Chronic Stress and Ovarian Function in Female Childhood Cancer Survivors
Objectives: To explore the relationships among perceived stress, biomarkers of hypothalamic–pituitary–adrenal (HPA) activity, gonadotropin levels, and anti-Müllerian hormone (AMH) in female childhood cancer survivors (CCSs).
Sample & Setting: 24 female CCSs from the Royal Hospital for Sick Children in Edinburgh, Scotland, were included in the study.
Methods & Variables: Perceived stress was measured using the Perceived Stress Scale. HPA activity was measured using salivary cortisol and hair cortisol. Ovarian function was measured using serum gonadotropin levels and serum AMH levels. Latent growth curve modeling was used to determine diurnal cortisol slope and intercept. Bayesian structural equation modeling was used to explore the relationship among perceived stress, biomarkers of HPA activity, and ovarian function.
Results: The authors found an inverse association between perceived stress and ovarian function and a positive association between biomarkers of HPA activity and ovarian function.
Implications for Nursing: Further research is needed to understand factors contributing to risk for post-treatment reproductive dysfunction in female CCSs.
Jump to a section
An estimated 1 in 1,000 adults aged younger than 35 years is a survivor of childhood cancer (Blumenfeld, 2012). Because of significant advances in the treatment of pediatric cancer, the five-year survival rate exceeds 80% in the United States (Phillips et al., 2015). With an increasing number of survivors, there has been growing recognition of the late effects of cancer treatment (Kremer et al., 2013). Among these, reproductive dysfunction is a major concern for cancer survivors and is highly correlated with quality of life in this population (Cherven, Mertens, Wasilewski-Masker, Williamson, & Meacham, 2015; Knopman, Papadopoulos, Grifo, Fino, & Noyes, 2010; Kondapalli et al., 2014; Letourneau, Chan, & Rosen, 2013). Among female childhood cancer survivors (CCSs), 6% experience acute ovarian failure, and another 23% experience a significant reduction in ovarian function (Salih et al., 2015).
In clinical practice, post-treatment ovarian function is assessed using a profile of hormones, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH), plus the presence or absence of menses. However, neither FSH nor menstrual cyclicity post–cancer treatment are reliable predictors of future fertility (Knight et al., 2015). Because risk of infertility related to premature ovarian failure is associated with the size of the ovarian follicle pool (ovarian reserve), a biomarker that more closely reflects the number of remaining follicles in the ovary would have significant clinical potential. Anti-Müllerian hormone (AMH) is a biomarker of the ovarian reserve; plasma levels reflect the continuous noncyclic growth of small follicles and, therefore, mirror the size of the remaining follicle pool (Jeppesen et al., 2013). AMH is prematurely reduced in CCSs (Anderson & Wallace, 2013; Charpentier et al., 2014; Miyoshi et al., 2013) and may be an early marker of significant gonadotoxicity post-treatment (Brougham et al., 2012; Lie Fong et al., 2009). Although studies have demonstrated the use of pretreatment AMH concentrations to predict risk of post-treatment ovarian dysfunction in cancer survivors (Anderson & Wallace, 2013; Lunsford, Whelan, McCormick, & McLaren, 2014), its predictive value is limited because of incomplete knowledge of factors that influence AMH concentrations (Abusief, Missmer, Ginsburg, Weeks, & Partridge, 2012; van Dorp et al., 2014).
Cancer survivors have high rates of psychological stress and hypothalamic–pituitary–adrenal (HPA) dysregulation (Oancea et al., 2014; Taylor, Absolom, Snowden, & Eiser, 2012; Zeltzer et al., 2009). One of the primary regulators of reproductive function is the HPA axis. Psychological stress impairs reproductive function by activating the HPA axis, which suppresses hypothalamic pituitary gonadal function (Kalantaridou et al., 2010; Louis et al., 2011; Lynch, Sundaram, Maisog, Sweeney, & Buck Louis, 2014; Whirledge & Cidlowski, 2013). Increased HPA activity is associated with elevated levels of glucocorticoids, such as cortisol. At homeostatic levels, cortisol contributes to steroid biosynthesis and maintenance of gonadotropin release; at elevated levels, it suppresses gonadotropin-releasing hormone secretion, reduces pulsatile LH secretion, and increases rates of follicle atresia (Breen & Mellon, 2014; Whirledge & Cidlowski, 2013). Because higher rates of psychological stress and reproductive dysfunction are observed in cancer survivors, the purpose of this study was to explore relationships among perceived stress, HPA activity, and ovarian function in a sample of female CCSs.
Methods
The sample for this exploratory cross-sectional study consisted of female CCSs aged from 16 to 35 years, who were previously treated for cancer at the Edinburgh Children’s Cancer Centre at the Royal Hospital for Sick Children in Scotland. The inclusion criteria were female patients treated for cancer at the Edinburgh Children’s Cancer Centre who were aged younger than 18 years at cancer diagnosis, were aged from 16 to 35 years at the time of the study visit, were at least one year from completing treatment, and signed written informed consent. The exclusion criteria were a positive pregnancy test, ovarian surgery in the past six months, hormonal therapy in the past three months (GnRH agonists, recFSH), alcohol or drug abuse/dependence according to ICD-10 criteria, or having received an investigational drug in the past three months.
The Marquette University Institutional Review Board and the South East Scotland Research Ethics Committee approved this study. Data management was compliant with the most current guidelines of the Health Insurance Portability and Accountability Act of 1996 and in full conformity with the principles of the Declaration of Helsinki, in accordance with the principles laid down in the Convention of the Council of Europe for the protection of human rights and dignity of the human being. Original patient names and hospital bar codes were deidentified by using a new, independent numbering system for all study-related documentation. A link-list was established and kept separate from other study documentation. The link-list was only accessible to the study investigators and was kept in a locked office at the Royal Hospital for Sick Children.
