Systematic Review of Nonpharmacologic Approaches for the Management of Gastrointestinal Symptoms
Problem Identification: To summarize and critique the literature for nonpharmacologic complementary approaches to manage gastrointestinal (GI) symptoms attributed to chemotherapy.
Literature Search: A literature search was conducted using CINAHL®, MEDLINE®, and PsycINFO® from database inception through January 2018.
Data Evaluation: Studies were independently appraised by each author regarding inclusion eligibility and summary of GI symptom outcomes and the nonpharmacologic complementary intervention.
Synthesis: 57 studies met inclusion criteria. GI symptoms most commonly evaluated as a chemotherapy outcome were nausea and vomiting and nausea alone. GI symptoms infrequently evaluated as outcomes included diarrhea, anticipatory nausea, and dysgeusia. Ten GI symptoms associated with chemotherapy were not evaluated by any study. Nonpharmacologic interventions included 15 different interventions.
Implications for Research: Studies evaluating nonpharmacologic interventions for managing chemotherapy-related GI symptoms have been growing but tend to focus on nausea and vomiting to the exclusion of other relevant GI symptoms. Studies evaluating nonpharmacologic effects on other GI symptoms may make great strides in reducing patient symptom burden.
Jump to a section
Patients with cancer receiving chemotherapy experience as many as 14 treatment-related symptoms, with each additional symptom resulting in an increase in symptom distress (Spichiger et al., 2011; Thiagarajan et al., 2016). Symptom management studies tend to focus on the more prevalent symptoms related to cancer chemotherapy, which include pain, fatigue, and sleep disturbance, but gastrointestinal (GI) symptoms have been shown to contribute to high symptom burden in this population. Although 19 GI symptoms are related to chemotherapy (i.e., oral mucositis, xerostomia, dysphagia, dysgeusia, anticipatory nausea, anticipatory vomiting, nausea, vomiting, anorexia, early satiety, pyrosis, bloating, eructation, flatulence, retching, diarrhea, constipation, rectal burning, and rectal itching) (see Table 1), symptom management literature predominantly focuses on nausea and vomiting. A study by Cherwin and Kwekkeboom (2016) demonstrated that, despite pharmacologic intervention, people with a hematologic cancer receiving chemotherapy experience as many as five concurrent GI symptoms, and 11 of 19 GI symptoms assessed met criteria to be considered clinically relevant (i.e., greater than 15% prevalence and moderate to severe duration, severity, or distress). Unrelieved GI symptoms contribute to depression, shortened survival, and poor quality of life (QOL) in people with cancer (Goodell & Nail, 2005). High symptom burden from GI symptoms, despite pharmacologic intervention, may indicate the need for novel methods of symptom management. 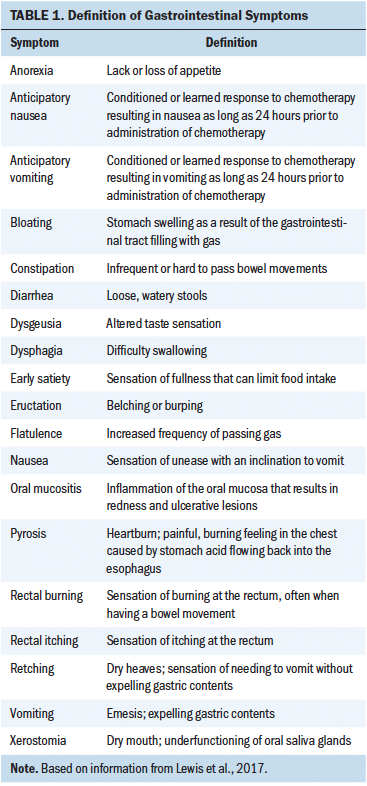
Modern health care is increasingly merging mainstream medicine with scientifically evaluated complementary therapies in a way that treats a person’s mind, body, and spirit (National Center for Complementary and Integrative Health, 2017). The National Institutes of Health Office of Cancer Complementary and Alternative Medicine has assigned classifications to the different forms of complementary therapies. These classifications include alternative medical systems (e.g., acupuncture, homeopathy), energy therapies (e.g., Qigong, Reiki, therapeutic touch), exercise therapies (e.g., tai chi, yoga), manipulative and body-based methods (e.g., chiropractic, massage), mind–body interventions (e.g., meditation, hypnosis, imagery), nutritional therapeutics (e.g., macrobiotic diet, vitamins), pharmacologic and biologic treatments (e.g., herbal therapies, off-label drugs), and spiritual therapies (e.g., prayer).
Although mainstream medicine is the most commonly used medical therapy for people in the United States, many Americans choose to use complementary therapy to supplement their medical care. As many as 33% of adults in the United States report using some form of complementary therapy (Clarke, Black, Stussman, Barnes, & Nahin, 2015). Most people who report using a complementary approach do so to support general wellness or to prevent disease (Stussman, Black, Barnes, Clarke, & Nahin, 2015). The general population reports a relatively high use of complementary therapies, but people with a diagnosis of cancer use complementary therapies in far greater numbers. A meta-analysis of complementary therapy application showed that 50% of people with cancer in the United States reported using some form of complementary or alternative therapy (Horneber et al., 2012). This higher use of complementary therapy may be attributed to the high symptom burden in cancer. People with cancer in need of additional symptom relief may benefit from adding a complementary approach to their pharmacologic regimens.
Despite the advancements in pharmacotherapy, GI symptoms in people with cancer remain prevalent and severe. Complementary therapies used in conjunction with mainstream medicine may offer additional relief from GI symptoms. Evidence has shown promising results for the use of complementary approaches to manage non-GI symptoms in people with cancer, including pain (Bardia, Barton, Prokop, Bauer, & Moynihan, 2006), fatigue (Finnegan-John, Molassiotis, Richardson, & Ream, 2013), and sleep disturbance (Langford, Lee, & Miaskowski, 2012). Other reviews have focused on examining the evidence for complementary therapies managing individual GI symptoms, such as oral mucositis (Clarkson et al., 2010), xerostomia (Furness, Bryan, McMillan, & Worthington, 2013), or nausea (Tipton et al., 2007), but there has yet to be a review of literature examining complementary approaches to manage GI symptoms as a whole. Because 19 different GI symptoms are associated with chemotherapy, clinicians should understand the available research on complementary management of these symptoms. However, it remains unclear which complementary therapies might be beneficial to offer to a patient with cancer experiencing GI symptoms. The purpose of this review is to summarize the evidence surrounding complementary therapy use for the management of GI symptoms in people receiving chemotherapy and to offer practice recommendations based on a critical appraisal of available research.
Methods
Literature Search
This systematic review was performed using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). Three databases (i.e., CINAHL®, MEDLINE®, and PsycINFO®) were searched from database inception through January 2018. Inclusion criteria for studies involved adult (aged 18 years or older) participants with a cancer diagnosis who were receiving chemotherapy exclusively, reported the effect of a nonpharmacologic intervention, and experienced at least one GI-related symptom as a chemotherapy outcome.
This literature review focuses on nonpharmacologic complementary therapies. Because ingested therapies can elicit a pharmacologic-like effect in the body, nutritional therapeutics (e.g., diet therapy, vitamins) and pharmacologic and biologic treatments (e.g., herbal therapies, off-label drugs) were excluded, as well as any remedies that were ingested (e.g., a mouthwash that is swallowed). The authors also excluded complementary approaches that required extensive medical intervention, such as intubation for the use of esophageal laser treatments. When searching MEDLINE and CINAHL, to limit the search to studies with an experimental design, the authors used a search filter by Haynes, McKibbon, Wilczynski, Walter, and Werre (2005). When searching PsycINFO, the authors adapted the experimental design filter reported by Cochrane for use with the ProQuest platform of PsycINFO to enable it to work with the American Psychology Association platform (Cochrane Work, n.d.).
