Satisfaction With an Interactive Voice Response System and Symptom Management Toolkit Intervention to Improve Adherence in Patients Prescribed an Oral Anticancer Agent
Objectives: To describe patient satisfaction with an interactive voice response (IVR) system to assess adherence and symptom management in patients newly prescribed an oral anticancer agent (OAA).
Sample & Setting: Patients prescribed a new OAA were recruited from six comprehensive cancer centers in the United States.
Methods & Variables: Cross-sectional analysis and descriptive statistics were used to summarize patient demographics and satisfaction with the IVR system and symptom management toolkit.
Results: Participants had a mean age of 61.82 years, and gastrointestinal cancer was most prevalent. Participants were either “very” or “highly” satisfied with the IVR weekly calling system to assess symptoms, the IVR system daily OAA adherence reminders, and the symptom management toolkit.
Implications for Nursing: Nurses often triage patient-reported issues with OAAs. Nurses are well positioned to lead IVR system symptom management interventions and to be actively involved in the development, implementation, and dissemination of IVR technologies through research and practice.
Jump to a section
Oral anticancer agents (OAAs) are increasingly being prescribed. Nearly half of all new cancer treatments approved by the U.S. Food and Drug Administration (FDA) in the past several years are in oral form (Center Watch, n.d.). Despite the noted convenience of OAAs (Barni et al., 2016; Given et al., 2011; Marshall et al., 2018; Siden et al., 2014), patients and their caregivers are often met with numerous challenges and must demonstrate self-efficacy in the management of complex OAA regimens, adherence, the management of symptoms/side effects, the monitoring and reporting of drug toxicities, the monitoring for potential drug–drug or food–drug interactions, and polypharmacy in the home environment without the close observation of oncology healthcare providers (Given et al., 2011, 2017; Marshall et al., 2018; Siden et al., 2014). The complexity of OAA regimens and their related symptoms, side effects, and toxicities can affect safe treatment administration and management (Greer et al., 2016). In addition, OAAs often have restricted therapeutic ranges and safety margins (Neuss et al., 2016), which necessitates adherence and leaves patients susceptible to uncontrolled symptoms and side effects (Given et al., 2011; Shimada et al., 2014; Spoelstra et al., 2013; Tipton, 2015). In a recent randomized controlled trial (RCT), patients experienced an average of five symptoms, and evaluation of medical records revealed numerous adverse events, such as anemia, bleeding, hemorrhage, confusion, hallucinations, thrombocytopenia, and febrile neutropenia that resulted in interruptions to (Given et al., 2017) and/or stoppages of treatment with OAAs (Marshall et al., 2020). Such challenges can negatively influence patient outcomes and have implications for how oncology healthcare providers deliver and communicate care because patients taking OAAs experience less face-to-face contact with oncology providers compared to patients receiving other cancer therapies.
Background
As cancer care shifts from oncology clinics to patients’ homes, new supportive interventions are needed to address the changing cancer care model. Interactive voice response (IVR) systems are one such intervention that can promote adherence and symptom management in patients prescribed OAAs. IVR is a technology that allows individuals to interact with host computers using their voices or the touch keys on a telephone. Individuals can either receive information from or provide information to the IVR system (Ruikar, 2016).
IVR has been used to promote pain management among patients with cancer and has demonstrated acceptability and feasibility (Besse et al., 2016; Knegtmans et al., 2020). Besse et al. (2016) conducted a four-week pilot study of a nurse-led intervention in which daily IVR mobile telephone calls were made to assess patient-reported pain among patients with cancer receiving palliative care. If patients rated their pain as moderate or high on the numeric rating scale, a nurse contacted the patients and adjusted pain treatment as appropriate. The results indicated that mean pain scores were significantly reduced postintervention, and all 13 participants reported satisfaction with the intervention. Limitations of this study were the small sample size, short intervention period, and limited representation of female participants (Besse et al., 2016). Knegtmans et al. (2020) conducted an intervention study using a pre–post study design. The control group (n = 54) received standard oncology care, whereas the intervention group (n = 54) received standard oncology care in addition to an intervention using an IVR system. The intervention group received pain assessment IVR messages on their mobile phone three times per week. If the patient-reported pain score on the numeric rating scale was 5 or higher, an oncology nurse adapted the pain treatment, including use of prescribed analgesics. The main objective of the study was to evaluate if IVR increased the registration of pain scores into the medical records of patients with cancer. Results of the study indicated that patients in the IVR intervention group had significantly more documentation of pain and pain treatment in their medical record than patients in the control group and were also significantly more likely to receive a prescription for pain medication. This study was limited because of the lack of randomization of participants, the demographics in the two groups were not similar with respect to gender and type of cancer, and the comparison or change in pain scores using IVR was not assessed (Knegtmans et al., 2020).