Instruments
Assessment of ovarian function included serum gonadotropin levels (LH and FSH), estradiol (E2), and AMH. Serum levels of these hormones are routinely measured during the annual follow-up appointment. LH, FSH, and E2 levels are measured to screen for gonadotropin deficiency and ovarian dysfunction post–cancer treatment (Metzger et al., 2013). The laboratory results were retrieved from participants’ medical records. Serum AMH levels were analyzed using the Beckman Coulter AMH Gen II ELISA. The AMH Gen II ELISA has a sensitivity of 0.57 pmol/L.
HPA activity was determined using measures of salivary cortisol and hair cortisol. Salivary cortisol has been used to measure HPA activity in cancer survivors and other populations (Du et al., 2013; Ho, Fong, Chan, & Chan, 2013; Sephton et al., 2013). Cortisol secretory activity follows a diurnal pattern, with levels peaking after awakening and lowest levels before going to bed (Kirschbaum & Hellhammer, 1989). Chronic stress is associated with alterations in the cortisol awakening response (Dedovic & Ngiam, 2015; Révész et al., 2013) and blunting of the morning–evening diurnal slope (Garland, Beck, Lipschitz, & Nakamura, 2015; Ho et al., 2013; Schrepf et al., 2015). A commercially available ELISA kit by Salimetrics® was used for the quantification of cortisol in saliva. This cortisol assay kit has a lower detection limit of 7 ng/dl. The mean intra-assay coefficient is 5.7%, and the mean inter-assay coefficient is 10%.
Chronic stress has also been associated with elevated levels of cortisol in hair (Russell, Koren, Rieder, & Van Uum, 2012; Wells et al., 2014). Hair has a predictable growth rate of 1 cm per month; the most proximal 1 cm segment to the scalp approximates the past month’s cortisol production, the second most proximal segment the month prior to that, and so on (Russell et al., 2012). Hair (3 cm) was processed for extraction of cortisol in the laboratory of Clemens-Kirschbaum in Dresden, Germany, as previously described (Kirschbaum, Tietze, Skoluda, & Dettenborn, 2009).
Perceived stress was measured using the Perceived Stress Scale (PSS-10) (Cohen, Kamarck, & Mermelstein, 1983). The PSS-10 is a 10-item self-report scale measuring life stress during the past four weeks and is the most widely used measure of this concept. The PSS-10 was designed for use in community samples of participants with education as low as junior high. Good to excellent reliability has been observed with the PSS-10 in varied populations (Cohen et al., 1983), with a Cronbach alpha ranging from 0.78–0.91 (Lee, 2012). The measure has been demonstrated to reliably measure stress appraisal in cancer survivors (Golden-Kreutz, Browne, Frierson, & Andersen, 2009).
Data Collection
Recruitment was conducted by sending letters of invitation to female CCSs in long-term follow-up two weeks prior to their annual review appointment. After providing informed consent, saliva-collecting devices and detailed instructions for collection of samples were provided to participants at their appointment. Participants were instructed not to eat, smoke, consume caffeine, or exercise for 30 minutes prior to sample collection (Schrepf et al., 2015). Participants provided three timed saliva samples for the analysis of salivary cortisol: the first sample was collected before going to bed on the night prior to the study visit, the second immediately upon awakening the morning of their visit before getting out of bed, and the third 30 minutes after the second sample. Participants were instructed to record the time of saliva collection on their saliva-collecting devices and to keep the saliva samples refrigerated until coming in for their study visit to preserve sample integrity. Participants brought the three saliva samples to their study visit. Samples were stored at the Royal Hospital for Sick Children at –20ºC. Within a week of collection, a research nurse picked them up and brought them to the Queens Medical Research Institute, where they were centrifuged and stored at –80ºC.
During the study visit, participants provided demographic information, including age, ethnicity/race, level of education, marital status, and employment status. The following clinical information was retrieved from participants’ medical records: cancer diagnosis, age at cancer diagnosis, chemotherapy/radiation received, and age at menarche. Participants then completed the PSS-10 (Cohen et al., 1983). Lastly, participants provided a sample of hair for measurement of hair cortisol. A small sample of hair, 20 mg (100–150 strands), was taken from the vertex posterior of the participant’s scalp using a new pair of thinning shears (Hoffman, Karban, Benitez, Goodteacher, & Laudenslager, 2014; Van Uum et al., 2008). Hair length was recorded, and proximal ends of the hair samples were marked. Hair samples were stored in aluminum foil for protection before processing (Hoffman et al., 2014) and labeled with a unique participant study ID.
Data Analysis and Interpretation
Means and standard deviations for all demographic and clinical variables were reported to describe the sample and reveal distributional characteristics. Latent growth curve modeling was used to define diurnal cortisol change over time (three time points). Change processes are determined by combining the underlying pattern of growth (slope) with the initial or baseline measurement (intercept). The intercept and slope were then incorporated into the overall model as independent predictors of ovarian function.
Bayesian structural equation modeling (SEM) was used to explore the relationship between perceived stress and HPA activity and to explore relationships between perceived stress, biomarkers of HPA activity, gonadotropin levels, and AMH (see Figure 1). Bayesian SEM was selected because it facilitates greater precision in estimating the posterior distribution of the parameters for small samples. The posterior distribution describes the best estimate of the parameters given the data and the level of uncertainty about the respective parameter by the width of it, represented by the credible interval (Yuan & MacKinnon, 2009). Bayesian SEM also offers the advantage of incorporating previous knowledge about the model’s parameters through the use of priors. The term “prior” is used to refer to the expected parameter distribution based on theory or previous research. Given the exploratory nature of this study, the authors examined the model parameters using weakly informed priors. Weakly informed priors are not intended to guide the parameters, but rather to provide information to delimitate the most likely data space for the parameters. The authors used a special case of SEM called path analysis. In path analysis, every variable in the model is directly measured or observed (Bryan, Schmiege, & Broaddus, 2007). The authors ran path analysis models with salivary cortisol intercept and slope, PSS-10, hair cortisol, body mass index, age, age at diagnosis, and age at menarche as predictors and with AMH, E2, FSH, and LH as outcomes. 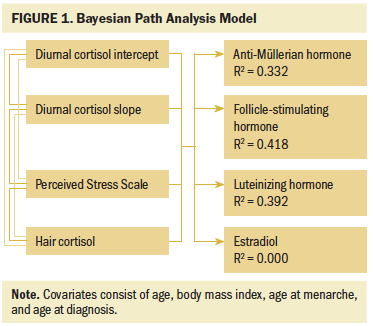
Data augmentation was used to account for missing data. Missing data were treated simultaneously as a parameter and data, filling any missing data points with the most likely value given the posterior distribution (Merkle, 2011). Data analysis was conducted in R (Core Team, 2017). Bayesian path analysis was run with the blavaan R package (Merkle & Rosseel, 2018), which estimates the model with the general Bayesian software JAGS (Plummer, 2003). Convergence of the Markov chains was determined using the potential scale reduction factor, also known as univariate R-hat (Gelman & Rubin, 1992). Model convergence was achieved when R-hat was lower than 1.1 for every parameter (Brooks & Gelman, 1998). The model was run with three chains, keeping the last 5,000 iterations from each chain to build the posterior distributions.
