Skeletal Muscle Mass Loss During Cancer Treatment: Differences by Race and Cancer Site
Objectives: To examine skeletal muscle mass change in a racially diverse sample of patients undergoing cancer treatment, determine significant predictors of muscle mass loss, and explore the interaction of race and cancer site.
Sample & Setting: A retrospective analysis was conducted for 212 patients seeking treatment at a university hospital clinic.
Methods & Variables: Skeletal muscle mass index (SMI) was determined by computed tomography at the time of cancer diagnosis and with cancer treatment.
Results: One hundred thirty-four patients (63%) had SMI loss with cancer treatment. Race and cancer site were found to be significant predictors of SMI loss. Compared to other racial groups, non-Hispanic Black (NHB) patients had the greatest SMI loss (p < 0.001) with cancer treatment. NHB patients with rectal cancer experienced the greatest SMI loss compared to patients of other races and cancer types.
Implications for Nursing: To improve survivorship care for patients with cancer, it is essential to develop strategies for assessing and managing skeletal muscle mass loss throughout treatment, particularly for NHB patients with rectal cancer.
Jump to a section
Oncology researchers have turned their attention during the past decade to the growing burden of skeletal muscle mass loss, an important factor driving healthcare needs and cancer survivorship care (Chang et al., 2018; Rier et al., 2016; Shachar et al., 2016). Low skeletal muscle mass in patients with cancer has been related to toxicities from chemotherapy, overall survival rates, and disease-free survival (Antoun et al., 2013; Pamoukdjian et al., 2018; Prado et al., 2007; Shachar et al., 2016). Cancer-related research and treatment efforts have considered various causes for muscle loss in patients with cancer (Williams et al., 2019). The prevalence of low skeletal muscle mass, which is variously defined based on several cut-points, such as 52.4 cm2/m2 for men and 38.5 cm2/m2 for women (Prado et al., 2008), is known to vary widely across cancer types, with a range of 5%–89% (Rier et al., 2016). Sex also affects the risk of low muscle mass; more men (61%) than women (41%) have shown loss of muscle mass after the first line of chemotherapy (Choi et al., 2015). In addition, increasing age is known to be related to greater muscle mass loss among patients with cancer (Williams et al., 2019).
These issues are particularly important considerations in cancer survivorship care because low muscle mass has been directly related not only to cancer treatment toxicities but also to postoperative complications, longer hospitalizations, and higher cancer recurrence and mortality (Mei et al., 2016; Miyamoto et al., 2015; Prado et al., 2008, 2009; Simonsen et al., 2018; Villaseñor et al., 2012). In addition, the lowered physical function and frailty associated with cancer and its treatment are known to accelerate muscle mass loss (Ethun et al., 2017; Galvão et al., 2009; Handforth et al., 2014; Morishita et al., 2012), which, in turn, leads to reduced quality of life, increased fatigue and depression, and reduced engagement in activities known to reduce the risk of recurrence (Luciani et al., 2008; Nipp et al., 2018; Prado et al., 2013).
Despite the fact that skeletal muscle mass loss is a common cancer-related symptom and a measurable predictor of cancer treatment toxicity, and that research has shown differences in skeletal muscle mass by race and cancer site (Deurenberg et al., 1998; Hull et al., 2011), few studies have compared skeletal muscle mass loss with cancer treatment while also considering interactions with race and cancer site. In a retrospective study involving 3,262 patients with colorectal cancer, skeletal muscle mass measured by computed tomography (CT) scan at diagnosis was higher among non-Hispanic Black (NHB) and Hispanic patients than among non-Hispanic White (NHW) and Asian patients (Feliciano et al., 2017). Similarly, in a study involving 2,914 patients with early-stage breast cancer, NHB patients showed lower odds of low skeletal muscle mass at diagnosis than patients of other races (Caan et al., 2017). With respect to cancer sites, a meta-analysis and systematic review involving 7,843 patients with cancer found the highest prevalence of skeletal muscle mass loss among patients with non-small cell lung cancer (74%), followed by patients with urothelial carcinoma (69%) (Shachar et al., 2016).