Search strategies were developed with the assistance of a health sciences librarian with expertise in conducting systematic reviews. Comprehensive strategies, including index and keyword methods, were devised for the databases used. The English-language filter was applied for CINAHL and PubMed. No other pre-established filters were used in an effort to maximize sensitivity. Searches were conducted from December 2016 to January 2017 and rerun in January 2018 to capture new records that became available during the screening process. To capture publications not indexed in the databases, reference lists and articles cited in the included studies were also reviewed.
Data Evaluation
Three reviewers individually assessed articles for eligibility, and each article included for full review was evaluated for risk of bias using the Cochrane Collaboration Tool for Assessing Risk of Bias (Higgins & Greene, 2011). The Cochrane Collaboration Tool for Assessing Risk of Bias evaluates each study based on the following criteria: adequate sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain is rated as high, low, or unclear risk of bias. Assessing the source of bias in a study allows the reviewer to identify how strong the presented evidence is and how reliable the conclusions are. Randomized, controlled trials with limited source of bias offer the highest grade of evidence for influencing practice.
Results
Selection of Articles
A total of 6,734 potentially relevant studies were identified through three databases and other sources (e.g., reference lists). Of these, 891 duplicates were excluded. After screening by title and abstract, an additional 5,523 articles were excluded. A total of 325 articles were included for a full-text review for eligibility. Of these, 268 articles were excluded for not meeting eligibility criteria (i.e., not original research, not adults, no nonpharmacologic intervention, no GI symptom measured, no randomization, participants not exclusively receiving chemotherapy, or withdrawn). A total of 57 studies were included for qualitative synthesis (see Figure 1). 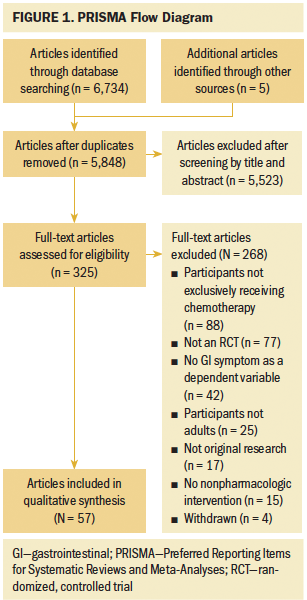
Nausea and Vomiting
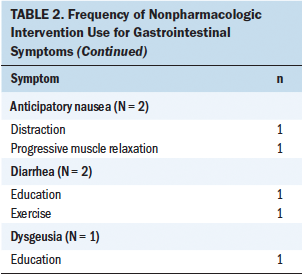
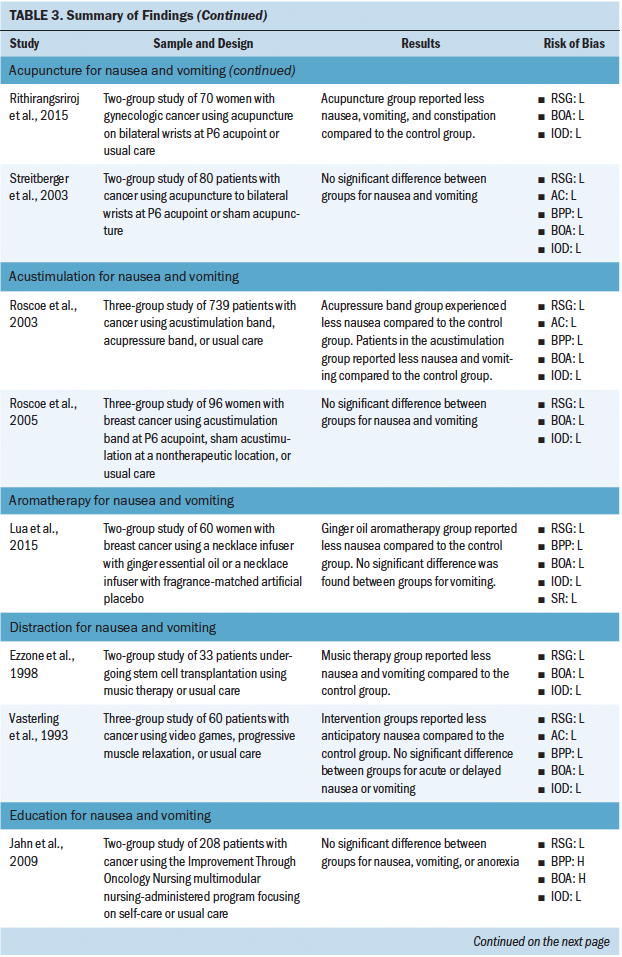
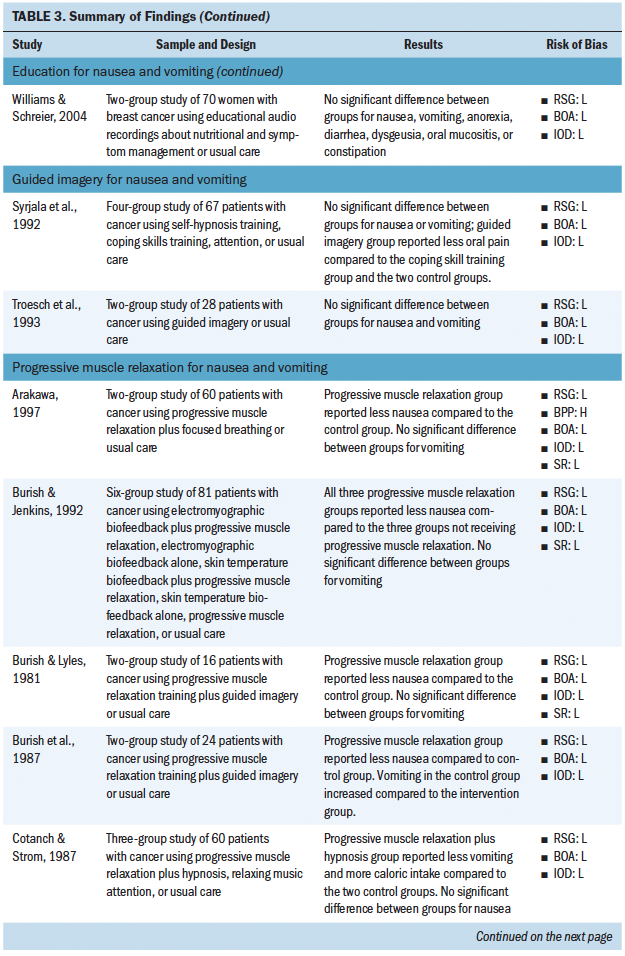
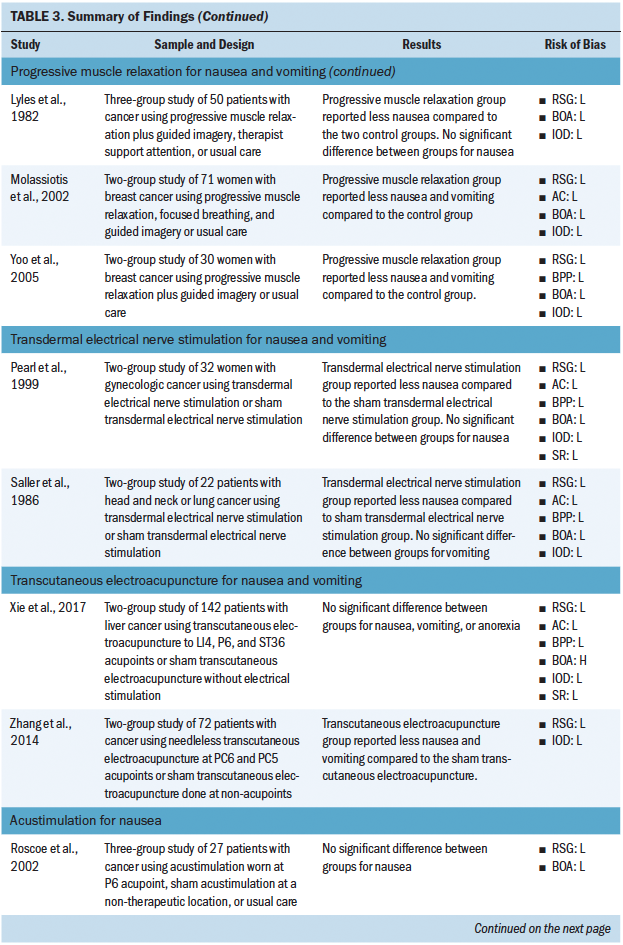
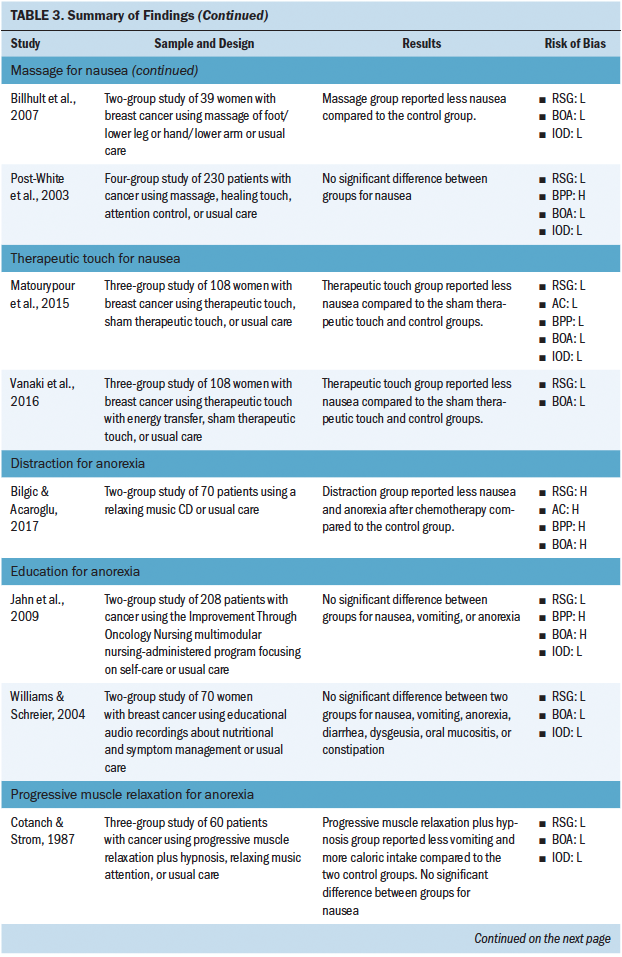
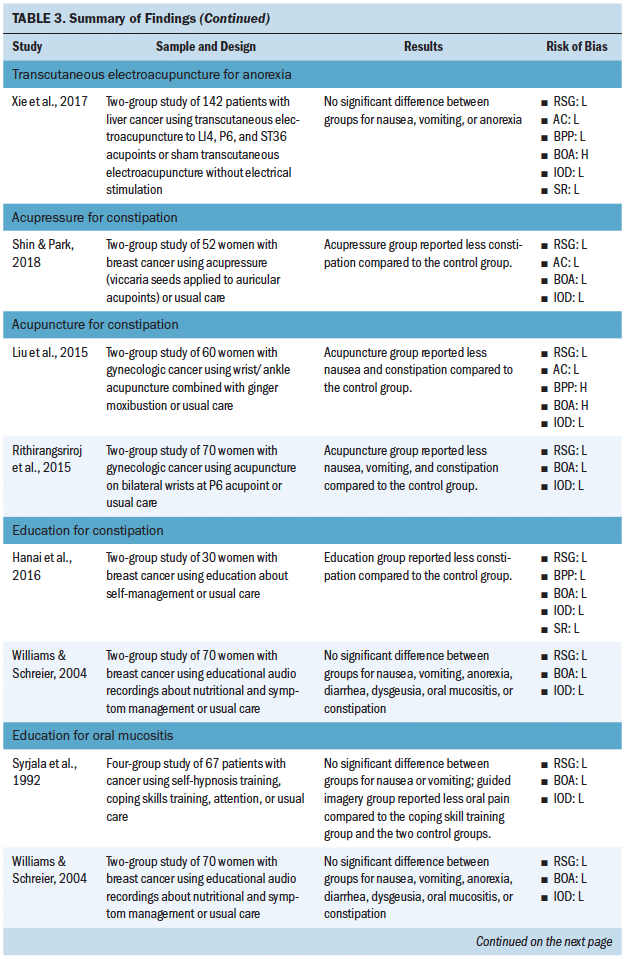
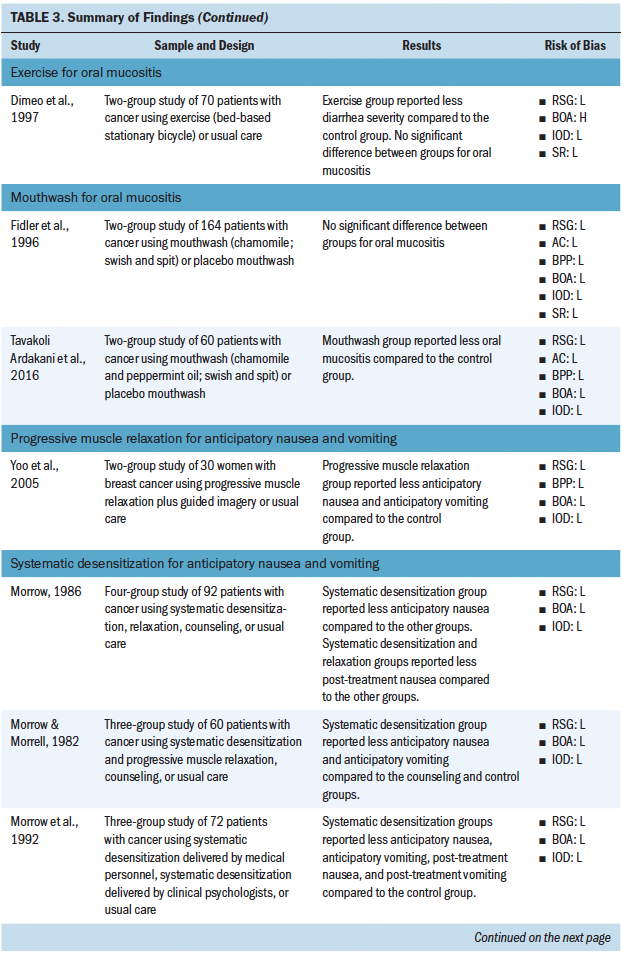
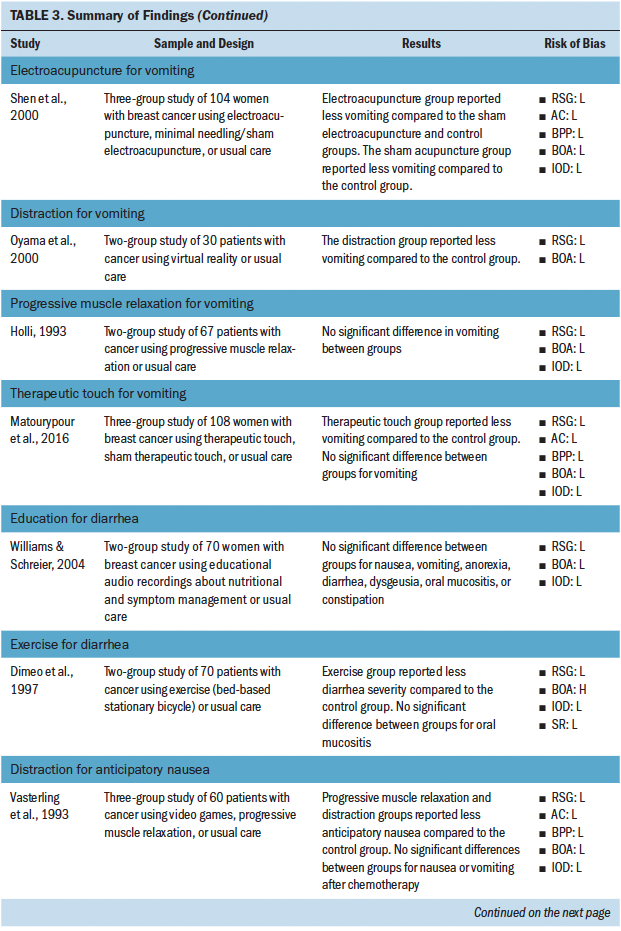
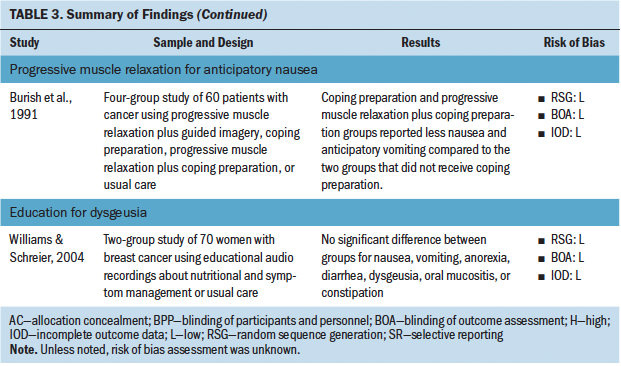
Most studies included in this review (n = 31) investigated the use of a nonpharmacologic complementary approach for nausea and vomiting (see Tables 2 and 3). The most common nonpharmacologic complementary interventions for nausea and vomiting included progressive muscle relaxation and acupressure. A handful of studies investigated acupuncture, acustimulation, distraction, education, guided imagery, transcutaneous electroacupuncture, transdermal electrical nerve stimulation, and aromatherapy. A majority of the studies found that the intervention had some effect on reducing nausea and vomiting. 
