Comparison of Fatigue and Quality of Life in Individuals With Pancreatogenic Diabetes After Total or Partial Pancreatectomy
Objectives: To compare fatigue and quality of life (QOL) between individuals with pancreatogenic diabetes after total pancreatectomy (TP) and pancreaticoduodenectomy (PD).
Sample & Setting: 50 individuals (14 after TP and 36 after PD) were recruited from a pancreatic surgical outpatient department. A final sample of 39 matched individuals (13 after TP and 26 after PD) were included in the final analysis.
Methods & Variables: A comparative cross-sectional approach was used. Variables were fatigue and QOL. The Fatigue Symptom Inventory and European Organisation for the Research and Treatment of Cancer Quality-of-Life Questionnaire—Core 30 were used. Data went through propensity score one-to-two matching. Generalized estimating equation was used to compare fatigue and QOL.
Results: The groups showed no statistically significant difference in fatigue intensity and overall QOL. The TP group had significantly longer fatigue duration, perceived higher interference of functioning, lower physical function, and a higher level of insomnia.
Implications for Nursing: Future studies with a larger sample and longitudinal design will help identify the trajectory of fatigue and QOL in individuals with pancreatogenic diabetes post-TP and PD.
Jump to a section
Pancreatic resection plays a crucial role in treating individuals with diseases of the pancreas, including chronic pancreatitis, pancreatic tumors (e.g., intraductal papillary mucinous neoplasm, pancreatic neuroendocrine tumor), and pancreatic malignancies (Heidt, Burant, & Simeone, 2007; Tillou et al., 2017). Pancreatic cancer is one of the most fatal malignant tumors worldwide (Ilic & Ilic, 2016). The onset of pancreatic cancer occurs in older adults aged an average of 65 years, and its five-year survival rate is much lower when compared to benign conditions, such as chronic pancreatitis (Raimondi, Lowenfels, Morselli-Labate, Maisonneuve, & Pezzilli, 2010). Pancreatic surgery is one of the only curative treatments for pancreatic malignancies (Zhang et al., 2016). Pancreaticoduodenectomy (PD), also known as the Whipple procedure, is the most common and traditional surgical method used in treating pancreatic cancer (Cid-Arregui & Juarez, 2015); however, pancreatic anastomosis–related complications and pancreatic cancer recurrence after PD decrease individuals’ quality of life and survival (Zhang et al., 2016). With the improvement in surgical techniques, individuals with diseases of the pancreas are provided with the option of a total pancreatectomy (TP) to assist in the complete resection of the malignancy or the region with a higher risk of malignancy to improve survival (Andrén-Sandberg, Ansorge, & Yadav, 2016).
Despite the promising advantages of TP, postoperative exocrine and endocrine insufficiency remains a downside of the procedure (Casadei et al., 2010). However, with the progress of medical and surgical care, individuals after TP receive better care for endocrine and exocrine insufficiency (Andrén-Sandberg et al., 2016; Jamil et al., 2012; Keim, Klar, Poll, & Schoenberg, 2009). In addition, with improvements in diagnostic technology, more individuals with diffuse pancreatic disease are diagnosed; therefore, increased performance of TP occurs (Farrell, 2015; Stark, Donahue, Reber, & Hines, 2016). However, TP remains a difficult decision for individuals and healthcare providers because pancreatogenic diabetes causes major concerns as one of the side effects of TP (Maeda & Hanazaki, 2011).
Pancreatogenic diabetes is defined as a type of diabetes associated with benign and malignant disease of the exocrine pancreas (e.g., pancreatitis, pancreatic neoplasms, pancreatic resection) (Cui & Andersen, 2011). In individuals who underwent PD for chronic pancreatitis or pancreatic cancer, the incidence of postoperative diabetes was 15% to 40% and 39%, respectively, whereas for individuals who had TP, the incidence of postoperative diabetes was 100% because of the resulting apancreatic status (Maeda & Hanazaki, 2011). Epelboym et al. (2014) mentioned that there was no statistically significant difference in the diabetic impact of TP and PD, and Casadei et al. (2016) concluded that individuals who underwent TP contracted diabetes, which was harder to control. Barbier et al. (2013) reported that 54% of individuals who had TP were readmitted to the hospital because of diabetes-associated issues and that diabetes has a significant negative impact on an individuals’ quality of life.
Fatigue is a persistent, multidimensional symptom affecting physical, mental, and emotional domains (Ryan et al., 2007; Scott, Lasch, Barsevick, & Piault-Louis, 2011) and disrupting daily life and emotional well-being (National Comprehensive Cancer Network [NCCN], 2018). It is one of the most prevalent symptoms in individuals after pancreatic surgery for malignancy (Heerkens et al., 2016) and chronic pancreatitis (Rückert et al., 2011), as well as in the diabetes population (Fritschi & Quinn, 2010). Two studies (Epelboym et al., 2014; Müller et al., 2007) compared fatigue as a secondary outcome in individuals who underwent pancreatic resection mostly for pancreatic cancer (65% to 92% of the study population). Using the symptom subscale of the European Organisation for the Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (EORTC QLQ-C30), the results showed no statistically significant difference in fatigue in the two groups (Epelboym et al., 2014; Müller et al., 2007). However, both studies measured fatigue from a one-dimensional perspective, and other fatigue characteristics (e.g., duration, interference) were not included. An in-depth evaluation of fatigue is needed to further understand the impact of fatigue in individuals with pancreatogenic diabetes after TP and PD.