In addition, IVR has been used to promote cancer awareness and screening (Tokosi et al., 2017), symptom management for patients receiving chemotherapy in ambulatory care centers (Beck et al., 2017), and symptom management for patients with cancer and comorbid conditions (Fadol et al., 2018). Beck et al. (2017) developed SymptomCare@Home, which is unique in that the system comprises a patient and provider interface. SymptomCare@Home links an IVR and web-based decision support system to provide ongoing symptom management for patients with cancer receiving chemotherapy in ambulatory cancer centers. This system allows patients to monitor symptoms daily, provides symptom management coaching, alerts the oncology provider if symptoms meet a specific threshold, and allows for nurse practitioners to follow up with patients. Strengths of this system, which used IVR for patients’ symptom management monitoring, are that it is based on empirical evidence that supports improved symptom outcomes from a RCT (Mooney et al., 2017), and it has been piloted to gain feedback from patients and oncology professionals to improve future iterations.
Fadol et al. (2018) performed a pilot study using IVR in 26 patients diagnosed with cancer and concurrent heart failure. Patients received weekly IVR telephone calls for three months that assessed symptoms using the MD Anderson Symptom Inventory–Heart Failure. If patients reported their symptoms at specific predetermined thresholds, an alert was sent to the oncology provider, which prompted a nurse call to the patients for triage. The nurse then decided which action to take, including notifying the physician, titrating medication, or sending the patients to the emergency department. During the course of the three-month study, over 100 critical threshold alerts were sent to providers. Most alerts were able to be managed via telephone; nine patients required intervention in either the clinic or emergency department. The study found no statically significant difference in symptom scores from baseline to three months; however, this research is important because many patients with cancer have comorbid conditions requiring treatment, and few interventions focus on more than one illness. This study also supports that symptoms can be managed via IVR with nursing care (Fadol et al., 2018).
Conceptual Framework
The parent study’s RCT was guided by the Information–Motivation–Behavioral Skills (IMB) model. The IMB model was developed to understand, predict, and promote adherence in patients with HIV who were prescribed highly active antiretroviral therapy (Fisher et al., 2006). The model emphasizes that information, motivation, and behavioral skills are determinants of adherence and assumes that the more that patients are informed, are motivated, and possess the ability to carry out adherence behaviors, the more likely they are to adhere to the medication regimen and experience beneficial health outcomes (Fisher et al., 2006). The IMB model has successfully predicted adherence to medications for patients with HIV and type 2 diabetes and for patients recovering from coronary artery bypass surgery (Fisher & Fisher, 2003; Fisher et al., 2006; Osborn & Egede, 2010; Zarani et al., 2010).
According to the IMB model, patients bring personal and disease factors that will influence their ability to incorporate OAA adherence and symptom management into daily activities. The parent study’s RCT examined the impact of tailored daily reminders (behavioral skills construct), strategies to manage symptoms via a symptom management toolkit (information construct), and telephone calls that queried the patient’s level of adherence during the past week and assessed symptoms (motivation construct). The hypothesis of the parent RCT was that motivational queries accompanied by information would produce a greater level of adherence (i.e., behavior) in the IVR experimental group.
Although IVR has been involved in oncology research, to date, the use of IVR has not been previously reported in patients managing adherence to OAAs and related symptoms, specifically. This article reports on patient satisfaction with an IVR system to promote adherence and symptom management in patients newly prescribed an OAA to determine features of the intervention that address the supportive needs of patients managing cancer care at home, as well as determine potential improvements for future interventions involving IVR systems or other technology-based interventions.
Methods
Sample, Setting, and Procedures
Institutional review board (IRB) approval was obtained from the affiliated university for the parent study and secondary analyses, as well as from all respective recruitment locations. Site recruiters from each of the comprehensive cancer centers in the midwestern United States were trained on the parent study’s protocol and identified patients newly prescribed an OAA from a list of 28 FDA-approved agents. Site recruiters were also trained on how to approach potential participants for recruitment. If patients agreed to participate in the study, site recruiters reviewed and obtained written consent. Participants were then given a folder that outlined and explained the study and how to contact the principal investigator or affiliated IRB. Site recruiters entered all participant information into a secure electronic database, and the study coordinator was notified to schedule the baseline interview.