Nausea
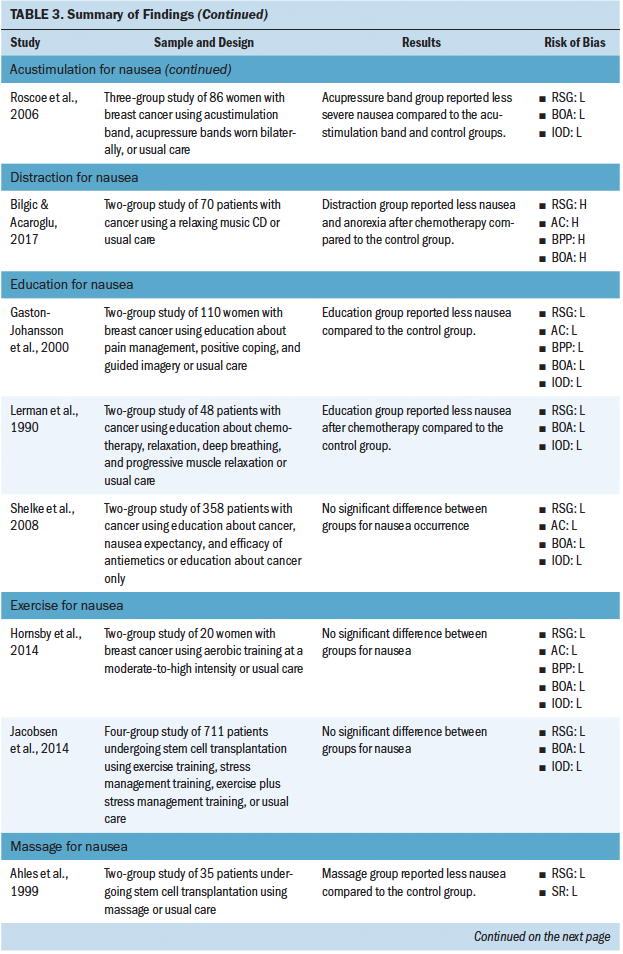
Thirteen studies investigated the use of nonpharmacologic complementary approaches for nausea alone. These interventions included massage, education, exercise, acustimulation, therapeutic touch, and distraction. A majority of these studies demonstrated a positive effect of the intervention for reducing nausea.
Anorexia
Five studies investigated the use of nonpharmacologic complementary approaches for anorexia. These interventions included education, distraction, progressive muscle relaxation, and transcutaneous electroacupuncture. Less than half of these studies demonstrated an effect of the intervention of reducing anorexia.
Constipation
Five studies investigated the use of nonpharmacologic complementary approaches for constipation. Interventions investigated included acupuncture, education, and acupressure. A majority of these studies demonstrated a positive effect of the intervention for reducing constipation.
Oral Mucositis
Five studies investigated the use of nonpharma-cologic complementary approaches for oral mucositis. Interventions included education, mouthwash, and exercise. Fewer than half of these studies demonstrated an effect of the intervention on reducing oral mucositis.
Anticipatory Nausea and Vomiting
Four studies investigated the use of nonpharmacologic complementary approaches for anticipatory nausea and vomiting. These interventions included systematic desensitization and progressive muscle relaxation. All of the studies demonstrated an effect on reducing anticipatory nausea and anticipatory vomiting.
Vomiting
Four studies investigated the use of nonpharmacologic complementary approaches for vomiting. These interventions included electroacupuncture, distraction, therapeutic touch, and progressive muscle relaxation. A majority demonstrated an effect of the intervention on reducing vomiting.
Diarrhea
Two studies investigated the use of nonpharmacologic complementary approaches for diarrhea. These interventions included education and exercise. One of the two studies demonstrated an effect of the intervention on reducing diarrhea.
Anticipatory Nausea
Two studies investigated the use of nonpharmacologic complementary approaches for anticipatory nausea alone. These interventions included distraction and progressive muscle relaxation. Both studies demonstrated an effect of the intervention on reducing anticipatory nausea.
Dysgeusia
One study investigated the use of a nonpharmacologic complementary approach for dysgeusia, which was education. This intervention was not shown to have an effect on reducing dysgeusia.
Nonpharmacologic Complementary Interventions
Most studies used a single intervention (e.g., acupuncture only), and few studies offered an intervention that combined more than one complementary approach (i.e., progressive muscle relaxation plus guided imagery). Overall, certain interventions were found to be more or less successful in having an effect on chemotherapy-associated GI symptoms. Of the studies using acupressure, progressive muscle relaxation, and acupuncture, 22 of 26 cases found a statistically significant effect of the intervention on GI symptoms. However, of the studies using an education-based intervention, the intervention was found to significantly affect GI symptoms in only 3 of 12 cases. Across all reviewed studies, sources of bias were low, but many reports lacked sufficient detail to assess source of bias. Sources of bias most consistently reported included not blinding participants and personnel and not blinding the outcome assessment. 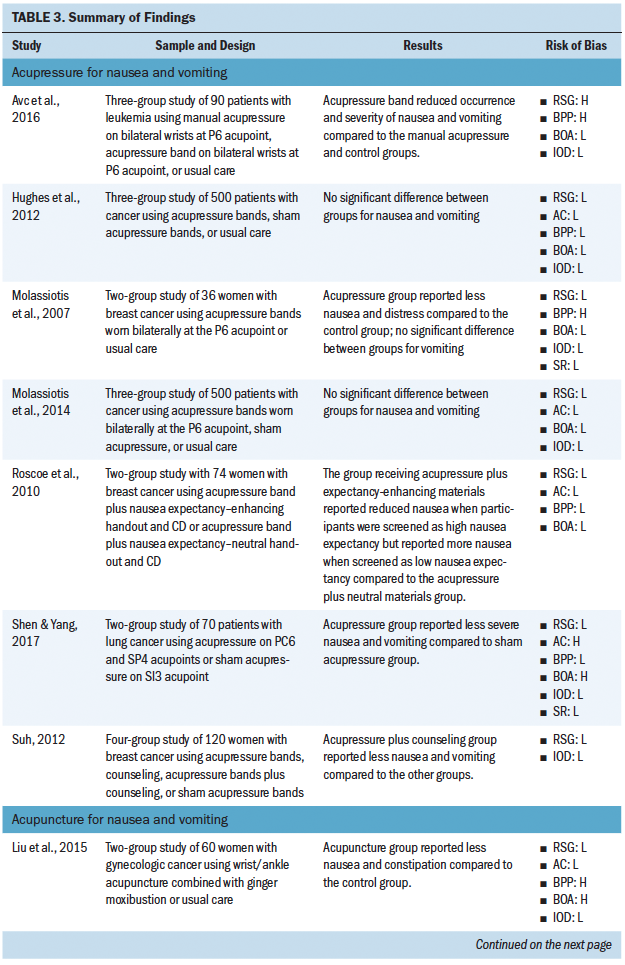
Discussion
Complementary therapy use has been growing, and many people find it to be a welcome addition to mainstream therapy. This is particularly true for patients with cancer because cancer treatments often produce many severe and distressing symptoms. Although pharmacologic therapy can reduce GI symptom severity, many GI symptoms remain problematic. Therefore, complementary nonpharmacologic therapies can offer additional relief where pharmacologic therapy cannot. New evidence is emerging demonstrating the effectiveness of nonpharmacologic complementary approaches to managing GI symptoms; however, to date, no summary has collected and critiqued that evidence. This review of the literature addresses this gap by summarizing the evidence provided by randomized, controlled trials investigating nonpharmacologic complementary therapy to manage GI symptoms.