Results
Twenty-four female CCSs (mean age = 21.79 years, SD = 5.68) participated in the study. The majority had at least a high school education (n = 15) and were unmarried (n = 19) (see Table 1). Childhood cancer diagnoses varied considerably and included osteosarcoma (n = 1), rhabdomyosarcoma (n = 5), Wilms tumor (n = 1), optic chiasmal glioma (n = 1), non-Hodgkin lymphoma (n = 3), Ewing sarcoma (n = 2), immature teratoma of the left ovary (n = 1), B-cell lymphoma (n = 1), Hodgkin lymphoma (n = 4), right temporal lobe pilocytic astrocytoma (n = 1), pilocytic astrocytoma of the conus (n = 1), left renal tumor (n = 1), undifferentiated sarcoma of posterior sacral region (n = 1), and acute lymphoblastic leukemia (n = 1). Cancer treatments included chemotherapy only (n = 11), radiation only (n = 1), chemotherapy and radiation (n = 8), or other treatment (n = 4). Mean length of treatment was 0.63 years (SD = 1.05). 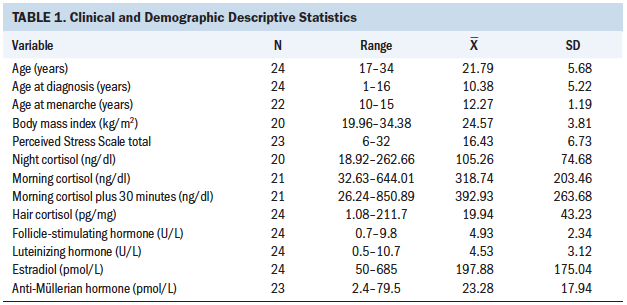
Latent Growth Curve
The average salivary cortisol intercept (corresponding to the night time point) was 105.25 ng/dl, with a variance of 48.53 ng/dl. On average, salivary cortisol levels increased 24.19 ng/dl per hour with a variance of 9.41 ng/dl. The latent growth curve demonstrated heterogeneity of variance over time (see Figure 2), with increased variability observed at the later time points. Some slopes were positive and others flat, creating an overdispersion at the later time points. 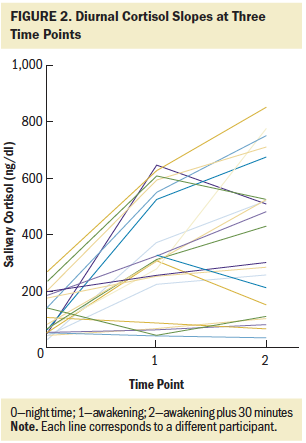
Bayesian Structural Equation Modeling
The authors found that the model predictors (i.e., salivary cortisol intercept, salivary cortisol slope, hair cortisol, and PSS-10) explained 42% of the variance of FSH, 39% of LH, 33% of AMH, and 0% of E2. The standardized regression coefficients allowed the authors to compare the effect of different predictors for each outcome. For AMH, age, age at diagnosis, perceived stress, and hair cortisol levels were the strongest predictors. For FSH, perceived stress was the strongest predictor, followed by the intercept of salivary cortisol and age at menarche. For LH, the authors found that the three predictors related to cortisol (i.e., salivary cortisol intercept, salivary cortisol slope, and hair cortisol) and age at diagnosis were the strongest predictors. For E2, all predictors were equivalent around 0, demonstrating that these predictors did not characterize the behavior of E2.
Discussion
In this small sample of female CCSs, the mean PSS-10 score was 16.43 (SD = 6.73), which is higher than the mean threshold for stress in the general population (13.7 for women) (Cohen et al., 1983). Surviving a life-threatening illness, such as pediatric cancer, represents a major early life stressor (Laufer et al., 2012). Several studies have demonstrated that CCSs report higher levels of stress than healthy controls (Brown, Madan-Swain, & Lambert, 2003; Oancea et al., 2014). Keir, Swartz, and Friedman (2007) demonstrated that long-term cancer survivors are just as likely as patients with cancer to report high levels of stress, suggesting that the time since diagnosis and treatment does not mitigate the psychological stress experienced by cancer survivors.
The authors observed higher mean night salivary cortisol concentrations (greater than 100 ng/dl) and flattened diurnal cortisol slopes in the current sample of female CCSs. In healthy individuals, cortisol concentrations peak in the morning about 30 minutes after awakening and decline throughout the day (Bergen et al., 2012). Higher nighttime cortisol levels and blunted cortisol slopes are abnormal diurnal patterns. It is hypothesized that these abnormal diurnal patterns induce systemic inflammation, which is associated with increased incidence of adverse long-term health outcomes for cancer survivors (e.g., cancer recurrence, chronic symptom experience) (Schrepf et al., 2015).