Conceptual frameworks for low muscle mass in general populations have been developed by Calvani et al. (2014) and Landi et al. (2016). These frameworks consider low muscle mass as an underlying biologic mechanism for physical frailty and the pathway by which frailty’s negative health outcomes occur (Calvani et al., 2014; Landi et al., 2016). In the oncology field, Williams et al. (2019) stated that multiple causes of muscle loss exist, including aging, cancer-related factors, and cancer treatment–related effects. Considering the importance of low muscle mass in oncology practice and research, this study’s theoretical design was drawn from Williams et al. (2019)’s multifactorial causes of low muscle mass, as well as from previous findings regarding low muscle mass based on sex-specific skeletal muscle mass index (SMI) cut-points (Prado et al., 2008) and race-/ethnicity-specific differences in muscle mass (Silva et al., 2010; Yoowannakul et al., 2018).
Given the existence of racial disparities in skeletal muscle mass and the range of cancer treatment regimens that depend on cancer site, an improved understanding of skeletal muscle mass loss with cancer treatment in various populations is needed to drive more effective survivorship care strategies. This study aimed to examine skeletal muscle mass with cancer treatment, to evaluate predictors of skeletal muscle mass loss, and to examine interactions of race and cancer site using data from 212 racially diverse patients with various cancer diagnoses.
Methods
Design and Sample
This study employed a retrospective analysis design that used de-identified data from a tumor registry at the University of Illinois Cancer Center in Chicago. The data subjected to secondary analysis were for 212 racially diverse patients aged 18 years or older who were diagnosed with a solid tumor cancer from April 2013 to December 2016 and treated at a single academic institution. Ethical approval for the study was obtained from the institutional review board of the University of Illinois at Chicago.
Data Collection
The 212 cancer cases were linked with electronic health records, from which the authors obtained data on patient demographic characteristics (including age, sex, and race) and patient clinical characteristics related to cancer diagnosis (including date of diagnosis, cancer stage, and cancer type). The target population size was determined according to G*Power 3.1 software using paired t-test analysis. Based on previous studies (Chang et al., 2018; Rier et al., 2016; Yamaoka et al., 2015), a sample size of 199 was assumed with an alpha of 0.05, medium effect size of 0.02, and power of 0.8. Therefore, the inclusion of 212 cases was sufficient and established in this study. Each patient had abdominal CT scans, which included images of the third lumbar spine vertebra (L3), at the time of cancer diagnosis and after cancer treatment. For patients with metastatic cancer, CT images were obtained at least two months after treatment initiation.
Derivation of Skeletal Muscle Mass Index
The CT images were retrieved using DICOM® (Digital Imaging and Communications in Medicine) software. The de-identified CT information was then sent to the University of Alberta in Edmonton, Canada, where body composition analysis was performed using the TomoVision sliceOmatic 5.0 software. This software enables researchers to calculate SMI (as cm2/m2) by measuring the skeletal muscle mass cross-sectional area (cm2) at L3 and then normalizing the results for patient height in meters squared (m2).
Data Analysis
Descriptive data are shown as means and standard deviations (SDs) for continuous variables and as proportions and percentages for categorical variables. Mean differences in SMI change with cancer treatment were estimated for race- and sex-specific comparisons using a paired t test. Multiple linear regression was employed to determine which of the demographic and clinical variables (i.e., age, race, sex, cancer site, and cancer stage) were predictors of SMI loss. Because the treatment period differed by cancer type, treatment period was controlled for in the multiple linear regression model for SMI loss. To predict SMI loss by race and cancer site, marginal effects analysis was applied. To account for missing data for variables, maximum likelihood estimation was performed. All statistical analyses were performed with STATA, version 14.2, with a statistical significance value of p < 0.05 established by two-sided tests.