Patients were eligible for participation in the study if they were aged 21 years or older, received a new prescription for an OAA from a list of 28 FDA-approved agents, were cognitively intact, were able to speak English, were able and willing to complete telephone calls, and obtained a score of 0–2 on the Eastern Cooperative Oncology Group Performance Status Scale (Oken et al., 1982) or a score of 50 or higher on the Karnofsky Performance Status Scale (Karnofsky & Burchenal, 1949). Patients who did not meet these inclusion criteria were excluded. The current study reports on a subset of patients from the parent study who were in the experimental arm of the RCT and completed the 12-week trial.
This cross-sectional secondary analysis, which used data derived from a National Cancer Institute–funded RCT (NCT02043184), evaluated patient satisfaction with an intervention using IVR to promote symptom management and OAA adherence during a 12-week period after initiating a new OAA (ClinicalTrials.gov, 2013). Patients were randomized to either the experimental or control arm following the baseline interview using a minimization approach. Patient allocations to the experimental or control arm were equalized based on cancer site, continuous versus intermittent OAA dosing, recruitment location, and depressive signs and symptoms. Both arms received weekly IVR calls for eight weeks that monitored adherence and symptom management. In addition, the experimental arm received daily OAA adherence reminders for four weeks tailored to their prescribed dosing regimen. If symptoms were rated a 4 or higher on a scale ranging from 1 to 9 (higher ratings indicated more severe symptoms), participants were referred to the symptom self-management recommendation strategies provided in the symptom management toolkit for eight weeks. The symptom management toolkit provides evidence-based strategies for self-managing symptoms that are commonly experienced by patients with cancer; the toolkit also includes a section on adherence (Given et al., 2013). The toolkit provides patients with a description of symptoms, common causes of symptoms, strategies to prevent or manage symptoms, tips to guide communication with oncology healthcare providers regarding symptoms, and when to seek medical attention (Given et al., 2013). Participants in the experimental arm had the option to extend IVR telephone calls for an additional four weeks (weeks 9–12 of the study) with daily calls, every-other-day calls, or no calls. Trained personnel interviewed all patients via telephone at baseline, 4, 8, and 12 weeks. At the completion of the 12-week interview, participants in control and experimental arms completed separate researcher-developed satisfaction surveys. Additional information regarding the parent study can be found at ClinicalTrials.gov (NCT02043184).
Data Collection
Data were collected by interviewers who were trained on the study’s protocol. Interviews were conducted via telephone. Initial interviews were completed within one week of patients initiating a new OAA, followed by interviews at 4, 8, and 12 weeks.