To the authors’ knowledge, no studies have examined hair cortisol levels in CCSs. In this small sample of 24 CCSs, the authors found a wide range of three-month hair cortisol concentrations (range = 1.08–211.7 pg/mg, mean = 19.94 pg/mg, SD = 43.23 pg/mg). There was one outlier in the sample (211.7 pg/mg); excluding the outlier, the range of hair cortisol concentrations was 1.08 pg/mg to 55.71 pg/mg. Age may contribute to changes in hair cortisol concentrations (Gow, Thomson, Rieder, Van Uum, & Koren, 2010); however, no association between age and cortisol concentrations was observed in the current sample. The mean hair cortisol concentration observed in this sample is lower than the mean reported in healthy samples (Stalder & Kirschbaum, 2012). In a meta-analysis of chronic stress and HPA activity, Miller, Chen, and Zhou (2007) reported that patterns of HPA activity in response to chronic stress vary by time since onset. When the eliciting stimulus of a chronic stressor first begins, there is an initial activation of the HPA axis, which results in elevated concentrations of cortisol. As time passes, this activity lessens, and cortisol secretion rebounds to below normal (Miller et al., 2007). This may explain the lower mean hair cortisol concentrations observed in this sample of CCSs. Because hypocortisolism and hypercortisolism are associated with adverse health outcomes, the finding of lower mean hair cortisol concentrations in this sample is notable and requires further investigation (Miller et al., 2007).
In this study of 24 CCSs, the authors found that the model predictors explained 33% of the variation in AMH levels, with the highest standardized regression coefficients observed for age, age at diagnosis, perceived stress, and hair cortisol (see Table 2). AMH was inversely associated with age, age at diagnosis, and perceived stress; with an increase of one standard deviation in age, age at diagnosis, and perceived stress score, AMH declined 5.66 pmol/L, 5.19 pmol/L, and 5.02 pmol/L, respectively. The inverse association between age and AMH is well established in the literature (Kelsey, Wright, Nelson, Anderson, & Wallace, 2011). Barton et al. (2013) also found that older age at diagnosis increased risk of post-treatment infertility in CCSs, but only in unadjusted models (Barton et al., 2013). The association among perceived stress, HPA activity, and the ovarian reserve has not been examined in CCSs. However, Pal, Bevilacqua, and Santoro (2010) examined the association between AMH levels and chronic psychosocial stress in 89 premenopausal infertile women. The study found that women with chronic stress demonstrated significantly lower AMH levels (p = 0.034) and were three times more likely to be diagnosed with diminished ovarian reserve (p = 0.025). Pal et al. (2010) concluded that chronic psychosocial stress was associated with diminished ovarian reserve. The study proposed HPA dysregulation as a plausible explanation for this association; because a biomarker of chronic stress was not included in the study, the study was unable to provide evidence supporting this theory. 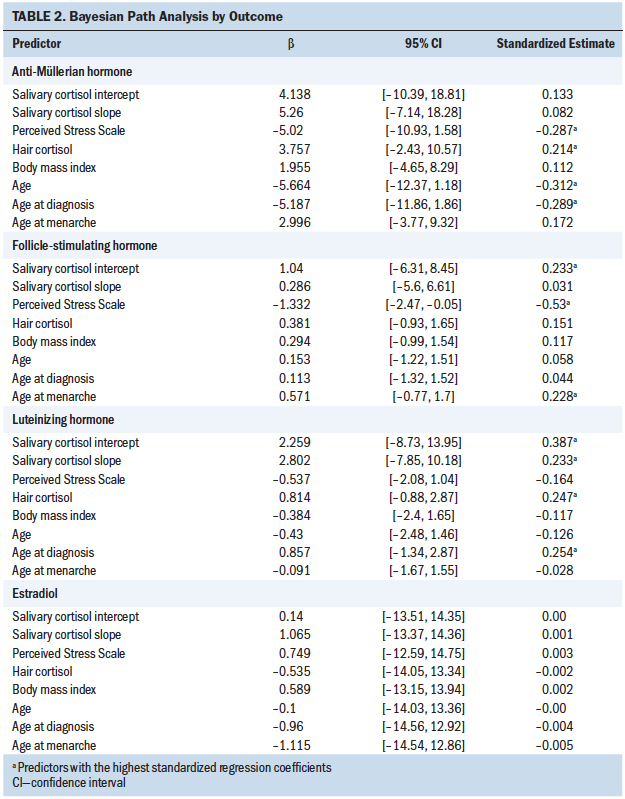
In the current study, the authors found that, with an increase of one standard deviation in hair cortisol concentrations, AMH increased 3.76 pmol/L. Although the standardized regression coefficients of the diurnal cortisol intercept and slope were lower, they were also positively associated with AMH levels. Taken together (positive associations among hair cortisol concentrations, diurnal cortisol intercept and slope, and AMH levels), these findings suggest a positive association between cortisol concentrations and the ovarian reserve, and support the hypothesis that threshold glucocorticoid levels are necessary to sustain ovarian function (Whirledge & Cidlowski, 2013). As stated previously, the authors found lower mean hair cortisol concentrations in the current sample than reported in healthy populations (Stalder & Kirschbaum, 2012).
More than half of the participants in the sample exhibited flattened diurnal cortisol slopes. Lower daily cortisol output and flattened diurnal cortisol slopes have been observed in cancer survivors. These findings are consistent with prior studies linking hypocortisolism to increased risk of experiencing late effects of cancer treatment (Bower et al., 2005; Cuneo et al., 2017; Ho et al., 2013; Schrepf et al., 2015; Sephton et al., 2013).
Because the authors were unable to time the collection of reproductive hormone levels according to menstrual cycle phase, a strong association among perceived stress and FSH and LH levels was not anticipated. Several possible explanations exist for the strong inverse association the authors observed irrespective of menstrual cycle phase. It may be that perceived stress has a significant effect on ovarian function regardless of menstrual cycle phase (Schliep et al., 2015). Another explanation may be that stress perception varies across the menstrual cycle, such that the degree to which a woman perceives her life to be stressful is, in part, influenced by fluctuations in gonadotropin levels (Duchesne & Pruessner, 2013).