Quality of life is defined as an individual’s subjective overall satisfaction with life, including their physical, psychological, and social well-being (Yao, 2002). Three studies in the published literature have proposed that individuals who undergo TP have comparable quality of life to those with partial pancreatic resections (Casadei et al., 2016; Epelboym et al., 2014; Müller et al., 2007). Among the three studies, one did not use methods to address possible confounders, despite the presence of a significant imbalance of benign diagnoses between the two groups (Casadei et al., 2016), and two used frequency matching (Epelboym et al., 2014; Müller et al., 2007) in which only the overall frequency of a certain confounder was made equal between the groups (de Graaf, Jager, Zoccali, & Dekker, 2011). To accurately account for potential confounders, reduce bias, and reach a more accountable comparison result, an adequate matching method in which matching is done on the individual level, such as with propensity score matching, should be used (Austin, 2011; Trutschel, Palm, Holle, & Simon, 2017). A propensity score–matched analysis in the comparison study of fatigue characteristics and quality of life between patients after TP and patients after PD will help to reduce selection bias and improve the internal validity of study outcomes (Stuart, 2010).
With the rise of the TP technique in treatment of pancreatic neoplasms (Andrén-Sandberg et al., 2016), it is important to decrease the knowledge gap regarding these individuals’ fatigue and quality of life for healthcare providers to be able to deliver optimal care. In addition, the multiple burdens of diabetes and fatigue could have a negative impact on the quality of life in individuals who had pancreatic resection for exocrine pancreatic diseases, particularly in those who had a more extended procedure, such as TP. Therefore, because of limitations in the comparison methodology of the existing literature on the fatigue and quality of life of people after TP and PD, and the lack of knowledge regarding in-depth fatigue characteristics of individuals with pancreatogenic diabetes after TP and PD, the purpose of this study was to describe and compare fatigue characteristics and quality of life in patients with pancreatogenic diabetes after TP and PD.
Methods
Design and Sample
A comparative cross-sectional approach was used. To accurately account for potential confounders, propensity score matching was applied (Austin, 2011). The data were collected via purposive sampling in an outpatient pancreatic surgical department. This study was approved by the institutional review board at the National Taiwan University Hospital in Taipei and was registered at ClinicalTrials.gov (identification number: NCT02985502). Eligibility criteria were the following:
• Having undergone TP or PD
• Being diagnosed with diabetes (before or after surgery)
• Being aged 20 years old or older
• Having no cognitive impairment
• Being able to communicate in Chinese
• Having agreed to participate in the study and signed the informed consent
Individuals who were undergoing active treatment for cancer other than pancreatic cancer were excluded. Screening of the individuals’ medical records for cognitive impairment–associated disease (e.g., dementia) was done to exclude those with cognitive impairment. Because the indication for total and partial pancreatectomy included not only cancer but also noncancer diagnoses in the current clinical setting, the authors included individuals with cancer and noncancer diagnoses to conform to clinical reality and the study aim.
A power analysis using G*Power, version 3.0, was conducted to determine the sample size needed. Because the generalized estimating equation is an extension of the generalized linear model (Zeger, Liang, & Albert, 1988), the authors used the sample size determination method for multiple linear regressions to estimate the sample size needed in this study. In addition, because of limited studies on populations having both cancer and diabetes, the authors conservatively used R2 = 0.3 in the power analysis based on previous studies on quality of life of individuals with cancer (Beijer, Kempen, Pijls-Johannesma, de Graeff, & Dagnelie, 2008) and with diabetes (Adriaanse, Drewes, van der Heide, Struijs, & Baan, 2016). With R2 = 0.3, 80% power, alpha = 0.05, and entering five covariates (age, gender, diabetes status before operation, tumor malignancy, and type of surgery) in the model, a total sample of 36 was needed to reach adequate power.
The questionnaires were completed with the assistance of a master’s-prepared research assistant during face-to-face interviews in a quiet, vacant room at the outpatient pancreatic surgical department; each interview took about 15 to 20 minutes to complete. The research assistant had three years of experience as an RN in the medical ward, one year of research assistant training, and interview skills training in the oncology ward and the pancreatic surgical outpatient department. All individuals who met the inclusion criteria were approached and asked to participate. From November 2016 to May 2017, of the 52 individuals approached, 2 individuals refused to enter the study. A total of 50 participants were recruited to this study; 14 underwent TP and 36 had PD.
Measures
The data were collected with a structured questionnaire that included the following items:
• Demographic and clinical characteristics
• The Chinese version of the Fatigue Symptom Inventory (FSI)
• The Chinese version of the EORTC QLQ-C30
Demographic and clinical characteristics: The demographic variables included age, gender, occupational status, and years of education. The clinical characteristics included postoperative time in months, body mass index, number of comorbidities, diabetes duration in months, hemoglobin A1c (HbA1c) level, functional status measured by the Karnofsky Performance Status Scale (KPS), diagnosis, smoking status, cancer stage, current chemotherapy treatment, and current diabetes treatment.
Fatigue Symptom Inventory: The FSI was used to evaluate participants’ subjective fatigue characteristics. The scale contains 14 items in four categories: fatigue intensity (4 items), interference (7 items), duration (2 items), and pattern (1 item). Fatigue intensity, interference, and duration were measured by an 11-point Likert-type scale, ranging from 0 (no fatigue at all) to 10 (extreme fatigue). The item “number of days fatigued last week” ranged from 0 to 7 days, and the item “daily pattern of fatigue” ranged from 0 to 4, with 0 indicating no fatigue at all, 1 indicating worst fatigue in the morning, 2 indicating worst fatigue in the afternoon, 3 indicating worst fatigue at night, and 4 indicating no specific fatigue pattern. Higher scores represented higher levels of fatigue. The FSI has demonstrated good validity and reliability in the population of patients with chronic illness, including cancer (Donovan & Jacobsen, 2010). The Chinese version of the FSI was positively correlated with the Cancer Fatigue Scale (r = 0.62) and was negatively and weakly correlated with the Herth Hope Index (r = –0.18), indicating good convergent and divergent validity; in addition, the scale has been tested in people with cancer in Taiwan with high internal consistency (Cronbach alpha = 0.92) (Shun, Beck, Pett, & Berry, 2006). The Cronbach alpha was 0.92 in the current study.
