evidence confirms the possibility of SC administration of mAbs in cancer treatment, with similar efficacy and safety profiles as those of IV administration. The incidence of local AEs was higher in the SC group in all studies. The reactions tend to be mild and transient, and the most commonly reported reactions are pain, erythema, and edema. The studies assessing patients’ preference for SC or IV formulations of trastuzumab and rituximab indicated that these events are well tolerated and that the reduction of administration time is well accepted.
Immunotherapy is a promising alternative for cancer treatment. Nurses should be up-to-date with opportunities to improve practice; they are responsible for administering therapy and providing guidance to patients. Knowing safety procedures during administration, AEs, and strategies for AEs’ prevention and treatment contributes to safe, quality care. Because they are new formulations, it is important to report any events, during or after the use of SC mAbs, to the regulatory institution responsible.
About the Author(s)
Anne Rodrigues Ferreira, RN, is a nurse in the multiprofessional residency program in oncology and Eliete Farias Azevedo, MHS, is a nurse, both at the National Cancer Institute in Rio de Janeiro, Brazil. No financial relationships to disclose. Both authors contributed to the conceptualization and design, provided the analysis, and contributed to the manuscript preparation. Ferreira completed the data collection. Ferreira can be reached at annerf.ferreira@gmail.com, with copy to ONFEditor@ons.org. (Submitted April 2018. Accepted July 4, 2018.)
References
Alinari L., Lapalombella, R., Andritsos, L., Baiocchi, R.A., Lin, T.S., & Byrd, J.C. (2007). Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene, 26, 3644–3653. https://doi.org/10.1038/sj.onc.1210380
Arthur, A.O. (2015). Innovations in subcutaneous infusions. Journal of Infusion Nursing, 38, 179–187. https://doi.org/10.1097/NAN.0000000000000099
Assouline, S., Buccheri, V., Delmer, A., Gaidano, G., McIntyre, C., Brewster, M., . . . Badoux, X. (2015). Pharmacokinetics and safety of subcutaneous rituximab plus fludarabine and cyclophosphamide for patients with chronic lymphocytic leukaemia. British Journal of Clinical Pharmacology, 80, 1001–1009. https://doi.org/10.1111/bcp.12662
Byrd, J.C., Peterson, B.L., Rai, K.R., Hurd, D., Hohl, R., Perry, M.C., . . . Larson, R.A. (2009). Fludarabine followed by alemtuzumab consolidation for previously untreated chronic lymphocytic leukemia: Final report of cancer and leukemia group B study 19901. Leukemia and Lymphoma, 50, 1589–1596. https://doi.org/10.1080/10428190903150839
Carlson, J., Cox, K., Bedwell, K., & Ku, M. (2015). Rituximab for subcutaneous delivery: Clinical management principles from a nursing perspective. International Journal of Nursing Practice, 21(Suppl. 3), 1–13. https://doi.org/10.1111/ijn.12413
Cocquyt, V.F., Martinez-Mena, C.L., Martens, M.T., D’Hondt, R.G., Graas, M.P.L., Evron, E., . . . Van De Walle, E.I. (2017). BELIS: Safety and tolerability of at home administration of trastuzumab (Herceptin®) subcutaneous for the treatment of patients with HER2-positive early breast cancer [Abstract P4-21-17]. Cancer Research, 77(4 Suppl.). https://doi.org/10.1158/1538-7445.SABCS16-P4-21-17
Davies, A., Merli, F., Mihaljević, B., Mercadal, S., Siritanaratkul, N., Solal-Céligny, P., . . . MacDonald, D. (2017). Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): A randomised, open-label, phase 3 trial. Lancet Haematology, 4, e272–e282. https://doi.org/10.1016/S2352-3026(17)30078-9
Davies, A., Merli, F., Mihaljevic, B., Siritanaratkul, N., Solal-Céligny, P., Barrett, M., . . . Macdonald, D. (2014). Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): Stage 1 analysis of a randomised phase 3 study. Lancet Oncology, 15, 343–352. https://doi.org/10.1016/S1470-2045(14)70005-1
Dychter, S.S., Harrigan, R., Bahn, J.D., Printz, M.A., Sugarman, B.J., DeNoia, E., . . . Maneval, D.C. (2014). Tolerability and pharmacokinetic properties of ondansetron administered subcutaneously with recombinant human hyaluronidase in minipigs and healthy volunteers. Clinical Therapeutics, 36, 211–224. https://doi.org/10.1016/j.clinthera.2013.12.013
European Medicines Agency. (2013). CHMP assessment report: Herceptin. Retrieved from https://www.ema.europa.eu/documents/variation-report/herceptin-h-c-278-…
European Medicines Agency. (2016). Assessment report: MabThera. Retrieved from https://www.ema.europa.eu/documents/variation-report/mabthera-h-c-165-x…
Hale, G., Rebello, P., Brettman, L.R., Fegan, C., Kennedy, B., Kimby, E., . . . Hillmen, P. (2004). Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood, 104, 948–955. https://doi.org/10.1182/blood-2004-02-0593
Ismael, G., Hegg, R., Muehlbauer, S., Heinzmann, D., Lum, B., Kim, S.B., . . . Jackisch, C. (2012). Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): A phase 3, open-label, multicentre, randomised trial. Lancet Oncology, 13, 869–878. https://doi.org/10.1016/S1470-2045(12)70329-7
Jackisch, C., Hegg, R., Stroyakovskiy, D., Ahn, J.S., Melichar, B., Chen, S.C., . . . Pivot, X. (2016). HannaH phase III randomised study: Association of total pathological complete response with event-free survival in HER2-positive early breast cancer treated with neoadjuvant-adjuvant trastuzumab after 2 years of treatment-free follow-up. European Journal of Cancer, 62, 62–75. https://doi.org/10.1016/j.ejca.2016.03.087
Jackisch, C., Müller, V., Maintz, C., Hell, S., & Ataseven, B. (2014). Subcutaneous administration of monoclonal antibodies in oncology. Geburtshilfe und Frauenheilkunde, 74, 343–349. https://doi.org/10.1055/s-0034-1368173
Jadad, A.R., Moore, R.A., Carroll, D., Jenkinson, C., Reynolds, D.J., Gavaghan, D.J., & McQuay, H.J. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials, 17, 1–12.