Although perceived stress was inversely associated with FSH and LH, the authors found that HPA activity (hair cortisol, salivary cortisol intercept, and salivary cortisol slope) was positively associated with FSH and LH levels. The findings support the role of glucocorticoids in regulating the hypothalamic–pituitary–gonadal axis (Whirledge & Cidlowski, 2013). Another explanation is that cortisol levels may fluctuate across the menstrual cycle, as has been suggested by other studies (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999; Nepomnaschy et al., 2011; Stephens, Mahon, McCaul, & Wand, 2016; Wolfram, Bellingrath, & Kudielka, 2011). Although preliminary, the findings from this small sample support an association among perceived stress, HPA activity, and gonadotropin levels in female CCSs. More research is needed to clarify the mechanism behind this association and to determine whether it varies depending on the temporal nature of the stressor and menstrual cycle phase.
Limitations
This exploratory study has several limitations. Because of the small sample, the authors were unable to adjust for hormonal contraceptive use in the overall model. Hormonal contraceptive use is associated with suppression of FSH and LH levels, as well as increased production of corticosteroid-binding globulin and subsequent decreases in unbound cortisol levels (Stephens et al., 2016). Further research with larger sample sizes is needed to control for the effects of contraceptive use on ovarian function and HPA activity in female CCSs. In addition, because of the wide variety of pediatric cancer diagnoses in this small sample, the authors could not adjust for cancer diagnosis- and treatment-related factors in the model. Several studies have found a significant effect of diagnosis- and treatment-related factors on post-treatment ovarian function, including the cancer pathology itself (van Dorp et al., 2014), the therapeutic regimen (Charpentier et al., 2014), and the length of exposure (El-Shalakany, Ali, Abdelmaksoud, Abd El-Ghany, & Hasan, 2013; Reinmuth et al., 2013). Another limitation is that, because of the great distance that many of the participants needed to travel, the authors were unable to time study visits to coincide with a specific menstrual cycle phase. For this reason, the authors were unable to adjust gonadotropin and reproductive hormone levels to account for menstrual cycle phase. Lastly, the study design was cross-sectional and relied on a convenience sample. The study’s findings require validation in larger longitudinal studies.
Implications for Nursing
Reproductive dysfunction is a major concern for cancer survivors and is highly correlated with quality of life in this vulnerable population (Cherven et al., 2015; Knopman et al., 2010; Kondapalli et al., 2014; Letourneau et al., 2013). However, studies have demonstrated that cancer survivors are about half as likely to be treated for infertility as their siblings (Barton et al., 2013). Although the reasons for this discrepancy are not fully understood, healthcare providers may perceive fertility treatments to be less successful in this population. The guidelines from the American Society for Clinical Oncology recommend that options for fertility preservation be discussed with patients with cancer at the earliest opportunity (Knight et al., 2015; Letourneau et al., 2013). Oncology nurses play an important role in advocating for cancer survivors, helping to initiate conversations regarding future fertility as early as possible, and discussing factors that may mitigate the risk for post-treatment reproductive dysfunction. The findings from this study suggest that there may be modifiable factors, such as chronic stress, that contribute to risk for infertility post-treatment. Oncology nurses can minimize the potential burden of late effects by educating patients regarding their risk and what actions can be taken prior to or after treatment to reduce this risk (McClellan et al., 2013). 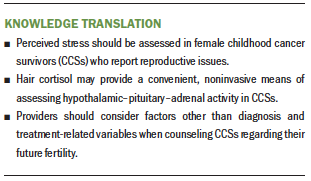
Conclusion
In this exploratory study, the authors sought to examine the association between chronic stress and ovarian function in survivors of childhood cancer. Although there is prior evidence suggesting stress affects gonadotropin hormone levels, the effects of chronic stress on the ovarian reserve and its biomarker AMH are poorly understood. The findings from this study provide preliminary evidence to suggest that perceived stress is negatively associated with ovarian function and that biomarkers of HPA activity are positively associated with ovarian function in female CCSs. Few studies have examined predictors of the ovarian reserve in CCSs apart from diagnosis- and treatment-related factors. The findings from this study provide the foundation for further research examining the risk for post-treatment reproductive dysfunction in female CCSs.
The authors gratefully acknowledge the support of the clinical team at the Royal Hospital for Sick Children, particularly Hamish Wallace, MD, and Rachel McAndrew, RN. The authors also acknowledge the support of Rebecca Reynolds, PhD, and Richard Anderson, MD, PhD, at the Queens Medical Research Institute for their assistance with completing the laboratory component of this study.
About the Author(s)
Theresa M. Hardy, PhD, RN, is a postdoctoral associate in the Rory Meyers College of Nursing at New York University in New York; and Mauricio Garnier-Villarreal, PhD, is a research assistant professor, Jennifer M. Ohlendorf, PhD, RN, is an assistant professor, and Donna O. McCarthy, PhD, RN, is a professor, all in the College of Nursing at Marquette University in Milwaukee, WI. This research was funded, in part, by a grant (1F31NR016621) from the National Institute of Nursing Research of the National Institutes of Health, and by a dissertation research grant to Hardy from the Oncology Nursing Society. Hardy, Ohlendorf, and McCarthy contributed to the conceptualization and design. Hardy completed the data collection. Hardy and Garnier-Villarreal provided statistical support. Hardy, Garnier-Villarreal, and Ohlendorf provided the analysis. All authors contributed to the manuscript preparation. Hardy can be reached at th89@nyu.edu, with copy to ONFEditor@ons.org. (Submitted October 2018. Accepted November 29, 2018.)