EORTC QLQ-C30: Quality of life was measured by the EORTC QLQ-C30. This questionnaire contains 30 items and consists of a global health status and quality-of-life scale (2 items); a symptom scale (13 items); and five functional scales, including physical functioning (5 items), role functioning (2 items), emotional functioning (4 items), cognitive functioning (2 items), and social functioning (2 items). The global health status and quality-of-life scale was measured by a seven-point Likert-type scale ranging from 1 (worst global health status and quality of life) to 7 (best global health status and quality of life). The symptom scale and functional scales were measured by a four-point Likert-type scale, with 1 indicating not at all, 2 indicating a little, 3 indicating quite a bit, and 4 indicating very much. All scores of the subscales were calculated to range from 0 to 100 according to the EORTC manual (Fayers et al., 2001). Higher scores on the global health status and quality-of-life scale and the functional scales represented a higher quality of life, whereas higher scores on the symptom scale indicated high levels of symptomatology. The EORTC QLQ-C30 has demonstrated good reliability and validity in the general population (Hjermstad, Fayers, Bjordal, & Kaasa, 1998), in individuals with cancer (Niezgoda & Pater, 1993), and in individuals with chronic pancreatitis (Bloechle, Izbicki, Knoefel, Kuechler, & Broelsch, 1995). The Cronbach alpha was 0.79 for the Chinese version of the EORTC QLQ-C30 (Chie, Yang, Hsu, & Lai, 2002). The questionnaire had positive correlation with the SF-36® (r = 0.7), which demonstrated good convergent validity (Chie, Yang, Hsu, & Yang, 2004). The Cronbach alpha was 0.82 in the current study.
Data Analysis
Data were entered and analyzed using IBM SPSS Statistics, version 24.0. Descriptive statistics were used to report the mean and standard deviation of the continuous variables; categorical variables were presented with counts and percentage. Nonparametric quantitative data were analyzed using the Mann-Whitney U test. The chi-squared test, or the Fisher’s exact test, were used to analyze categorical variables. A significance level of 0.05 was used in this study.
The propensity score matching method was used to reduce confounding between the two operative groups (Austin, 2011; Trutschel et al., 2017). In the case of small study samples or low prevalence of treatment, propensity score matching is an appropriate method to account for confounders (Pirracchio, Resche-Rigon, & Chevret, 2012). The propensity score was estimated using logistic regression. Age and gender were reported to be important predictors of quality of life in both the diabetes (Wan et al., 2016) and cancer populations (Zimmermann et al., 2011). Diabetes status (Adriaanse et al., 2016) and tumor malignancy (Velanovich & Wollner, 2011) were both factors that also influenced quality of life. The factors (i.e., age, gender, diabetes status before operation, and tumor malignancy) mentioned previously that could be potential confounders that may influence the results of comparing quality of life between patients undergoing TP and patients undergoing PD were entered into the propensity model. One-to-two matching of patients undergoing TP and patients undergoing PD, respectively, was used because the number of TPs performed was much less than the number of PDs in the current clinical setting. The data went through greedy nearest neighbor matching without replacement under a caliper width of 0.15 (Yang & Chen, 2016). Numeric and graphic diagnostics were performed to check the balance of the matched pairs, including generalized estimating equation (GEE), standardized mean difference (SMD), and distribution of the propensity scores (Austin, 2008). As opposed to the traditional statistical test of a hypothesis, SMD greater than 0.25 was used as the indicator of distribution unbalance to avoid potential bias caused by sample size (Stuart, 2010). The GEE, a multilevel analysis, was used for comparing fatigue characteristics and quality of life of the resulting propensity score matched pairs according to Austin (2011).