Results
Sample Characteristics
Table 1 describes the demographic and clinical characteristics of the study population. Of the 212 patients with cancer included for analysis, 128 (60%) were men and 132 (62%) self-identified as NHB. Patients’ mean age was 63.8 years (SD = 11). More than 30% of patients overall (n = 65) had lung cancer, and more than half of the patient population had advanced-stage cancer, with stage IV cancer identified in more than one-third (n = 83, 39%) and stage III cancer in almost one-third (n = 59, 28%) of patients. 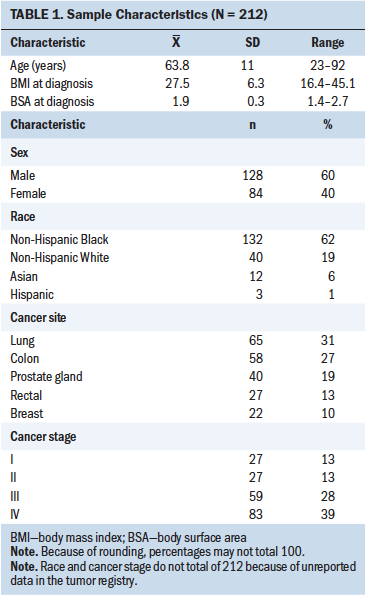
Skeletal Muscle Mass Loss
Figure 1 shows the distribution of absolute SMI change with cancer treatment by race. The mean change in SMI loss among all patients was 2.6 (SD = 6.4) cm2/m2. The highest SMI loss for an individual patient was 22.6 cm2/m2. Overall, 33% of patients in the study had improved skeletal muscle mass with cancer treatment, and 67% experienced a decrease in skeletal muscle mass. Accordingly, change in SMI across patients in all three racial groups (NHB, NHW, and other) was widely distributed. Patients who demonstrated the greatest loss in SMI (n = 7) were NHB, and, overall, the NHW patients experienced less SMI loss than the NHB patients. 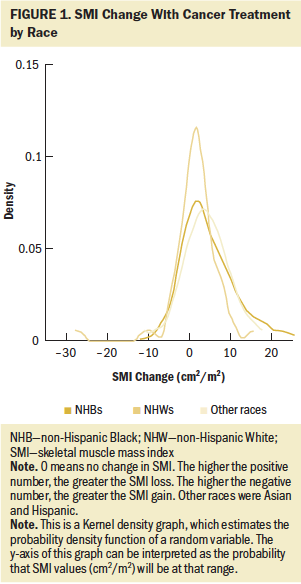
Table 2 presents a comparison of SMI changes with cancer treatment by gender. Based on the paired t test, there was a significant difference between men and women in SMI change with treatment (p < 0.001). 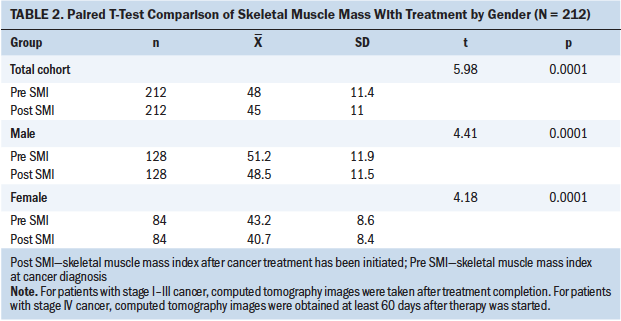
Predictors of Skeletal Muscle Mass Change
The multiple regression that examined the SMI loss group (n = 134) was significant, with model fit statistics significant at F(12, 121) = 2.15, p < 0.05, with 9% of the variance in SMI loss explained. Two predictors, race and cancer site, were found to be significant. The SMI loss of NHB patients with cancer showed 3.287 cm2/m2 more muscle loss than the reference group (NHW patients). In addition, the groups of patients with breast, lung, colon, and prostate cancer had less SMI loss compared with the SMI loss of patients with rectal cancer (see Table 3). Therefore, race (NHB) and cancer site (rectal cancer) were significant predictors for explaining the highest amount of SMI loss. 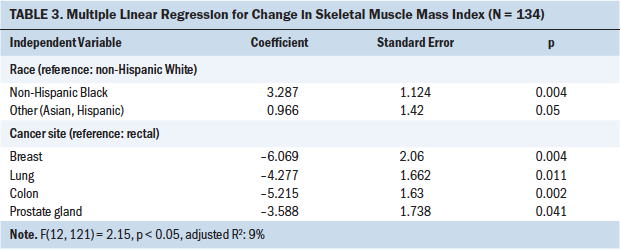
To examine SMI loss by race and cancer site with the estimated regression model, the authors used predictive margins and marginal effects analysis, as shown in Table 4. NHB patients with rectal cancer experienced the highest SMI loss, and NHB patients with breast cancer had the lowest SMI loss. Figure 2 shows that, compared to NHB patients, the group of NHW patients had lower SMI loss across all cancer sites. 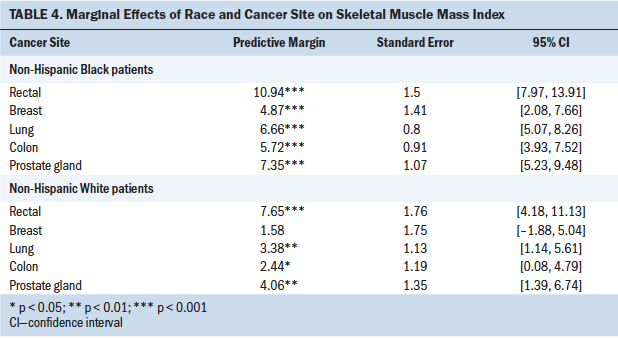
Discussion