References
Abusief, M.E., Missmer, S.A., Ginsburg, E.S., Weeks, J.C., & Partridge, A.H. (2012). Relationship between reproductive history, anthropometrics, lifestyle factors, and the likelihood of persistent chemotherapy-related amenorrhea in women with premenopausal breast cancer. Fertility and Sterility, 97, 154–159. https://doi.org/10.1016/j.fertnstert.2011.10.005
Anderson, R.A., & Wallace, W.H. (2013). Antimüllerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertility and Sterility, 99, 1469–1475. https://doi.org/10.1016/j.fertnstert.2013.03.014
Barton, S.E., Najita, J.S., Ginsburg, E.S., Leisenring, W.M., Stovall, M., Weathers, R.E., . . . Diller, L. (2013). Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncology, 14, 873–881. https://doi.org/10.1016/S1470-2045(13)70251-1
Bergen, A.W., Mallick, A., Nishita, D., Wei, X., Michel, M., Wacholder, A., . . . Andrews, J.A. (2012). Chronic psychosocial stressors and salivary biomarkers in emerging adults. Psychoneuroendocrinology, 37, 1158–1170. https://doi.org/10.1016/j.psyneuen.2011.11.010
Blumenfeld, Z. (2012). Chemotherapy and fertility. Best Practice and Research. Clinical Obstetrics and Gynaecology, 26, 379–390. https://doi.org/10.1016/j.bpobgyn.2011.11.008
Bower, J.E., Ganz, P.A., Dickerson, S.S., Petersen, L., Aziz, N., & Fahey, J.L. (2005). Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology, 30, 92–100. https://doi.org/10.1016/j.psyneuen.2004.06.003
Breen, K.M., & Mellon, P.L. (2014). Influence of stress-induced intermediates on gonadotropin gene expression in gonadotrope cells. Molecular and Cellular Endocrinology, 385, 71–77. https://doi.org/10.1016/j.mce.2013.08.014
Brooks, S.P., & Gelman, A. (1998). General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics, 7, 434–455. https://doi.org/10.2307/1390675
Brougham, M.F., Crofton, P.M., Johnson, E.J., Evans, N., Anderson, R.A., & Wallace, W.H. (2012). Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: A prospective study. Journal of Clinical Endocrinology and Metabolism, 97, 2059–2067. https://doi.org/10.1210/jc.2011-3180
Brown, R.T., Madan-Swain, A., & Lambert, R. (2003). Posttraumatic stress symptoms in adolescent survivors of childhood cancer and their mothers. Journal of Traumatic Stress, 16, 309–318. https://doi.org/10.1023/A:1024465415620
Bryan, A., Schmiege, S.J., & Broaddus, M.R. (2007). Mediational analysis in HIV/AIDS research: Estimating multivariate path analytic models in a structural equation modeling framework. AIDS and Behavior, 11, 365–383. https://doi.org/10.1007/s10461-006-9150-2
Charpentier, A.M., Chong, A.L., Gingras-Hill, G., Ahmed, S., Cigsar, C., Gupta, A.A., . . . Hodgson, D.C. (2014). Anti-Müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. Journal of Cancer Survivorship, 8, 548–554. https://doi.org/10.1007/s11764-014-0364-4
Cherven, B.O., Mertens, A., Wasilewski-Masker, K., Williamson, R., & Meacham, L.R. (2015). Infertility education: Experiences and preferences of childhood cancer survivors. Journal of Pediatric Oncology Nursing, 33, 257–264. https://doi.org/10.1177/1043454215607342
Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. https://doi.org/10.2307/2136404
Core Team, R. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.r-project.org
Cuneo, M.G., Schrepf, A., Slavich, G.M., Thaker, P.H., Goodheart, M., Bender, D., . . . Lutgendorf, S.K. (2017). Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology, 84, 139–142. https://doi.org/10.1016/j.psyneuen.2017.06.019
Dedovic, K., & Ngiam, J. (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment, 11, 1181–1189. https://doi.org/10.2147/NDT.S62289
Du, Y.J., Zhang, H.Y., Li, B., Wu, X., Lv, Y.B., Jin, H.L., . . . Dong, J.C. (2013). Sputum interleukin-6, tumor necrosis factor-a and salivary cortisol as new biomarkers of depression in lung cancer patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 47, 69–76. https://doi.org/10.1016/j.pnpbp.2013.08.004
Duchesne, A., & Pruessner, J.C. (2013). Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology, 38, 3155–3159. https://doi.org/10.1016/j.psyneuen.2013.08.009
El-Shalakany, A.H., Ali, M.S., Abdelmaksoud, A.A., Abd El-Ghany, S., & Hasan, E.A. (2013). Ovarian function in female survivors of childhood malignancies. Pediatric Hematology and Oncology, 30, 328–335. https://doi.org/10.3109/08880018.2013.778927
Garland, E.L., Beck, A.C., Lipschitz, D.L., & Nakamura, Y. (2015). Dispositional mindfulness predicts attenuated waking salivary cortisol levels in cancer survivors: A latent growth curve analysis. Journal of Cancer Survivorship, 9, 215–222. https://doi.org/10.1007/s11764-014-0402-2
Gelman, A., & Rubin, D.B. (1992). Inference from iterative simulation using multiple sequences. Statistical Science, 7, 457–472. https://doi.org/10.1214/ss/1177011136
Golden-Kreutz, D.M., Browne, M.W., Frierson, G.M., & Andersen, B.L. (2009). Assessing stress in cancer patients: A second-order factor analysis model for the perceived stress scale. Assessment, 11, 216–223. https://doi.org/10.1177/1073191104267398
Gow, R., Thomson, S., Rieder, M., Van Uum, S., & Koren, G. (2010). An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International, 196, 32–37. https://doi.org/10.1016/j.forsciint.2009.12.040
Ho, R.T., Fong, T.C., Chan, C.K., & Chan, C.L. (2013). The associations between diurnal cortisol patterns, self-perceived social support, and sleep behavior in Chinese breast cancer patients. Psychoneuroendocrinology, 38, 2337–2342. https://doi.org/10.1016/j.psyneuen.2013.05.004
Hoffman, M.C., Karban, L.V, Benitez, P., Goodteacher, A., & Laudenslager, M.L. (2014). Chemical processing and shampooing impact cortisol measured in human hair. Clinical and Investigative Medicine, 37(4), E252–E257.