This systematic review demonstrates that evidence supports the use of complementary approaches to manage certain GI symptoms. In total, 15 different complementary approaches were used to manage nine unique GI symptoms. Although this evidence is a meaningful step toward improved GI symptom management, many bothersome GI symptoms had little to no evidence to support the use of complementary approaches. Forty-eight of the 57 studies included in this review focused on nausea or vomiting, and only 19 studies focused on GI symptoms other than nausea or vomiting. Although nausea and vomiting are prevalent, severe, and distressing symptoms, they do not comprise the entirety of the chemotherapy symptom experience. Research has shown that GI symptoms like xerostomia, anorexia, bloating, and early satiety are prevalent, severe, and distressing in patients undergoing chemotherapy (Cherwin & Kwekkeboom, 2016; Thiagarajan et al., 2016). However, these symptoms are often overlooked in intervention studies. More research is needed investigating the effectiveness of nonpharmacologic therapies in preventing or relieving GI symptoms other than nausea and vomiting.
Although research on nonpharmacologic interventions has become more prevalent, more progress is needed. Thirty-three of 57 studies reviewed were published more than 10 years ago. Major advancements in chemotherapy have been made in that time, including the use of immunotherapy. Immunotherapy treatment regimens come with a different set of side effects as compared to the older, cytotoxic treatment regimens. Although this new class of cancer therapy tends to be better tolerated than older-generation cytotoxic therapies, it is still associated with many severe and distressing side effects, including a number of GI toxicities like dysgeusia, oral mucositis, xerostomia, and diarrhea (Rapoport et al., 2017). Studies need to account for the changing face of cancer treatment–related symptoms and evaluate how effective nonpharmacologic complementary therapies can be against these symptoms.
The available evidence has some methodologic limitations. Of the 326 full-text articles assessed for eligibility, about one-fourth had to be excluded because they were not randomized, controlled trails. There is a shortage of high-grade evidence describing the effectiveness of nonpharmacologic interventions for managing chemotherapy-related GI symptoms. In addition, of the 57 articles included for full review, a majority had a small sample size. The sample sizes for articles included for full review ranged from 16–739, but 42 of 57 studies had a sample size of less than 100. Although most of the studies reviewed showed a positive effect on GI symptoms as a result of a nonpharmacologic intervention, a number of studies did not show a difference in GI outcomes as a result of the intervention, or the intervention effect was not sustained. This may be a result of many of the studies not being powered appropriately to detect a difference caused by the intervention. More large-scale, randomized, controlled trials are needed to determine which nonpharmacologic interventions elicit an effect on GI symptom outcomes. Of the sources of bias most commonly found in the articles reviewed, not blinding the participant/personnel and not blinding the outcome assessment were the most common. It is often difficult to blind a participant or interventionist to the intervention when it is a complementary therapy like acupuncture or transdermal electrical nerve stimulation. However, more randomized, controlled trials are employing sham interventions in an effort to blind the participants to the intervention. Blinding the outcome assessment can be more problematic because assessing symptoms most often requires the person to self-report, making concealment difficult.
The results of the current literature review mirror the results of a 2008 review of nonpharmacologic strategies for managing common chemotherapy side effects (Lotfi-Jam et al., 2008). Lofti-Jam et al. (2008) concluded that, although many nonpharmacologic strategies have evidence to support their use for managing chemotherapy-related symptoms, much of the evidence was of lower quality, calling their results into question and making clinical application difficult. Despite the limitations of the studies reviewed in the current article, nonpharmacologic complementary therapy use is supported by evidence, generally inexpensive, safe for use, and well-liked by participants. Some of the interventions need a trained practitioner or assistance from a healthcare provider (e.g., acupuncture, transcutaneous electrical stimulation), but many of these interventions are self-care strategies that can be used at any time. The Oncology Nursing Society ([ONS], 2017) Putting Evidence Into Practice (PEP) recommends certain complementary approaches for managing treatment-related GI symptoms, such as hypnosis, progressive muscle relaxation, and guided imagery for nausea and vomiting. Although many complementary approaches are not yet specifically recommended for practice, practice recommendations will change to reflect this as more high-grade evidence is produced.
Limitations
Although inclusion criteria limited studies to randomized, controlled trials, the review of bias revealed that many studies had a high risk of bias regarding blinding of personnel or outcome assessment. This knowledge of who had the active intervention on the part of the participants and interventionists may have artificially skewed the results of the intervention to have a larger effect size than may have been actually present. A second limitation relates to the large number of studies that needed to be excluded from this review. Many studies were not considered for this review because the study design did not employ random assignment, the participants were not receiving chemotherapy exclusively, or the complementary therapy was an ingestible herbal remedy. This means that the articles reviewed were a relatively small selection of the total available research on complementary therapies, and the results are only applicable to a small subset of people with solid tumors. Future reviews should summarize evidence for herbal therapies, using people with hematologic cancers and people receiving other treatment regimens, such as radiation therapy or stem cell transplantations.
Implications for Practice
This review of evidence suggests that nonpharma-cologic complementary approaches are effective for managing chemotherapy-related GI symptoms in people with cancer. The National Center for Complementary and Integrative Health (2016) strategic plan has made care improvement for hard-to-manage symptoms a primary objective. GI symptoms can be classified as hard to manage because recent evidence has suggested that, despite the availability of pharmacologic interventions, more than 25% of patients with a hematologic cancer receiving chemotherapy experienced GI symptoms like nausea, xerostomia, anorexia, bloating, flatulence, dysgeusia, and constipation (Cherwin & Kwekkeboom, 2016). Complementary approaches can offer additional relief from GI symptoms when pharmacologic therapies are not sufficient. Oncology nurses should be aware of current practice recommendations to discuss complementary treatment approaches with their patients. ONS (2018) PEP committees review current literature on interventions and publish summaries of the strengths and limitations of that literature. Twenty symptom topics comprise the PEP summaries, five of which are GI symptoms (i.e., anorexia, chemotherapy-induced nausea and vomiting, constipation, diarrhea, and mucositis). Summaries like these are needed because a survey found that, although nurses like complementary therapy options for their patients, they feel unfamiliar with the research and are unsure of how to discuss these therapies with their patients (Hall, Leach, Brosnan, & Collins, 2017). Literature reviews, such as the one provided in the current article and the ONS PEP resources, are useful tools for practicing nurses to become familiar with evidence surrounding complementary therapy use in their patient population.