Results
Participant Characteristics
The majority of the individuals were men and unemployed, with a mean age of 62.1 years in the TP group and 67.5 years in the PD group. Most were diagnosed with pancreatic ductal adenocarcinoma (71.4% in the TP group and 63.9% in the PD group). The most common type of surgical procedure was the pylorus-preserving PD (42%). All individuals in the TP group were treated with insulin, and 75% of the participants in the PD group were prescribed oral hypoglycemic agents for their diabetes.
The demographic and clinical disease characteristics for all the individuals and the matched individuals are shown in Table 1. A total of 50 individuals participated in the study, of whom 14 had TP and 36 had PD. After the propensity score matching process, 13 participants from the TP group and 26 from the PD group were considered suitable for comparison. Before propensity score matching, in demographic and clinical characteristics, both groups were comparable only in gender (SMD = 0.22), KPS (SMD = 0.06), diagnosis (SMD = 0.14), smoking status (SMD = 0.01), and cancer stage (SMD = 0.19); the rest of the demographic and disease characteristics were not comparable. After the propensity score matching procedure, the distributions of the demographic and disease characteristics in the two groups were comparable, except for postoperative time (SMD = 1.21), HbA1c (SMD = 0.3), and current treatment of diabetes (SMD = 1.7). As a result, in the fatigue and quality-of-life comparison procedure, postoperative time, HbA1c, and current treatment of diabetes were entered as adjustment variables in the GEE analysis. 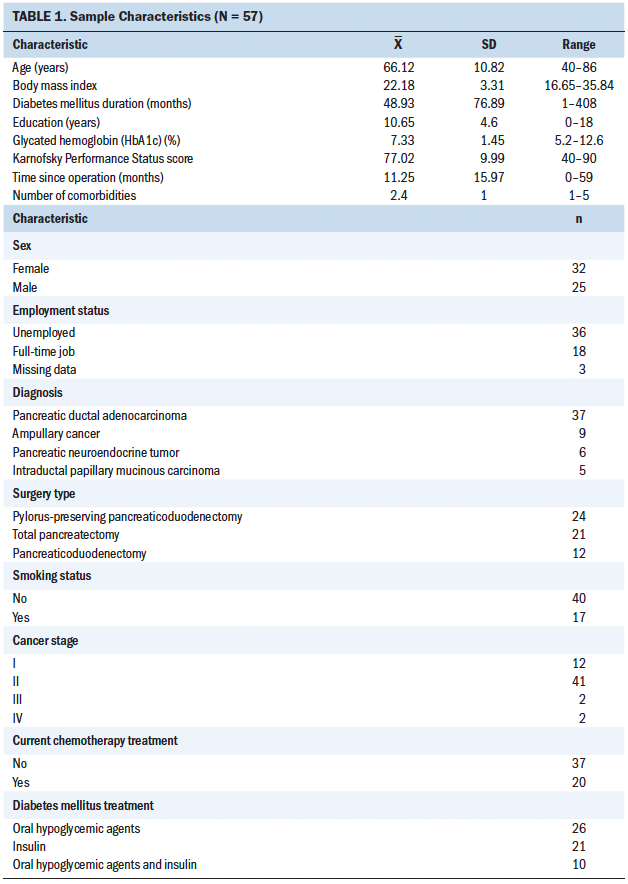
Matched-Pairs Analysis of Fatigue Characteristics
The matched-pairs analysis of the individuals’ fatigue characteristics is shown in Table 2. There were no statistically significant differences in total fatigue score and fatigue intensity between the TP and PD group. However, the TP group had a higher number of days experiencing fatigue in a week than the PD group (beta = 2.36, p = 0.019). In addition, the TP group reported a greater proportion of the day fatigued than the PD group (beta = 2.21, p = 0.021). In terms of perceived interference with functioning because of fatigue, the TP group reported higher scores in general daily activities (beta = 2.13, p = 0.009), work activity (beta = 2.17, p = 0.009), ability to concentrate (beta = 1.37, p = 0.012), relations with others (beta = 1.31, p = 0.013), enjoyment of life (beta = 2.66, p = 0.005), and overall perceived interference (beta = 1.52, p = 0.035) than the PD group. 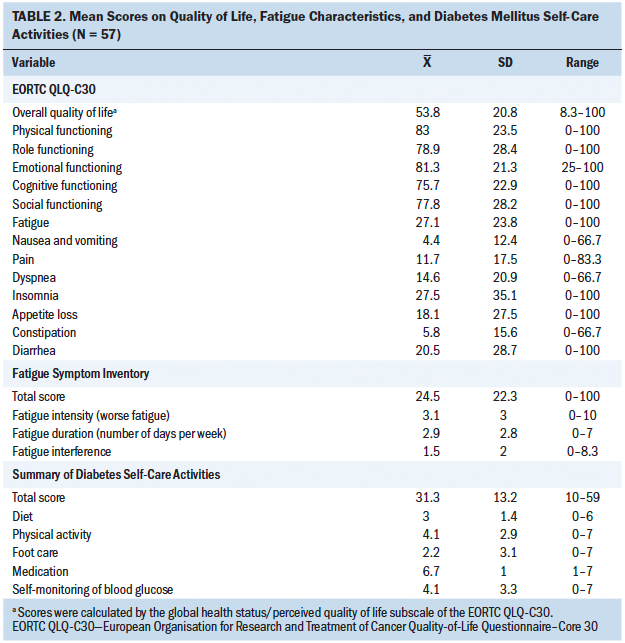
Matched-Pairs Analysis of Quality of Life
The matched-pairs analysis of the individuals’ quality of life is shown in Table 3. In comparison with individuals who had PD, patients who had TP showed a tendency for lower overall quality of life and functional scales. However, only physical function (beta = –22.67, p = 0.001) showed a statistically significant difference between the two groups. The TP group had lower physical function than the PD group. In terms of the symptom scales, the TP group demonstrated a tendency for higher levels of symptoms. However, only the symptom scales for insomnia (beta = 54.88, p < 0.001) were statistically significantly worse in the TP group. 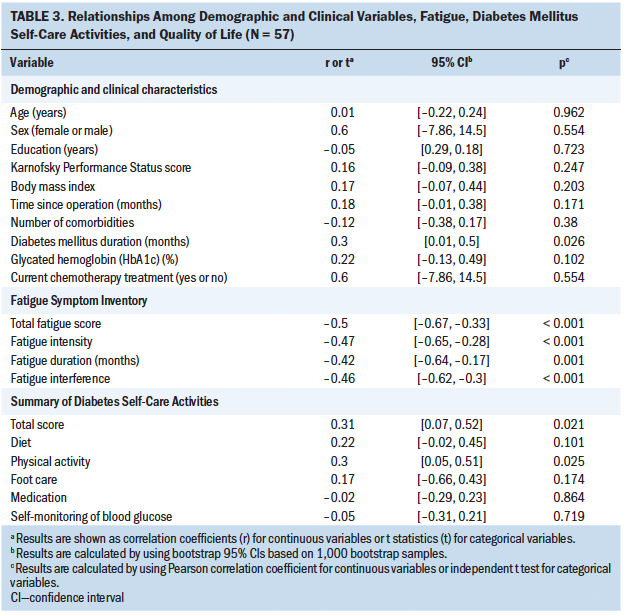
Discussion
The purpose of this study was to describe and compare fatigue characteristics and quality of life in individuals with pancreatogenic diabetes after TP and PD. To the authors’ knowledge, the present study is the first to compare both fatigue characteristics and quality of life in these patients. The authors found that the two groups had comparable fatigue intensity and overall quality of life. However, the TP group had lower physical function, a higher level of insomnia, longer fatigue duration, and perceived higher fatigue interference on functioning in general daily activities, work activity, ability to concentrate, relations with others, and enjoyment of life than the PD group.