Changes in skeletal muscle mass with cancer treatment have not been widely studied by race or by cancer site. In examining skeletal muscle mass by using SMI with cancer treatment, this study found differences according to race and cancer site. Of note, NHB patients experienced appreciable losses in skeletal muscle mass, which were significantly greater than losses observed in NHW patients. Inclusion of cancer site to models showed that NHB patients with rectal cancer experienced the greatest loss of skeletal muscle mass (an average of 10.94 cm2/m2) with treatment. To the best of the authors’ knowledge, no previous study has compared skeletal muscle mass values with cancer treatment while specifically examining its interaction with race and cancer site. These study findings provide preliminary guidance for identifying groups at high risk for muscle mass loss. To improve care and outcomes, patients in these groups will need to be carefully assessed and managed during cancer treatment. 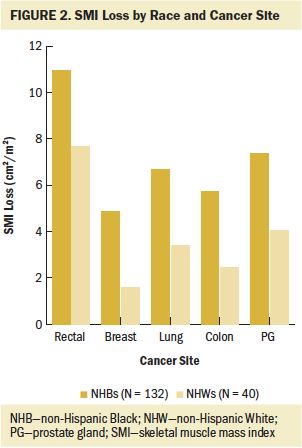
Overall, this study’s findings demonstrate that the prevalence of SMI loss with cancer treatment, assessed using CT scans, differs among patients of different races. The authors found that NHB men had the highest SMI before cancer treatment began, a finding similar to that of a previous descriptive study of 3,262 patients with colorectal cancer, which reported that NHB patients had higher skeletal muscle mass than NHW patients (Xiao et al., 2019). Specifically, the descriptive study reported that NHW men had the lowest SMI among men of all races, and NHW women had lower SMI than NHB, Hispanic, or Latino women (Xiao et al., 2019).
In an important contribution to the understanding of SMI changes, the current analysis revealed that the effects of cancer treatment on SMI differ by race and cancer site, and that race and cancer site are predictive of SMI loss with treatment. NHB patients with cancer showed the highest SMI before treatment, yet they experienced the greatest muscle mass loss with cancer treatment. This suggests that NHB patients with cancer may be particularly vulnerable to skeletal muscle mass loss. Considering body composition differences by race (Deurenberg et al., 1998; Goodpaster et al., 2006; Heymsfield et al., 2016; Hull et al., 2011) and known racial disparities in health (Cossrow & Falkner, 2004; Egede, 2006; Richardson & Norris, 2010), NHB patients with cancer should be carefully monitored for muscle mass loss during treatment. Loss of skeletal muscle mass has been related to postoperative complications and lower survival rates (Joglekar et al., 2015; Pamoukdjian et al., 2018; Shachar et al., 2016), and racial differences in muscle mass changes during cancer treatment should be further explored for their implications for survivorship care.