The further dissemination of high-grade research (i.e., randomized, controlled trials with large sample sizes) of complementary strategies to manage GI symptoms will help to strengthen the ONS PEP recommendations for use of these interventions. As of December 2018, only chemotherapy-induced nausea and vomiting has nonpharmacologic complementary therapies classified as Likely to be Effective, and only mucositis has nonpharmacologic complementary therapies classified as Recommended for Practice. For the other GI symptoms reviewed by the PEP committees, many of the interventions discussed in this review of literature are still classified as Effectiveness not Established, primarily because of the lack of high-grade evidence. A need exists for studies exploring GI symptoms beyond nausea and vomiting because treatment-related symptoms can be highly varied. Building the body of evidence will influence practice, which will in turn make more complementary approaches available to patients, ultimately reducing the treatment burden they face. 
Conclusion
The purpose of this systematic review was to summarize and critique the literature reporting nonpharmacologic management of chemotherapy-related GI symptoms. In particular, this review focused on high-grade evidence; therefore, only randomized, controlled trials were included. The results of this systematic review demonstrate that evidence supports the use of nonpharmacologic management of GI symptoms in chemotherapy, but a wide variety of interventions can be used for several GI symptoms, primarily nausea and vomiting. More research is needed to evaluate nonpharmacologic interventions for GI symptoms beyond nausea and vomiting.
The authors gratefully acknowledge Jennifer Deberg, MLS, for her assistance in conducting the literature search.
About the Author(s)
Catherine Cherwin, PhD, RN, is an assistant professor, and Lynn Nakad, BSN, RN, and Alaa Albashayreh, MSN, RN, are graduate research assistants, all in the College of Nursing at the University of Iowa in Iowa City. No financial relationships to disclose. Cherwin and Nakad contributed to the conceptualization and design and provided statistical support. All authors completed the data collection, provided the analysis, and contributed to the manuscript preparation. Cherwin can be reached at catherine-cherwin@uiowa.edu, with copy to ONFEditor@ons.org. (Submitted May 2018. Accepted June 27, 2018.)
References
Ahles, T.A., Tope, D.M., Pinkson, B., Walch, S., Hann, D., Whedon, M., . . . Silbarfarb, P.M. (1999). Massage therapy for patients undergoing autologous bone marrow transplantation. Journal of Pain and Symptom Management, 18, 157–163.
Arakawa, S. (1997). Relaxation to reduce nausea, vomiting, and anxiety induced by chemotherapy in Japanese patients. Cancer Nursing, 20, 342–349.
Avc, H.S., Ovayolu, N., & Ovayolu, Ö. (2016). Effect of acupressure on nausea-vomiting in patients with acute myeloblastic leukemia. Holistic Nursing Practice, 30, 257–262. https://doi.org/10.1097/HNP.0000000000000161
Bardia, A., Barton, D.L., Prokop, L.J., Bauer, B.A., & Moynihan, T.J. (2006). Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. Journal of Clinical Oncology, 24, 5457–5464. https://doi.org/10.1200/jco.2006.08.3725
Bilgic, S., & Acaroglu, R. (2017). Effects of listening to music on the comfort of chemotherapy patients. Western Journal of Nursing Research, 39, 745–762. https://doi.org/10.1177/0193945916660527
Billhult, A., Bergbom, I., & Stener-Victorin, E. (2007). Massage relieves nausea in women with breast cancer who are undergoing chemotherapy. Journal of Alternative and Complementary Medicine, 13, 53–57. https://doi.org/10.1089/acm.2006.6049
Burish, T.G., Carey, M.P., Krozely, M.G., & Greco, F.A. (1987). Conditioned side effects induced by cancer chemotherapy: Prevention through behavioral treatment. Journal of Consulting and Clinical Psychology, 55, 42–48.
Burish, T.G., & Jenkins, R.A. (1992). Effectiveness of biofeedback and relaxation training in reducing the side effects of cancer chemotherapy. Health Psychology, 11, 17–23.
Burish, T.G., & Lyles, J.N. (1981). Effectiveness of relaxation training in reducing adverse reactions to cancer chemotherapy. Journal of Behavioral Medicine, 4, 65–78.
Burish, T.G., Snyder, S.L., & Jenkins, R.A. (1991). Preparing patients for cancer chemotherapy: Effect of coping preparation and relaxation interventions. Journal of Consulting and Clinical Psychology, 59, 518–525.
Cherwin, C., & Kwekkeboom, K. (2016). Prevalence, duration, severity, and distress of chemotherapy-related gastrointestinal symptoms in patients with a hematologic malignancy. Oncology Nursing Forum, 43, 561–571. https://doi.org/10.1188/16.ONF.43-05AP
Clarke, T.C., Black, L.I., Stussman, B.J., Barnes, P.M., & Nahin, R.L. (2015). Trends in the use of complementary health approaches among adults: United States, 2002–2012. National Health Statistics Reports, 79, 1–16.
Clarkson, J.E., Worthington, H.V., Furness, S., McCabe, M., Khalid, T., & Meyer, S. (2010). Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews, 8, CD001973. https://doi.org/10.1002/14651858.CD001973.pub4
Cochrane Work. (n.d.). Identifying RCTs in PsycINFO2. Retrieved from http://work.cochrane.org/psycinfo
Cotanch, P.H., & Strom, S. (1987). Progressive muscle relaxation as antiemetic therapy for cancer patients. Oncology Nursing Forum, 14, 33–37.
Dimeo, F., Fetscher, S., Lange, W., Mertelsmann, R., & Keul, J. (1997). Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood, 90, 3390–3394.
Ezzone, S., Baker, C., Rosselet, R., & Terepka, E. (1998). Music as an adjunct to antiemetic therapy. Oncology Nursing Forum, 25, 1551–1556.
Fidler, P., Loprinzi, C.L., O’Fallon, J.R., Leitch, J.M., Lee, J.K., Hayes, D.L., . . . Michalak, J.C. (1996). Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer, 77, 522–525. https://doi.org/10.1002/(SICI)1097-0142(19960201)77:3<522::AID-CNCR14>3…
Finnegan-John, J., Molassiotis, A., Richardson, A., & Ream, E. (2013). A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integrative Cancer Therapies, 12, 276–290. https://doi.org/10.1177/1534735413485816
Furness, S., Bryan, G., McMillan, R., & Worthington, H.V. (2013). Interventions for the management of dry mouth: Non-pharmacological interventions. Cochrane Database of Systematic Reviews, 8, CD009603. https://doi.org/10.1002/14651858.CD009603.pub2
Gaston-Johansson, F., Fall-Dickson, J.M., Nanda, J., Ohly, K.V., Stillman, S., Krumm, S., & Kennedy, M.J. (2000). The effectiveness of the comprehensive coping strategy program on clinical outcomes in breast cancer autologous bone marrow transplantation. Cancer Nursing, 23, 277–285.
Goodell, T.T., & Nail, L.M. (2005). Operationalizing symptom distress in adults with cancer: A literature synthesis [Online exclusive]. Oncology Nursing Forum, 32, E42–E47. https://doi.org/10.1188/05.ONF.E42-E47
Hall, H., Leach, M., Brosnan, C., & Collins, M. (2017). Nurses’ attitudes towards complementary therapies: A systematic review and meta-synthesis. International Journal of Nursing Studies, 69, 47–56. https://doi.org/10.1016/j.ijnurstu.2017.01.008
Hanai, A., Ishiguro, H., Sozu, T., Tsuda, M., Arai, H., Mitani, A., & Tsuboyama, T. (2016). Effects of a self-management program on antiemetic-induced constipation during chemotherapy among breast cancer patients: A randomized controlled clinical trial. Breast Cancer Research and Treatment, 155, 99–107. https://doi.org/10.1007/s10549-015-3652-4
Haynes, R.B., McKibbon, K.A., Wilczynski, N.L., Walter, S.D., & Werre, S.R. (2005). Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: Analytical survey. BMJ, 330, 1179. https://doi.org/10.1136/bmj.38446.498542.8F
Higgins, J., & Green, S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions [v.5.1.0]. Retrieved from http://handbook-5-1.cochrane.org/
Holli, K. (1993). Ineffectiveness of relaxation on vomiting induced by cancer chemotherapy. European Journal of Cancer, 29A, 1915–1916.