The present study showed that individuals after TP and PD had comparable overall quality of life, which was similar to the findings of previous studies (Casadei et al., 2016; Epelboym et al., 2014; Müller et al., 2007). The reason for this may be the result of improved TP technology (e.g., better anastomotic technique and perioperative care [Andrén-Sandberg et al., 2016]) and exocrine and endocrine insufficiency management (Heidt et al., 2007; Maeda & Hanazaki, 2011). Despite the comparable results between TP and PD, compared to the reference values provided by the EORTC group (including 750 samples in the liver, bile, and pancreas cancer group, and 23,553 samples in the general cancer group), individuals after TP in the current study had better quality of life (mean = 57.69, SD = 26.45) than the liver, bile, and pancreas cancer group (mean = 55.9, SD = 25.1) but had worse quality of life than the general cancer group (mean = 61.3, SD = 24.2) (Aaronson et al., 1993; Fayers et al., 2001). The reason for this difference in quality of life may be because, compared to the liver, bile, and pancreas cancer group studied by the EORTC (Aaronson et al., 1993; Fayers et al., 2001), who were mostly individuals with stage IV cancer, individuals in the present study were mostly in the earlier stages of pancreatic cancer. Patients in this study had worse quality of life than the general cancer population, which emphasizes the need for more comprehensive studies and interventions regarding quality of life for individuals with pancreatogenic diabetes after pancreatic resection for pancreatic diseases.
The authors noticed that fatigue seemed comparable in the two groups in the EORTC QLQ-C30 symptom subscale analysis; however, from the FSI analysis, the authors observed that the two groups had comparable fatigue intensity but that the TP group had longer fatigue duration and higher perceived fatigue interference with functioning. This finding emphasizes the importance of evaluating patients’ fatigue duration and perceived interference with functioning. According to previous literature, the evaluation of fatigue interference plays a crucial role in the reporting of fatigue and the implementation of fatigue education in clinical practice (Shun, Lai, & Hsiao, 2009). Perceived fatigue interference in enjoyment of life and relationships with others is positively correlated with emotional representation of fatigue that influences individuals’ coping behaviors and strategies to mitigate fatigue (Donovan & Ward, 2005). Prolonged fatigue and fatigue interference in general daily activities, work activities, and the ability to concentrate also may facilitate symptom recognition and play an important role in coping with fatigue (Corbett, Groarke, Walsh, & McGuire, 2016; Yu, Lee, & Man, 2010). From previous literature and the current study results, initiating a discussion about fatigue duration and interference with individuals after TP may be pivotal in communication and education about fatigue.
Individuals who had TP had lower physical function than those who had PD in the current study, which was inconsistent with previous studies (Epelboym et al., 2014; Müller et al., 2007). This difference in physical function may be associated with the time after resection, as well as the influence of individuals in the study who were diagnosed with chronic pancreatitis. The physical function of individuals after TP tends to improve two years after surgery (Belyaev, Herzog, Chromik, Meurer, & Uhl, 2013; Heerkens et al., 2016). In this study, the average time after surgery was 11.85 months, whereas it was 23 months and 45 months in the studies by Müller et al. (2007) and Epelboym et al. (2014), respectively. In other words, the finding in this study may indicate that within two years of pancreatic resection, individuals after TP had lower physical function than those who had PD, a finding that was not found in previous studies because their data collection time point was not within two years of surgery. As for the possible influence of chronic pancreatitis on physical function, individuals after TP in this study had lower physical function (mean = 77.95, SD = 23.16) than individuals with chronic pancreatitis (mean = 78.2, SD = 2.9) (Rückert et al., 2011). Because a higher percentage of individuals with chronic pancreatitis was included in the PD group than in the TP group, it is possible that the disparity in physical function between the TP and the PD group was because of the influence of the individuals with chronic pancreatitis.
This study showed that patients have more insomnia after TP compared those after PD, which was inconsistent with the results from previous studies that revealed comparable symptoms of insomnia between TP and PD groups (Epelboym et al., 2014; Müller et al., 2007). This difference may be the result of pancreatogenic diabetes and fatigue experienced in patients after TP. It is known that fatigue is highly correlated with insomnia (Roscoe et al., 2007). The present study showed that individuals after TP suffered from longer durations of fatigue and perceived more functional interference of fatigue than individuals after PD. In addition, most of the participants were diagnosed with cancer. According to previous literature, cancer may cause interference in individuals’ cortisol levels during the daytime and lead to circadian cycle disruption (Ryan et al., 2007). In the current study, participants in the TP group complained about excessive daytime sleep because of constant fatigue; consequently, excessive daytime sleep may have caused their nighttime insomnia. Of note, individuals after TP experience diabetes similar to insulin-dependent diabetes, in which individuals may suffer more from frequent hypoglycemic incidents than patients with type 2 diabetes because of their sensitivity to insulin injections (Cui & Andersen, 2011; Maeda & Hanazaki, 2011; Makuc, 2016). In a review, Fritschi and Quinn (2010) reported that acute episodes of hypoglycemia and daily injections of insulin correlate with prolonged daytime fatigue in type 1 diabetes. From the previously mentioned literature, not only cancer itself, but also pancreatogenic diabetes may contribute to prolonged fatigue and insomnia in individuals post-TP.
Limitations
This study has some limitations. Despite the effort of using propensity scoring as a way to reduce the impact of confounding factors, there may be potential confounders (e.g., medications used, time effect, disease diagnosed) that the authors could not control because of the nature of an observational study. The time after TP surgery was shorter than that after PD. This phenomenon came from the natural course of pancreatic resection and pancreatogenic diabetes in which patients after TP immediately suffer from diabetes while patients after partial pancreatic resection experience diabetes following a period of time after the operation (Hart et al., 2016; Maeda & Hanazaki, 2011). In the present study, patients after PD developed diabetes an average of 11.06 months after surgery. This created a risk of bias for which statistical methods could not completely adjust. This was a single-center study with a rather small sample size using a cross-sectional study design. The results may not be generalizable to all individuals after pancreatic surgery. The results in this study should be interpreted with caution because a heterogeneous population in which individuals who underwent pancreatic resection for benign (i.e., chronic pancreatitis) and malignant conditions of the pancreas were included.