This study also contributed to addressing important knowledge gaps in skeletal muscle mass loss by cancer site. For example, the current analyses revealed that NHB patients with rectal cancer experienced greater skeletal muscle mass loss compared to NHB patients with cancers at other sites. Those with breast cancer experienced the lowest amount of SMI loss. In addition, the analyses evaluated SMI change among patients with colon and rectal cancer separately and showed that patients with rectal cancer had greater skeletal muscle mass loss than patients with colon cancer. These findings expand on current research that has focused predominantly on analyzing the prevalence of muscle mass loss among patients with colon and rectal cancer as a single group.
These findings are important because, in a previous retrospective study, 39% of patients with advanced rectal cancer had low skeletal muscle mass, and low muscle mass was a significant prognostic factor for overall survival (Choi et al., 2018). In another study (Levolger et al., 2018), loss of skeletal muscle mass during neoadjuvant chemotherapy was found to be an independent prognostic factor for disease-free survival and distant metastasis–free survival among patients with advanced rectal cancer. It is important to acknowledge that individuals diagnosed with colorectal cancer often experience malnutrition (Hu et al., 2015; Yamano et al., 2016) resulting from bowel obstruction, malabsorption, and cancer treatment (Ryan et al., 2016). The coexistence of low skeletal muscle mass and malnutrition has been related to lower survival rates compared to only one of those conditions being present (Vashi et al., 2019). In another retrospective analysis examining individuals with colorectal cancer, those with low skeletal muscle mass had shorter durations of recurrence-free survival and overall survival than patients not exhibiting low skeletal muscle mass, as well as longer hospital stays and higher incidence of postoperative morbidity, infection, and mortality (Sun et al., 2018).
These findings highlight the need for interventions among individuals with colorectal cancer that involve personalized nutrition regimens and resistance exercise to preserve skeletal muscle mass. In particular, resistance exercise has been shown to be a promising strategy for preserving muscle mass and improving treatment effectiveness in patients with various cancer types (Adams et al., 2016; Naito et al., 2019; Yamamoto et al., 2017). Development of early interventions for groups at high risk, such as NHB patients undergoing treatment and specifically NHB patients with rectal cancer, will help to maintain muscle mass and enhance survivorship care and outcomes.
Limitations
The current study had several limitations. This was a retrospective study using available cancer cases that included CT images at diagnosis and with cancer treatment. Serial CT images are not standard of care, and the patients whose files contain these images may have been more malnourished, more symptomatic from their cancer treatment, or otherwise at higher risk for poor outcomes. This represents potential sampling bias. However, such a bias is unlikely to fully explain the higher SMI loss found among NHB versus NHW patients. Another limitation is that the authors were not able to take treatment into consideration. Chemotherapy is known to significantly alter body composition, and it is possible that the finding of a greater decrease in SMI among NHB patients with rectal cancer reflects more aggressive treatment regimens among these patients.
Although this was a retrospective study using tumor registry data for 212 patients with cancer who received treatment at one cancer center, the sample was heterogeneous in terms of racial composition and cancer site. In future studies, a prospective research design that includes samples from multiple cancer centers should be used to broaden the generalizability of results; such research should compare results by cancer type and cancer treatment, as well as by race/ethnicity. The study examined clinical and CT scan data that could be extracted from the tumor registry; therefore, potential confounding factors, such as physical, functional, and physiological factors, as well as health-related behavior during cancer treatment, could not be included. Additional potential factors that can independently affect a patient’s SMI include age at diagnosis, treatment, treatment period, disease recurrence status, comorbidities, and social determinants of health. Future studies would benefit from examining data that incorporate more of these potentially relevant factors with respect to changes in skeletal muscle mass. In addition, because skeletal muscle mass loss does not necessarily result in declines in muscle strength, physical function, or performance, future studies should also include physical function tests.