Horneber, M., Bueschel, G., Dennert, G., Less, D., Ritter, E., & Zwahlen, M. (2012). How many cancer patients use complementary and alternative medicine: A systematic review and metaanalysis. Integrative Cancer Therapies, 11, 187–203. https://doi.org/10.1177/1534735411423920
Hornsby, W.E., Douglas, P.S., West, M.J., Kenjale, A.A., Lane, A.R., Schwitzer, E.R., . . . Jones, L.W. (2014). Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: A phase II randomized trial. Acta Oncologica, 53, 65–74. https://doi.org/10.3109/0284186x.2013.781673
Hughes, J., Molassiotis, A., Russell, W., Breckons, M., Lloyd-Williams, M., & Richardson, J. (2012). The effectiveness and cost effectiveness of acupressure for chemotherapy-related nausea. BMC Complementary and Alternative Medicine, 12(Suppl. 1), O4. https://doi.org/10.1186/1472-6882-12-S1-O4
Jacobsen, P.B., Le-Rademacher, J., Jim, H., Syrjala, K., Wingard, J.R., Logan, B., . . . Lee, S.J. (2014). Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biology of Blood and Marrow Transplantation, 20, 1530–1536. https://doi.org/10.1016/j.bbmt.2014.05.027
Jahn, P., Renz, P., Stukenkemper, J., Book, K., Kuss, O., Jordan, K., . . . Landenberger, M. (2009). Reduction of chemotherapy-induced anorexia, nausea, and emesis through a structured nursing intervention: A cluster-randomized multicenter trial. Supportive Care in Cancer, 17, 1543–1552. https://doi.org/10.1007/s00520-009-0698-z
Langford, D.J., Lee, K., & Miaskowski, C. (2012). Sleep disturbance interventions in oncology patients and family caregivers: A comprehensive review and meta-analysis. Sleep Medicine Reviews, 16, 397–414. https://doi.org/10.1016/j.smrv.2011.07.002
Lerman, C., Rimer, B., Blumberg, B., Cristinzio, S., Engstrom, P.F., MacElwee, N., . . . Seay, J. (1990). Effects of coping style and relaxation on cancer chemotherapy side effects and emotional responses. Cancer Nursing, 13, 308–315.
Lewis, S.L., Bucher, L., Heitkemper, M.M., Harding, M.M., Kwong, J., & Roberts, D. (2017). Medical-surgical nursing: Assessment and management of clinical problems (10th ed.). St. Louis, MO: Mosby.
Liu, Y.Q., Sun, S., Dong, H.J., Zhai, D.X., Zhang, D.Y., Shen, W., . . . Yu, C.Q. (2015). Wrist-ankle acupuncture and ginger moxibustion for preventing gastrointestinal reactions to chemotherapy: A randomized controlled trial. Chinese Journal of Integrative Medicine, 21, 697–702. https://doi.org/10.1007/s11655-014-2009-x
Lotfi-Jam, K., Carey, M., Jefford, M., Schofield, P., Charleson, C., & Aranda, S. (2008). Nonpharmacologic strategies for managing common chemotherapy adverse effects: A systematic review. Journal of Clinical Oncology, 26, 5618–5629. https://doi.org/10.1200/jco.2007.15.9053
Lua, P.L., Salihah, N., & Mazlan, N. (2015). Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complementary Therapies in Medicine, 23, 396–404. https://doi.org/10.1016/j.ctim.2015.03.009
Lyles, J.N., Burish, T.G., Krozely, M.G., & Oldham, R.K. (1982). Efficacy of relaxation training and guided imagery in reducing the aversiveness of cancer chemotherapy. Journal of Consulting and Clinical Psychology, 50, 509–524.
Matourypour, P., Vanaki, Z., Zare, Z., Mehrzad, V., Dehghan, M., & Ranjbaran, M. (2016). Investigating the effect of therapeutic touch on the intensity of acute chemotherapy-induced vomiting in breast cancer women under chemotherapy. Iranian Journal of Nursing and Midwifery Research, 21, 255–260. https://doi.org/10.4103/1735-9066.180373
Matourypour, P., Zare, Z., Mehrzad, V., Musarezaie, A., Dehghan, M., & Vanaki, Z. (2015). An investigation of the effects of therapeutic touch plan on acute chemotherapy-induced nausea in women with breast cancer in Isfahan, Iran, 2012–2013. Journal of Education and Health Promotion, 4, 61. https://doi.org/10.4103/2277-9531.162380
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
Molassiotis, A., Helin, A.M., Dabbour, R., & Hummerston, S. (2007). The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complementary Therapies in Medicine, 15, 3–12. https://doi.org/10.1016/j.ctim.2006.07.005
Molassiotis, A., Russell, W., Hughes, J., Breckons, M., Lloyd-Williams, M., Richardson, J., . . . Ryder, W.D. (2014). The effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: A randomized controlled trial. Journal of Pain and Symptom Management, 47, 12–25. https://doi.org/10.1016/j.jpainsymman.2013.03.007
Molassiotis, A., Yung, H.P., Yam, B.M., Chan, F.Y., & Mok, T.S. (2002). The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: A randomised controlled trial. Supportive Care in Cancer, 10, 237–246. https://doi.org/10.1007/s00520-001-0329-9
Morrow, G.R. (1986). Effect of the cognitive hierarchy in the systematic desensitization treatment of anticipatory nausea in cancer patients: A component comparison with relaxation only, counseling, and no treatment. Cognitive Therapy and Research, 10, 421–446. https://doi.org/10.1007/BF01173295
Morrow, G.R., Asbury, R., Hammon, S., Dobkin, P., Caruso, L., Pandya, K., & Rosenthal, S. (1992). Comparing the effectiveness of behavioral treatment for chemotherapy-induced nausea and vomiting when administered by oncologists, oncology nurses, and clinical psychologists. Health Psychology, 11, 250–256.
Morrow, G.R., & Morrell, C. (1982). Behavioral treatment for the anticipatory nausea and vomiting induced by cancer chemotherapy. New England Journal of Medicine, 307, 1476–1480. https://doi.org/10.1056/nejm198212093072402
National Center for Complementary and Integrative Health. (2016). NCCIH 2016 strategic plan. Retrieved from https://nccih.nih.gov/about/strategic-plans/2016
National Center for Complementary and Integrative Health. (2017). Objectives and strategies. Retrieved from https://nccih.nih.gov/about/research/objectives
Oncology Nursing Society. (2017). Chemotherapy-induced nausea and vomiting. Retrieved from https://www.ons.org/practice-resources/pep/chemotherapy-induced-nausea-…
Oncology Nursing Society. (2018). Symptom interventions. Retrieved from https://www.ons.org/explore-resources?display=source&source=1506&ref=RO
Oyama, H., Kaneda, M., Katsumata, N., Akechi, T., & Ohsuga, M. (2000). Using the bedside wellness system during chemotherapy decreases fatigue and emesis in cancer patients. Journal of Medical Systems, 24, 173–182.
Pearl, M.L., Fischer, M., McCauley, D.L., Valea, F.A., & Chalas, E. (1999). Transcutaneous electrical nerve stimulation as an adjunct for controlling chemotherapy-induced nausea and vomiting in gynecologic oncology patients. Cancer Nursing, 22, 307–311.
Post-White, J., Kinney, M.E., Savik, K., Gau, J.B., Wilcox, C., & Lerner, I. (2003). Therapeutic massage and healing touch improve symptoms in cancer. Integrative Cancer Therapies, 2, 332–344. https://doi.org/10.1177/1534735403259064
Rapoport, B.L., van Eeden, R., Sibaud, V., Epstein, J.B., Klastersky, J., Aapro, M., & Moodley, D. (2017). Supportive care for patients undergoing immunotherapy. Supportive Care in Cancer, 25, 3017–3030. https://doi.org/10.1007/s00520-017-3802-9
Rithirangsriroj, K., Manchana, T., & Akkayagorn, L. (2015). Efficacy of acupuncture in prevention of delayed chemotherapy induced nausea and vomiting in gynecologic cancer patients. Gynecologic Oncology, 136, 82–86. https://doi.org/10.1016/j.ygyno.2014.10.025
Roscoe, J.A., Jean-Pierre, P., Morrow, G.R., Hickok, J.T., Issell, B., Wade, J.L., & King, D.K. (2006). Exploratory analysis of the usefulness of acupressure bands when severe chemotherapy-related nausea is expected. Journal of the Society for Integrative Oncology, 4, 16–20.