Implications for Nursing Practice and Research
Compared to individuals with pancreatogenic diabetes after PD, individuals after TP experience more insomnia, lower physical function, longer fatigue duration, and more interference from fatigue. The authors suggest that nurses who care for individuals after TP should pay extra attention to their fatigue, insomnia, and physical function. Because the three conditions are associated with each other, it is recommended that nurses assess them concurrently. The findings in this study also emphasize the importance of a comprehensive fatigue assessment, including fatigue intensity, duration, and perceived interference with functioning, in this population. Educational interventions led by nurses can help reduce fatigue intensity, fatigue interference, and overall fatigue (Bennett et al., 2016). General strategies for fatigue management, such as energy conservation techniques, including delegating and prioritizing daily tasks, could benefit patients with prolonged fatigue (NCCN, 2018). Nonpharmacologic interventions, such as exercise and sleep hygiene programs, can help improve fatigue symptoms and quality of life (Campos, Hassan, Riechelmann, & Del Giglio, 2011). Despite the important findings from this study, the authors also acknowledge that individuals with benign and malignant conditions of the pancreas were included. Because the postoperative period and management differ in the cancer and noncancer populations (e.g., adjuvant therapy), nurses should provide assessment and intervention for patients after pancreatic resection according to their diagnosis and needs.
In terms of future research, the propensity score matching analysis is a good method for addressing confounders in comparison studies; however, studies with a larger initial sample size will be optimal for this method because of the loss of samples during the matching procedure. A longitudinal design to help identify the trajectory of fatigue characteristics and quality of life in individuals with pancreatogenic diabetes after TP is recommended. Future study with nursing interventions targeting fatigue management, physical function, and insomnia in individuals after TP is suggested to effectively improve their quality of life. 
Conclusion
The present study suggested a similarity in fatigue intensity and overall quality of life in individuals after TP and PD. However, with in-depth fatigue assessment, patients after TP demonstrated a longer duration of fatigue and perceived more interference with functioning than individuals after PD. Individuals who had TP also had lower physical function and higher symptoms of insomnia than individuals after PD. Therefore, nursing interventions could help improve their fatigue, insomnia, and physical function. In clinical settings, nurses play important roles in fatigue assessment and symptom management. Future research on interventions that can provide patients with adequate education regarding fatigue management is imperative for this population to have better sleep, physical function, and quality of life.
About the Author(s)
Hsuan-Ju Kuo, RN, MSN, is a student in the School of Nursing in the College of Medicine at National Taiwan University in Taipei; Yu-Wen Tien, PhD, is a professor in the Department of Surgery at National Taiwan University Hospital in Taipei; and Nien-Tzu Chang, RN, PhD, is an assistant professor, Yun-Jen Chou, RN, MSN, is a doctoral student, and Shiow-Ching Shun, RN, PhD, is a professor, all in the School of Nursing, all in the College of Medicine at National Taiwan University. No financial relationships to disclose. Kuo, Chang, and Shun contributed to the conceptualization and design. Kuo and Tien completed the data collection. Kuo, Chang, Chou, and Shun provided statistical support. Kuo and Shun provided the analysis. Kuo, Chang, Chou, and Shun contributed to the manuscript preparation. Shun can be reached at scshun@ntu.edu.tw, with copy to ONFEditor@ons.org. (Submitted September 2018. Accepted February 26, 2019.)
References
Aaronson, N.K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N.J., . . . Takeda, F. (1993). The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376.
Adriaanse, M.C., Drewes, H.W., van der Heide, I., Struijs, J.N., & Baan, C.A. (2016). The impact of comorbid chronic conditions on quality of life in type 2 diabetes patients. Quality of Life Research, 25, 175–182. https://doi.org/10.1007/s11136-015-1061-0
Andrén-Sandberg, Å., Ansorge, C., & Yadav, T.D. (2016). Are there indications for total pancreatectomy in 2016? Digestive Surgery, 33, 329–334. https://doi.org/10.1159/000445018
Austin, P.C. (2008). Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiology and Drug Safety, 17, 1218–1225. https://doi.org/10.1002/pds.1674
Austin, P.C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research, 46, 399–424. https://doi.org/10.1080/00273171.2011.568786
Barbier, L., Jamal, W., Dokmak, S., Aussilhou, B., Corcos, O., Ruszniewski, P., . . . Sauvanet, A. (2013). Impact of total pancreatectomy: Short- and long-term assessment. HPB, 15, 882–892. https://doi.org/10.1111/hpb.12054
Beijer, S., Kempen, G.I., Pijls-Johannesma, M.C., de Graeff, A., & Dagnelie, P.C. (2008). Determinants of overall quality of life in preterminal cancer patients. International Journal of Cancer, 123, 232–235. https://doi.org/10.1002/ijc.23497
Belyaev, O., Herzog, T., Chromik, A.M., Meurer, K., & Uhl, W. (2013). Early and late postoperative changes in the quality of life after pancreatic surgery. Langenbeck’s Archives of Surgery, 398, 547–555. https://doi.org/10.1007/s00423-013-1076-3
Bennett, S., Pigott, A., Beller, E.M., Haines, T., Meredith, P., & Delaney, C. (2016). Educational interventions for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews, 11. https://doi.org/10.1002/14651858.CD008144.pub2
Bloechle, C., Izbicki, J.R., Knoefel, W.T., Kuechler, T., & Broelsch, C.E. (1995). Quality of life in chronic pancreatitis—Results after duodenum-preserving resection of the head of the pancreas. Pancreas, 11, 77–85.