Jeppesen, J.V, Anderson, R.A., Kelsey, T.W., Christiansen, S.L., Kristensen, S.G., Jayaprakasan, K., . . . Andersen, C.Y. (2013). Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Molecular Human Reproduction, 19, 519–527. https://doi.org/10.1093/molehr/gat024
Kalantaridou, S.N., Zoumakis, E., Makrigiannakis, A., Lavasidis, L.G., Vrekoussis, T., & Chrousos, G.P. (2010). Corticotropin-releasing hormone, stress and human reproduction: An update. Journal of Reproductive Immunology, 85, 33–39. https://doi.org/10.1016/j.jri.2010.02.005
Keir, S.T., Swartz, J.J., & Friedman, H.S. (2007). Stress and long-term survivors of brain cancer. Supportive Care in Cancer, 15, 1423–1428. https://doi.org/10.1007/s00520-007-0292-1
Kelsey, T.W., Wright, P., Nelson, S.M., Anderson, R.A., & Wallace, W.H. (2011). A validated model of serum anti-müllerian hormone from conception to menopause. PLoS ONE, 6(7), e22024. https://doi.org/10.1371/journal.pone.0022024
Kirschbaum, C., & Hellhammer, D.H. (1989). Salivary cortisol in psychobiological research: an overview. Neuropsychobiology, 22, 150–169.
Kirschbaum, C., Kudielka, B.M., Gaab, J., Schommer, N.C., & Hellhammer, D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162.
Kirschbaum, C., Tietze, A., Skoluda, N., & Dettenborn, L. (2009). Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34, 32–37. https://doi.org/10.1016/j.psyneuen.2008.08.024
Knight, S., Lorenzo, A., Maloney, A.M., Srikanthan, A., Donen, R., Greenblatt, E., & Gupta, A. (2015). An approach to ferility preservation in prepubertal and postpubertal females: A critical review of current literature. Pediatric Blood and Cancer, 62, 935–939. https://doi.org/10.1002/pbc.25440
Knopman, J.M., Papadopoulos, E.B., Grifo, J.A., Fino, M.E., & Noyes, N. (2010). Surviving childhood and reproductive-age malignancy: Effects on fertility and future parenthood. Lancet Oncology, 11, 490–498. https://doi.org/10.1016/S1470-2045(09)70317-1
Kondapalli, L.A., Dillon, K.E., Sammel, M.D., Ray, A., Prewitt, M., Ginsberg, J.P., & Gracia, C.R. (2014). Quality of life in female cancer survivors: Is it related to ovarian reserve? Quality of Life Research, 23, 585–592. https://doi.org/10.1007/s11136-013-0473-y
Kremer, L.C., Mulder, R.L., Oeffinger, K.C., Bhatia, S., Landier, W., Levitt, G., . . . Hudson, M.M. (2013). A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects Of Childhood Cancer Guideline Harmonization Group. Pediatric Blood and Cancer, 60, 543–549. https://doi.org/10.1002/pbc.24445
Laufer, D., Ansermet, F., von der Weid, N., Beck Popovic, M., Torrisi, R., & Pierrehumbert, B. (2012). Endocrine response and perceived stress test during an experimental challenge task in adult survivors of a childhood cancer. Pediatric Blood and Cancer, 59, 138–143. https://doi.org/10.1002/pbc.24044
Lee, E.H. (2012). Review of the psychometric evidence of the perceived stress scale. Asian Nursing Research, 6(4), 121–127. https://doi.org/10.1016/j.anr.2012.08.004
Letourneau, J., Chan, S.W., & Rosen, M.P. (2013). Accelerating ovarian age: Cancer treatment in the premenopausal woman. Seminars in Reproductive Medicine, 31, 462–468. https://doi.org/10.1055/s-0033-1356482
Lie Fong, S., Laven, J.S., Hakvoort-Cammel, F.G., Schipper, I., Visser, J.A., Themmen, A.P., . . . van den Heuvel-Eibrink, M.M. (2009). Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Human Reproduction, 24, 982–990. https://doi.org/10.1093/humrep/den487
Louis, G.M., Lum, K.J., Sundaram, R., Chen, Z., Kim, S., Lynch, C.D., . . . Pyper, C. (2011). Stress reduces conception probabilities across the fertile window: Evidence in support of relaxation. Fertility and Sterility, 95, 2184–2189. https://doi.org/10.1016/j.fertnstert.2010.06.078
Lunsford, A.J., Whelan, K., McCormick, K., & McLaren, J.F. (2014). Antimüllerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertility and Sterility, 101, 227–231. https://doi.org/10.1016/j.fertnstert.2013.08.052
Lynch, C.D., Sundaram, R., Maisog, J.M., Sweeney, A.M., & Buck Louis, G.M. (2014). Preconception stress increases the risk of infertility: Results from a couple-based prospective cohort study—The LIFE study. Human Reproduction, 29, 1067–1075. https://doi.org/10.1093/humrep/deu032
McClellan, W., Klemp, J.R., Krebill, H., Ryan, R., Nelson, E.L., Panicker, J., . . . Stegenga, K. (2013). Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncology Nursing Forum, 40, 254–262. https://doi.org/10.1188/13.ONF.254-262
Merkle, E.C. (2011). A comparison of imputation models for Bayesian factor analysis models. Journal of Educational and Behavioral Statistics, 36, 257–276. https://doi.org/10.3102/1076998610375833
Merkle, E.C., & Rosseel, Y. (2018). Blavaan: Bayesian structural equation models via parameter expansion. Journal of Statistical Software, 85(4), 1–30. https://doi.org/10.18637/jss.v085.i04
Metzger, M.L., Meacham, L.R., Patterson, B., Casillas, J.S., Constine, L.S., Hijiya, N., . . . Green, D.M. (2013). Female reproductive health after childhood, adolescent, and young adult cancers: Guidelines for the assessment and management of female reproductive complications. Journal of Clinical Oncology, 31, 1239–1247. https://doi.org/10.1200/JCO.2012.43.5511
Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. https://doi.org/10.1037/0033-2909.133.1.25