Roscoe, J.A., Matteson, S.E., Morrow, G.R., Hickok, J.T., Bushunow, P., Griggs, J., . . . Smith, J. (2005). Acustimulation wrist bands are not effective for the control of chemotherapy-induced nausea in women with breast cancer. Journal of Pain and Symptom Management, 29, 376–384. https://doi.org/10.1016/j.jpainsymman.2004.07.007
Roscoe, J.A., Morrow, G.R., Bushunow, P., Tian, L., & Matteson, S. (2002). Acustimulation wristbands for the relief of chemotherapy-induced nausea. Alternative Therapies in Health and Medicine, 8(4), 56–57.
Roscoe, J.A., Morrow, G.R., Hickok, J.T., Bushunow, P., Pierce, H.I., Flynn, P.J., . . . Atkins, J.N. (2003). The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting. A University of Rochester Cancer Center Community Clinical Oncology Program multicenter study. Journal of Pain and Symptom Management, 26, 731–742.
Roscoe, J.A., O’Neill, M., Jean-Pierre, P., Heckler, C.E., Kaptchuk, T.J., Bushunow, P., . . . Smith, B. (2010). An exploratory study on the effects of an expectancy manipulation on chemotherapy-related nausea. Journal of Pain and Symptom Management, 40, 379–390. https://doi.org/10.1016/j.jpainsymman.2009.12.024
Saller, R., Hellenbrecht, D., Bühring, M., & Hess, H. (1986). Enhancement of the antiemetic action of metoclopramide against cisplatin-induced emesis by transdermal electrical nerve stimulation. Journal of Clinical Pharmacology, 26, 115–119.
Shelke, A.R., Roscoe, J.A., Morrow, G.R., Colman, L.K., Banerjee, T.K., & Kirshner, J.J. (2008). Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: A University of Rochester cancer center community clinical oncology program study. Journal of Pain and Symptom Management, 35, 381–387. https://doi.org/10.1016/j.jpainsymman.2007.05.008
Shen, C.H., & Yang, L.Y. (2017). The effects of ccupressure on meridian energy as well as nausea and vomiting in lung cancer patients receiving chemotherapy. Biological Research for Nursing, 19, 145–152. https://doi.org/10.1177/1099800416683801
Shen, J., Wenger, N., Glaspy, J., Hays, R.D., Albert, P.S., Choi, C., & Shekelle, P.G. (2000). Electroacupuncture for control of myeloablative chemotherapy-induced emesis: A randomized controlled trial. JAMA, 284, 2755–2761.
Shin, J., & Park, H. (2018). Effects of auricular acupressure on constipation in patients with breast cancer receiving chemotherapy: A randomized control trial. Western Journal of Nursing Research, 40, 67–83. https://doi.org/10.1177/0193945916680362
Spichiger, E., Müller-Fröhlich, C., Denhaerynck, K., Stoll, H., Hantikainen, V., & Dodd, M. (2011). Prevalence of symptoms, with a focus on fatigue, and changes of symptoms over three months in outpatients receiving cancer chemotherapy. Swiss Medical Weekly, 141, w13303. https://doi.org/10.4414/smw.2011.13303
Streitberger, K., Friedrich-Rust, M., Bardenheuer, H., Unnebrink, K., Windeler, J., Goldschmidt, H., & Egerer, G. (2003). Effect of acupuncture compared with placebo-acupuncture at P6 as additional antiemetic prophylaxis in high-dose chemotherapy and autologous peripheral blood stem cell transplantation: A randomized controlled single-blind trial. Clinical Cancer Research, 9, 2538–2544.
Stussman, B.J., Black, L.I., Barnes, P.M., Clarke, T.C., & Nahin, R.L. (2015). Wellness-related use of common complementary health approaches among adults: United States, 2012. National Health Statistics Reports, 4(85), 1–12.
Suh, E.E. (2012). The effects of P6 acupressure and nurse-provided counseling on chemotherapy-induced nausea and vomiting in patients with breast cancer [Online exclusive]. Oncology Nursing Forum, 39, E1–E9. https://doi.org/10.1188/12.ONF.E1-E9
Syrjala, K.L., Cummings, C., & Donaldson, G.W. (1992). Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: A controlled clinical trial. Pain, 48, 137–146.
Tavakoli Ardakani, M., Ghassemi, S., Mehdizadeh, M., Mojab, F., Salamzadeh, J., Ghassemi, S., & Hajifathali, A. (2016). Evaluating the effect of Matricaria recutita and Mentha piperita herbal mouthwash on management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, double blind, placebo controlled clinical trial. Complementary Therapies in Medicine, 29, 29–34. https://doi.org/10.1016/j.ctim.2016.08.001
Thiagarajan, M., Chan, C.M., Fuang, H.G., Beng, T.S., Atiliyana, M.A., & Yahaya, N.A. (2016). Symptom prevalence and related distress in cancer patients undergoing chemotherapy. Asian Pacific Journal of Cancer Prevention, 17, 171–176.
Tipton, J.M., McDaniel, R.W., Barbour, L., Johnston, M.P., Kayne, M., LeRoy, P., & Ripple, M.L. (2007). Putting evidence into practice: Evidence-based interventions to prevent, manage, and treat chemotherapy-induced nausea and vomiting. Clinical Journal of Oncology Nursing, 11, 69–78. https://doi.org/10.1188/07.cjon.69-78
Troesch, L.M., Rodehaver, C.B., Delaney, E.A., & Yanes, B. (1993). The influence of guided imagery on chemotherapy-related nausea and vomiting. Oncology Nursing Forum, 20, 1179–1185.
Vanaki, Z., Matourypour, P., Gholami, R., Zare, Z., Mehrzad, V., & Dehghan, M. (2016). Therapeutic touch for nausea in breast cancer patients receiving chemotherapy: Composing a treatment. Complementary Therapies in Clinical Practice, 22, 64–68. https://doi.org/10.1016/j.ctcp.2015.12.004
Vasterling, J., Jenkins, R.A., Tope, D.M., & Burish, T.G. (1993). Cognitive distraction and relaxation training for the control of side effects due to cancer chemotherapy. Journal of Behavioral Medicine, 16, 65–80.
Williams, S.A., & Schreier, A.M. (2004). The effect of education in managing side effects in women receiving chemotherapy for treatment of breast cancer [Online exclusive]. Oncology Nursing Forum, 31, E16–23. https://doi.org/10.1188/04.ONF.E16-E23
Xie, J., Chen, L.H., Ning, Z.Y., Zhang, C.Y., Chen, H., Chen, Z., . . . Zhu, X.Y. (2017). Effect of transcutaneous electrical acupoint stimulation combined with palonosetron on chemotherapy-induced nausea and vomiting: A single-blind, randomized, controlled trial. Chinese Journal of Cancer, 36, 6. https://doi.org/10.1186/s40880-016-0176-1
Yoo, H.J., Ahn, S.H., Kim, S.B., Kim, W.K., & Han, O.S. (2005). Efficacy of progressive muscle relaxation training and guided imagery in reducing chemotherapy side effects in patients with breast cancer and in improving their quality of life. Supportive Care in Cancer, 13, 826–833. https://doi.org/10.1007/s00520-005-0806-7
Zhang, X., Jin, H.F., Fan, Y.H., Lu, B., Meng, L.N., & Chen, J.D. (2014). Effects and mechanisms of transcutaneous electroacupuncture on chemotherapy-induced nausea and vomiting. Evidence-Based Complementary and Alternative Medicine, 2014, 860631. https://doi.org/10.1155/2014/860631