Campos, M.P., Hassan, B.J., Riechelmann, R., & Del Giglio, A. (2011). Cancer-related fatigue: A practical review. Annals of Oncology, 22, 1273–1279. https://doi.org/10.1093/annonc/mdq458
Casadei, R., Monari, F., Buscemi, S., Laterza, M., Ricci, C., Rega, D., . . . Minni, F. (2010). Total pancreatectomy: Indications, operative technique, and results: A single centre experience and review of literature. Updates in Surgery, 62, 41–46. https://doi.org/10.1007/s13304-010-0005-z
Casadei, R., Ricci, C., Taffurelli, G., Guariniello, A., Di Gioia, A., Di Marco, M., . . . Minni, F. (2016). Is total pancreatectomy as feasible, safe, efficacious, and cost-effective as pancreaticoduodenectomy? A single center, prospective, observational study. Journal of Gastrointestinal Surgery, 20, 1595–1607. https://doi.org/10.1007/s11605-016-3201-4
Chie, W.C., Yang, C.H., Hsu, C., & Lai, C.C. (2002). Introduction of the EORTC Disease-Specific Quality of Life Questionnaires for cancer patients. Formosan Journal of Medicine, 6, 220–227. https://doi.org/10.6320/FJM.2002.6(2).13
Chie, W.C., Yang, C.H., Hsu, C., & Yang, P.C. (2004). Quality of life of lung cancer patients: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and QLQ-LC13. Quality of Life Research, 13, 257–262. https://doi.org/10.1023/B:QURE.0000015295.74812.06
Cid-Arregui, A., & Juarez, V. (2015). Perspectives in the treatment of pancreatic adenocarcinoma. World Journal of Gastroenterology, 21, 9297–9316. https://doi.org/10.3748/wjg.v21.i31.9297
Corbett, T., Groarke, A., Walsh, J.C., & McGuire, B.E. (2016). Cancer-related fatigue in post-treatment cancer survivors: Application of the common sense model of illness representations. BMC Cancer, 16, 919. https://doi.org/10.1186/s12885-016-2907-8
Cui, Y., & Andersen, D.K. (2011). Pancreatogenic diabetes: Special considerations for management. Pancreatology, 11, 279–294. https://doi.org/10.1159/000329188
de Graaf, M.A., Jager, K.J., Zoccali, C., & Dekker, F.W. (2011). Matching, an appealing method to avoid confounding? Nephron Clinical Practice, 118, c315–c318. https://doi.org/10.1159/000323136
Donovan, H.S., & Ward, S. (2005). Representations of fatigue in women receiving chemotherapy for gynecologic cancers. Oncology Nursing Forum, 32, 113–116. https://doi.org/10.1188/05.ONF.113-116
Donovan, K.A., & Jacobsen, P.B. (2010). The Fatigue Symptom Inventory: A systematic review of its psychometric properties. Supportive Care in Cancer, 19, 169–185. https://doi.org/10.1007/s00520-010-0989-4
Epelboym, I., Winner, M., DiNorcia, J., Lee, M.K., Lee, J.A., Schrope, B., . . . Allendorf, J.D. (2014). Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. Journal of Surgical Research, 187, 189–196. https://doi.org/10.1016/j.jss.2013.10.004
Farrell, J.J. (2015). Prevalence, diagnosis and management of pancreatic cystic neoplasms: Current status and future directions. Gut and Liver, 9, 571–589. https://doi.org/10.5009/gnl15063
Fayers, P., Aaronson, N.K., Bjordal, K., Groenvold, M., Curran, D., & Bottomley, A. (2001). EORTC QLQ-C30 scoring manual (3rd ed.). Brussels, Belgium: European Organisation for Research and Treatment of Cancer.
Fritschi, C., & Quinn, L. (2010). Fatigue in patients with diabetes: A review. Journal of Psychosomatic Research, 69, 33–41. https://doi.org/10.1016/j.jpsychores.2010.01.021
Hart, P.A., Bellin, M.D., Andersen, D.K., Bradley, D., Cruz-Monserrate, Z., Forsmark, C.E., . . . Chari, S.T. (2016). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterology and Hepatology, 1, 226–237. https://doi.org/10.1016/s2468-1253(16)30106-6
Heerkens, H.D., Tseng, D.S., Lips, I.M., van Santvoort, H.C., Vriens, M.R., Hagendoorn, J., . . . Molenaar, I.Q. (2016). Health-related quality of life after pancreatic resection for malignancy. British Journal of Surgery, 103, 257–266. https://doi.org/10.1002/bjs.10032
Heidt, D.G., Burant, C., & Simeone, D.M. (2007). Total pancreatectomy: Indications, operative technique, and postoperative sequelae. Journal of Gastrointestinal Surgery, 11, 209–216. https://doi.org/10.1007/s11605-006-0025-7
Hjermstad, M.J., Fayers, P.M., Bjordal, K., & Kaasa, S. (1998). Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ=C30 (+ 3). Journal of Clinical Oncology, 16, 1188–1196. https://doi.org/10.1200/JCO.1998.16.3.1188
Ilic, M., & Ilic, I. (2016). Epidemiology of pancreatic cancer. World Journal of Gastroenterology, 22, 9694–9705. https://doi.org/10.3748/wjg.v22.i44.9694
Jamil, L.H., Chindris, A.M., Gill, K.R., Scimeca, D., Stauffer, J.A., Heckman, M.G., . . . Wallace, M.B. (2012). Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: An exploratory study. HPB Surgery, 2012, 381328. https://doi.org/10.1155/2012/381328
Keim, V., Klar, E., Poll, M., & Schoenberg, M.H. (2009). Postoperative care following pancreatic surgery: Surveillance and treatment. Deutsches Arzteblatt International, 106, 789–794. https://doi.org/10.3238/arztebl.2009.0789
Maeda, H., & Hanazaki, K. (2011). Pancreatogenic diabetes after pancreatic resection. Pancreatology, 11, 268–276. https://doi.org/10.1159/000328785
Makuc, J. (2016). Management of pancreatogenic diabetes: Challenges and solutions. Diabetes, Metabolic Syndrome, and Obesity: Targets and Therapy, 9, 311–315. https://doi.org/10.2147/DMSO.S99701
Müller, M.W., Friess, H., Kleeff, J., Dahmen, R., Wagner, M., Hinz, U., . . . Buchler, M.W. (2007). Is there still a role for total pancreatectomy? Annals of Surgery, 246, 966–974. https://doi.org/10.1097/SLA.0b013e31815c2ca3
National Comprehensive Cancer Network. (2018). NCCN clinical practice guidelines in oncology: Cancer-related fatigue [v.2.2018]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
Niezgoda, H.E., & Pater, J.L. (1993). A validation study of the domains of the core EORTC quality of life questionnaire. Quality of Life Research, 2, 319–325.