Measures
Patient demographic, cancer, and cancer treatment characteristics were obtained during the baseline interview.
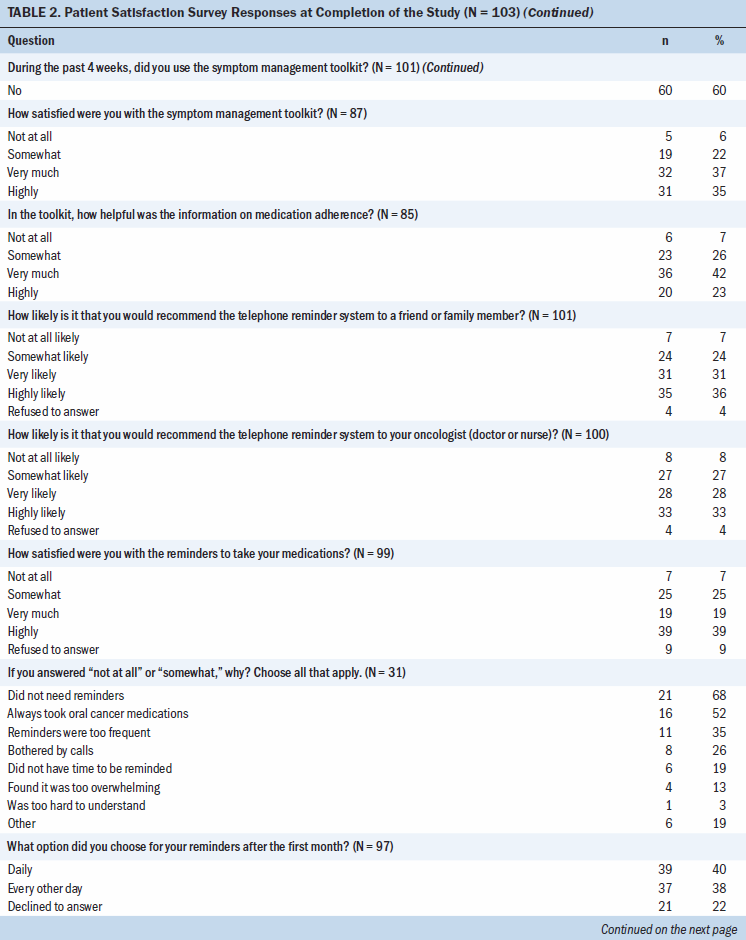
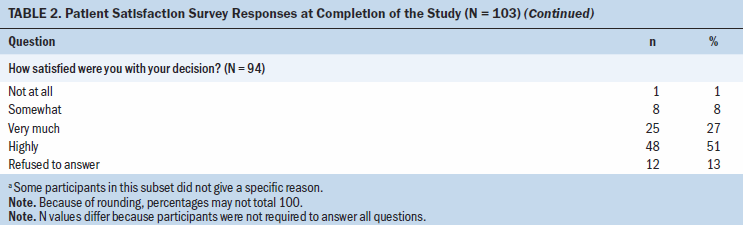
At the end of the 12-week interview, participants in the experimental arm of the trial completed a 12-week patient satisfaction survey specific to the following: (a) participation in the study, (b) the two components of the intervention using the IVR system that consisted of a weekly telephone calling system to assess symptoms and daily OAA adherence reminders, and (c) the symptom management toolkit. The researcher-developed satisfaction survey consisted of 13 items with Likert-type response scales.
Question 1 evaluated overall satisfaction with participation in the study. If patients responded “not all” or “somewhat” to question 1, they were prompted to give a reason for their response. Patients were also given the opportunity to refuse to answer any of the satisfaction survey questions. Question 2 evaluated if participants encountered any problems with the IVR system, and if they answered yes, participants were subsequently able to list specific problems they experienced. Question 3 evaluated how satisfied participants were with the weekly telephone calling system to assess symptoms. If participants responded “not at all” or “somewhat” to this question, they were asked why (e.g., being bothered by calls, found it overwhelming, calls were too frequent or not frequent enough, did not have time for calls, hard to understand, did not need the call, other). Questions 4 and 5 assessed how likely participants were to recommend the weekly telephone calling system for symptom management to a friend or family member and/or oncology doctor or nurse. Question 6 assessed whether patients used the symptom management toolkit within the past four weeks (weeks 9–12 of the study), and question 7 evaluated how satisfied participants were with the symptom management toolkit. Question 8 evaluated how helpful the information on adherence in the toolkit was. Question 9 evaluated satisfaction with the daily telephone adherence reminder system, and questions 10 and 11 evaluated how likely participants were to recommend the daily telephone adherence reminder system to a friend/family member or oncologist (doctor or nurse). Question 12 asked which option participants chose for daily adherence reminder calls after the first four weeks of the study (daily or every other day). Lastly, question 13 asked participants how satisfied they were with their decision for daily reminders after the fourth week of the study. Responses to the survey questions were rated on a four-point Likert-type scale (1 = not at all, 2 = somewhat, 3 = very much, and 4 = highly).
Analysis
Descriptive statistics were used to summarize the distribution of patient demographic, disease, and cancer treatment characteristics, and patient satisfaction with the two components of the IVR system and the symptom management toolkit. Statistical analyses were performed using IBM SPSS Statistics, version 24.0.