Miyoshi, Y., Ohta, H., Namba, N., Tachibana, M., Miyamura, T., Miyashita, E., . . . Ozono, K. (2013). Low serum concentrations of anti-Müllerian hormone are common in 53 female childhood cancer survivors. Hormone Research in Paediatrics, 79, 17–21. https://doi.org/10.1159/000346139
Nepomnaschy, P.A., Altman, R.M., Watterson, R., Co, C., McConnell, D.S., & England, B.G. (2011). Is cortisol excretion independent of menstrual cycle day? A longitudinal evaluation of first morning urinary specimens. PLOS ONE, 6(3), e18242. https://doi.org/10.1371/journal.pone.0018242
Oancea, S.C., Brinkman, T.M., Ness, K.K., Krull, K.R., Smith, W.A., Srivastava, D.K., . . . Gurney, J.G. (2014). Emotional distress among adult survivors of childhood cancer. Journal of Cancer Survivorship, 8, 293–303. https://doi.org/10.1007/s11764-013-0336-0
Pal, L., Bevilacqua, K., & Santoro, N.F. (2010). Chronic psychosocial stressors are detrimental to ovarian reserve: A study of infertile women. Journal of Psychosomatic Obstetrics and Gynaecology, 31, 130–139. https://doi.org/10.3109/0167482X.2010.485258
Phillips, S.M., Padgett, L.S., Leisenring, W.M., Stratton, K.K., Bishop, K., Krull, K.R., . . . Mariotto, A.B. (2015). Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiology, Biomarkers and Prevention, 24, 653–663. https://doi.org/10.1158/1055-9965.EPI-14-1418
Plummer, M. (2003). JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Retrieved from http://www.r-project.org/conferences/DSC-2003
Reinmuth, S., Hohmann, C., Rendtorff, R., Balcerek, M., Holzhausen, S., Müller, A., . . . Borgmann-Staudt, A. (2013). Impact of chemotherapy and radiotherapy in childhood on fertility in adulthood: The FeCt-survey of childhood cancer survivors in Germany. Journal of Cancer Research and Clinical Oncology, 139, 2071–2078. https://doi.org/10.1007/s00432-013-1527-9
Révész, D., Verhoeven, J.E., Milaneschi, Y., de Geus, E.J., Wolkowitz, O.M., & Penninx, B.W. (2013). Dysregulated physiological stress systems and accelerated cellular aging. Neurobiology of Aging, 35, 1422–1430. https://doi.org/10.1016/j.neurobiolaging.2013.12.027
Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. https://doi.org/10.1016/j.psyneuen.2011.09.009
Salih, S.M., Elsarrag, S.Z., Prange, E., Contreras, K., Osman, R.G., Eikoff, J.C., & Puccetti, D. (2015). Evidence to incorporate inclusive reproductive health measures in guidelines for childhood and adolescent cancer survivors. Journal of Pediatric and Adolescent Gynecology, 28, 95–101. https://doi.org/10.1016/j.jpag.2014.05.012
Schliep, K.C., Mumford, S.L., Vladutiu, C.J., Ahrens, K.A., Perkins, N.J., Sjaarda, L.A., . . . Schisterman, E.F. (2015). Perceived stress, reproductive hormones, and ovulatory function: A prospective cohort study. Epidemiology, 26, 177–184. https://doi.org/10.1097/EDE.0000000000000238
Schrepf, A., Thaker, P.H., Goodheart, M.J., Bender, D., Slavich, G.M., Dahmoush, L., . . . Lutgendorf, S.K. (2015). Diurnal cortisol and survival in epithelial ovarian cancer. Psycho-neuroendocrinology, 53, 256–267. https://doi.org/10.1016/j.psyneuen.2015.01.010
Sephton, S.E., Lush, E., Dedert, E.A., Floyd, A.R., Rebholz, W.N., Dhabhar, F.S., . . . Salmon, P. (2013). Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior, and Immunity, 30(Suppl.), S163–S170. https://doi.org/10.1016/j.bbi.2012.07.019
Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair—State of the art and future directions. Brain, Behavior, and Immunity, 26, 1019–1029. https://doi.org/10.1016/j.bbi.2012.02.002
Stephens, M.A., Mahon, P.B., McCaul, M.E., & Wand, G.S. (2016). Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology, 66, 47–55. https://doi.org/10.1016/j.psyneuen.2015.12.021
Taylor, N., Absolom, K., Snowden, J., & Eiser, C. (2012). Need for psychological follow-up among young adult survivors of childhood cancer. European Journal of Cancer Care, 21, 52–58. https://doi.org/10.1111/j.1365-2354.2011.01281.x
van Dorp, W., van den Heuvel-Eibrink, M.M., de Vries, A.C., Pluijm, S.M., Visser, J.A., Pieters, R., & Laven, J.S. (2014). Decreased serum anti-Müllerian hormone levels in girls with newly diagnosed cancer. Human Reproduction, 29, 337–342. https://doi.org/10.1093/humrep/det442
Van Uum, S.H., Sauvé, B., Fraser, L.A., Morley-Forster, P., Paul, T.L., & Koren, G. (2008). Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress, 11, 483–488. https://doi.org/10.1080/10253890801887388
Wells, S., Tremblay, P.F., Flynn, A., Russell, E., Kennedy, J., Rehm, J., . . . Graham, K. (2014). Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress, 17, 334–342. https://doi.org/10.3109/10253890.2014.930432
Whirledge, S., & Cidlowski, J.A. (2013). A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology, 154, 4450–4468.
Wolfram, M., Bellingrath, S., & Kudielka, B.M. (2011). The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology, 36, 905–912. https://doi.org/10.1016/j.psyneuen.2010.12.006
Yuan, Y., & MacKinnon, D.P. (2009). Bayesian mediation analysis. Psychological Methods, 14, 301–322. https://doi.org/10.1037/a0016972
Zeltzer, L.K., Recklitis, C., Buchbinder, D., Zebrack, B., Casillas, J., Tsao, J.C., . . . Krull, K. (2009). Psychological status in childhood cancer survivors: A report from the childhood cancer survivor study. Journal of Clinical Oncology, 27, 2396–2404. https://doi.org/10.1200/JCO.2008.21.1433