Despite these limitations, this was the first study to compare skeletal muscle mass changes with cancer treatment by race and cancer site, and it was able to determine that skeletal muscle mass loss differs by race and cancer site. These potential prognostic factors deserve careful clinical attention and further research. Given the dramatic change in NHB patients’ muscle mass over the course of cancer treatment, NHB patients with cancer should be considered a vulnerable group at high risk for skeletal muscle mass loss during treatment.
Implications for Nursing
This study identified significant predictors of muscle mass loss with cancer treatment and interactions of race, cancer site, and muscle mass loss in racially diverse patients undergoing cancer treatment. The findings have implications for development of future directions for oncology nursing research and practice focused on supporting individuals with cancer who experience muscle mass loss. Of note, more than two-thirds of the patients included in this study had decreases in skeletal muscle mass, and the NHB patients with rectal cancer experienced the greatest SMI loss; by way of comparison, NHW patients showed less SMI loss across all cancer sites. This study highlights the critical importance of monitoring particular groups of patients with cancer (e.g., NHB patients with rectal cancer) for skeletal muscle mass loss as a routine part of survivorship care. To help prevent skeletal muscle mass loss in such patients, early intervention made possible by monitoring is critical. Because skeletal muscle mass loss with cancer treatment is an emerging issue in the oncology field, oncology nurses need to translate this growing body of knowledge into careful assessment and management of patients’ muscle mass as part of their clinical practice. 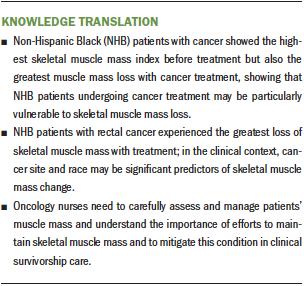
Conclusion
The study findings reveal that the extent of skeletal muscle mass lost during cancer treatment varies by race and cancer site. NHB patients with rectal cancer appeared to be the most vulnerable to skeletal muscle loss, compared to NHW patients. These findings provide preliminary insights for healthcare professionals to develop patient-centered strategies to preserve skeletal muscle mass during cancer treatment. These findings also suggest a possible role for oncologic rehabilitation programs to optimize skeletal muscle mass during and after completion of cancer treatment.
The authors gratefully acknowledge Carla Prado, PhD, RD, for her assistance with the database analysis and additional expertise for this study. They also gratefully acknowledge Dana Sohmer, MS, Winnie Mar, MD, Joshua Mendoza-Elias, MD, Alicia Hulbert, MD, and Lane Lerner, MD, for abstracting the computed tomography records and assisting with merging and cleaning the files.
About the Author(s)
Min Kyeong Jang, PhD, RN, KOAPN, is a postdoctoral fellow in the Cancer Center and in the College of Nursing, Chang Gi Park, PhD, is a research assistant professor in the College of Nursing, Susan Hong, MD, MPH, is the director of cancer survivorship in the College of Medicine, Deepika Laddu, PhD, is an assistant professor in the Department of Physical Therapy in the College of Applied Health Sciences, Hongjin Li, PhD, MS, BSN, is a postdoctoral fellow in the Cancer Center and in the College of Nursing, Esther Rhee, DO, is a clinical physician in the Cancer Survivorship Program and a clinical assistant professor in the Department of Medicine, and Ardith Z. Doorenbos, PhD, RN, FAAN, is a nursing collegiate professor in the College of Nursing and the director of palliative care in the Cancer Center, all at the University of Illinois at Chicago.This work was supported by the National Institute of Nursing Research of the National Institutes of Health (K24NR015340). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jang, Park, Hong, Laddu, Li, and Doorenbos contributed to the conceptualization and design. Jang and Hong completed the data collection. Jang, Park, and Hong provided the analysis. Jang, Park, Laddu, and Doorenbos provided statistical support. All authors contributed to the manuscript preparation. Jang can be reached at mjang21@uic.edu, with copy to ONFEditor@ons.org. (Submitted February 2020. Accepted March 31, 2020.)