Pirracchio, R., Resche-Rigon, M., & Chevret, S. (2012). Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Medical Research Methodology, 12(70), 1–10. https://doi.org/10.1186/1471-2288-12-70
Raimondi, S., Lowenfels, A.B., Morselli-Labate, A.M., Maisonneuve, P., & Pezzilli, R. (2010). Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Practice and Research: Clinical Gastroenterology, 24, 349–358. https://doi.org/10.1016/j.bpg.2010.02.007
Roscoe, J.A., Kaufman, M.E., Matteson-Rusby, S.E., Palesh, O.G., Ryan, J.L., Kohli, S., . . . Morrow, G.R. (2007). Cancer-related fatigue and sleep disorders. Oncologist, 12(Suppl. 1), 35–42. https://doi.org/10.1634/theoncologist.12-S1-35
Rückert, F., Distler, M., Hoffmann, S., Hoffmann, D., Pilarsky, C., Dobrowolski, F., . . . Grutzmann, R. (2011). Quality of life in patients after pancreaticoduodenectomy for chronic pancreatitis. Journal of Gastrointestinal Surgery, 15, 1143–1150. https://doi.org/10.1007/s11605-011-1539-1
Ryan, J.L., Carroll, J.K., Ryan, E.P., Mustian, K.M., Fiscella, K., & Morrow, G.R. (2007). Mechanisms of cancer-related fatigue. Oncologist, 12(Suppl. 1), 22–34. https://doi.org/10.1634/theoncologist.12-S1-22
Scott, J.A., Lasch, K.E., Barsevick, A.M., & Piault-Louis, E. (2011). Patients’ experiences with cancer-related fatigue: A review and synthesis of qualitative research. Oncology Nursing Forum, 38, E191–E203. https://doi.org/10.1188/11.ONF.E191-E203
Shun, S.C., Beck, S.L., Pett, M.A., & Berry, P.H. (2006). Psychometric testing of three Chinese fatigue instruments in Taiwan. Journal of Pain and Symptom Management, 32, 155–167. https://doi.org/10.1016/j.jpainsymman.2006.02.011
Shun, S.C., Lai, Y.H., & Hsiao, F.H. (2009). Patient-related barriers to fatigue communication in cancer patients receiving active treatment. Oncologist, 14, 936–943. https://doi.org/10.1634/theoncologist.2009-0048
Stark, A., Donahue, T.R., Reber, H.A., & Hines, O.J. (2016). Pancreatic cyst disease: A review. JAMA, 315, 1882–1893. https://doi.org/10.1001/jama.2016.4690
Stuart, E.A. (2010). Matching methods for causal inference: A review and a look forward. Statistical Science, 25, 1–21. https://doi.org/10.1214/09-STS313
Tillou, J.D., Tatum, J.A., Jolissaint, J.S., Strand, D.S., Wang, A.Y., Zaydfudim, V., . . . Brayman, K.L. (2017). Operative management of chronic pancreatitis: A review. American Journal of Surgery, 214, 347–357. https://doi.org/10.1016/j.amjsurg.2017.03.004
Trutschel, D., Palm, R., Holle, B., & Simon, M. (2017). Methodological approaches in analysing observational data: A practical example on how to address clustering and selection bias. International Journal of Nursing Studies, 76, 36–44. https://doi.org/10.1016/j.ijnurstu.2017.06.017
Velanovich, V., & Wollner, I. (2011). Quality of life and performance status in patients with pancreatic and periampullary tumors. International Journal of Clinical Oncology, 16, 401–407. https://doi.org/10.1007/s10147-011-0200-z
Wan, E.Y., Fung, C.S., Choi, E.P., Wong, C.K., Chan, A.K., Chan, K.H., & Lam, C.L. (2016). Main predictors in health-related quality of life in Chinese patients with type 2 diabetes mellitus. Quality of Life Research, 25, 2957–2965. https://doi.org/10.1007/s11136-016-1324-4
Yang, Q.Y., & Chen, Y.M. (2016). Medical statistics and SPSS analysis methods and application (2nd ed.). Taipei: Wunan.
Yao, K.P. (2002). Development and applications of the WHOQOL-Taiwan version. Formosan Journal of Medicine, 6, 193–200.
Yu, D.S., Lee, D.T., & Man, N.W. (2010). Fatigue among older people: A review of the research literature. International Journal of Nursing Studies, 47, 216–228. https://doi.org/10.1016/j.ijnurstu.2009.05.009
Zeger, S.L., Liang, K.Y., & Albert, P.S. (1988). Models for longitudinal data: A generalized estimating equation approach. Biometrics, 44, 1049–1060.
Zhang, Q., Zeng, L., Chen, Y., Lian, G., Qian, C., Chen, S., . . . Huang, K. (2016). Pancreatic cancer epidemiology, detection, and management. Gastroenterology Research and Practice, 2016, 8962321. https://doi.org/10.1155/2016/8962321
Zimmermann, C., Burman, D., Swami, N., Krzyzanowska, M.K., Leighl, N., Moore, M., . . . Tannock, I. (2011). Determinants of quality of life in patients with advanced cancer. Supportive Care in Cancer, 19, 621–629. https://doi.org/10.1007/s00520-010-0866-1