Leveque, D. (2014). Subcutaneous administration of anticancer agents. Anticancer Research, 34, 1579–1586.
Lugtenburg, P.J., Avivi, I., Berenschot, H.W., Ilhan, O., Marolleau, J.P., Nagler, A., . . . Pfeundschuh, M. (2017). Rituximab SC and IV plus CHOP show similar efficacy and safety in the randomised mabease study in first-line DLBCL. Hematological Oncology, 35(Suppl. 2), 185–186. https://doi.org/10.1002/hon.2438_46
Melnyk, B., & Fineout-Overholt, E. (2011). Evidence-based practice in nursing and healthcare: A guide to best practice (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Pivot, X., Gligorov, J., Müller, V., Curigliano, G., Knoop, A., Verma, S., . . . Fallowfield, L. (2014). Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: Final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Annals of Oncology, 25, 1979–1987. https://doi.org/10.1093/annonc/mdu364
Ponzetti, C., Canciani, M., Farina, M., Era, S., & Walzer, S. (2016a). Administrative risk quantification of subcutaneous and intravenous therapies in Italian centers utilizing the failure mode and effects analysis approach. ClinicoEconomics and Outcomes Research, 8, 353–359. https://doi.org/10.2147/CEOR.S97323
Ponzetti, C., Canciani, M., Farina, M., Era, S., & Walzer, S. (2016b). Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin’s lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. ClinicoEconomics and Outcomes Research, 8, 227–233. https://doi.org/10.2147/CEOR.S97319
Rummel, M., Kim, T.M., Plenteda, C., Capochiani, E., Mendoza, M., Smith, R., . . . Grigg, A. (2015). Prefmab: Final analysis of patient satisfaction with subcutaneous versus intravenous rituximab in previously untreated Cd20+ diffuse large B-cell lymphoma or follicular lymphoma. Value in Health, 18(7), A469. https://doi.org/10.1016/j.jval.2015.09.1237
Salar, A., Avivi, I., Bittner, B., Bouabdallah, R., Brewster, M., Catalani, O., . . . Tumyan, G. (2014). Comparison of subcutaneous versus intravenous administration of rituximab as maintenance treatment for follicular lymphoma: Results from a two-stage, phase IB study. Journal of Clinical Oncology , 32, 1782–1791. https://doi.org/10.1200/JCO.2013.52.2631
Stilgenbauer, S., Zenz, T., Winkler, D., Bühler, A., Schlenk, R.F., Groner, S., . . . Döhner, H. (2009). Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: Clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. Journal of Clinical Oncology, 27, 3994–4001. https://doi.org/10.1200/JCO.2008.21.1128
U.S. Food and Drug Administration. (2017). Rituxan Hycela™ [Prescribing information]. Washington, DC: Author. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761064s000lbl…
Wiley, K., LeFebvre, K.B., Wall, L., Baldwin-Medsker, A., Nguyen, K., Marsh, L., & Baniewicz, D. (2017). Immunotherapy administration: Oncology Nursing Society recommendations. Clinical Journal of Oncology Nursing, 21(Suppl. 2), 5–7. https://doi.org/10.1188/17.CJON.S2.5-7
Wynne, C., Harvey, V., Schwabe, C., Waaka, D., McIntyre, C., & Bittner, B. (2013). Comparison of subcutaneous and intravenous administration of trastuzumab: A phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. Journal of Clinical Pharmacology, 53, 192–201. https://doi.org/10.1177/0091270012436560