Results
A total of 137 participants were randomized to the experimental arm of the parent study’s RCT following the baseline interview. The 12-week trial was completed by 106 participants, and 103 participants completed the 12-week patient satisfaction survey. Reasons for attrition from the baseline interview to the 12-week satisfaction survey included being too ill (n = 6), lost to follow-up (n = 3), entered hospice (n = 1), death (n = 10), changed their mind (n = 2), OAA was withdrawn (n = 9), or refused/did not complete the 12-week satisfaction survey (n = 3). Descriptive statistics for the participants who completed the satisfaction survey are provided in Table 1. Participants had a mean age of 61.82 years (SD = 11.32). All participants were White. Gastrointestinal (n = 29) and breast (n = 24) cancers were most prevalent, and most participants were diagnosed with stage IV cancer. Kinase inhibitors were the most prevalent OAA prescribed (n = 53). 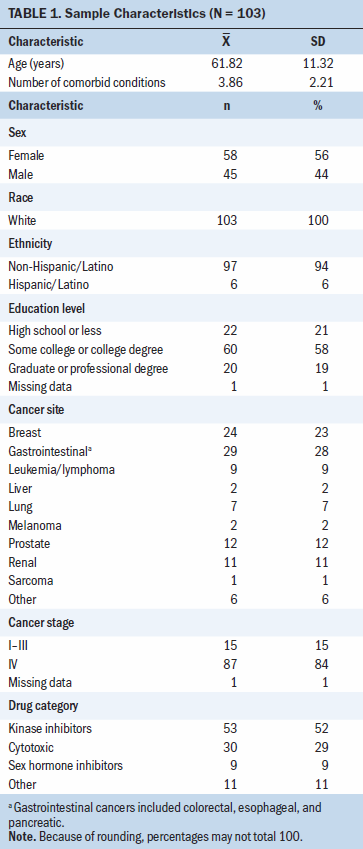
Overall Satisfaction With Study Participation
The results of the satisfaction survey at the conclusion of the 12-week study demonstrated overall satisfaction with participation in the study, with 86 participants stating they were either very satisfied or highly satisfied (see Table 2). Of the 17 participants who reported being somewhat satisfied with their overall participation in the study, 40% had hoped for fewer calls, and 60% noted that the system called more than once per week, began the next questions before the participants had a chance to answer the previous questions, was not as fast as they had hoped, would not allow the participant to change their answer, or did not allow them to report things that they wanted. 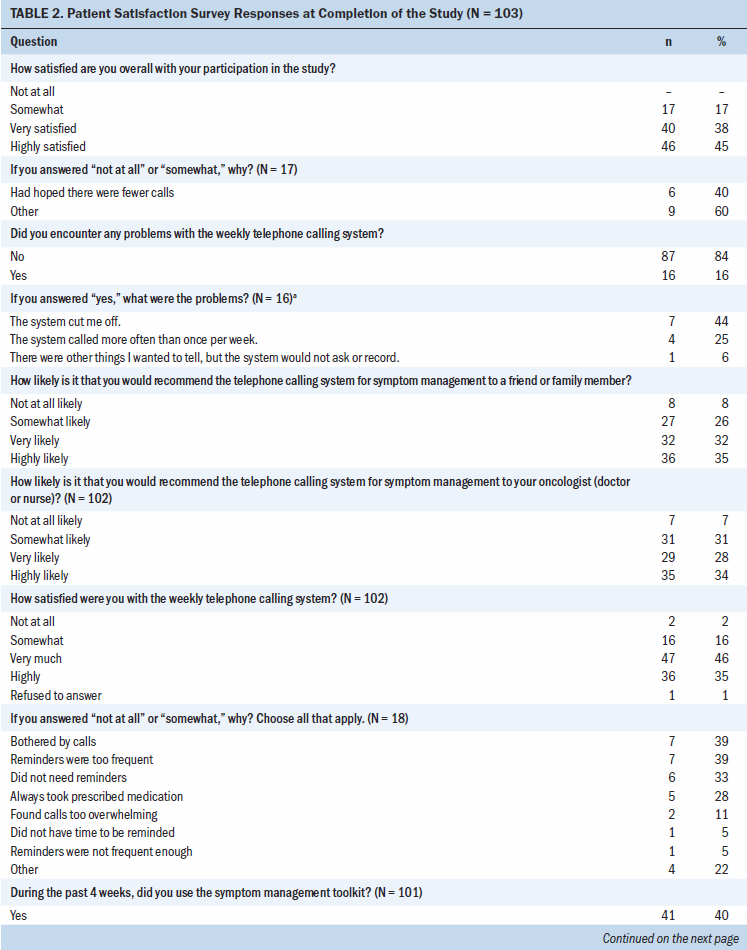
Satisfaction With Weekly Calling System to Assess Symptoms
Overall satisfaction with the IVR weekly calling system that assessed symptoms revealed that 83 participants were either very much or highly satisfied. Despite any reported issues with the IVR system, participants were either very likely or highly likely to recommend the IVR weekly calling system to family members/friends (66%) or their oncologist or nurse (62%).
Satisfaction With Daily Reminder Calls for Adherence
Participants also reported on their satisfaction with the daily telephone reminder system for adherence. Most participants reported being somewhat (n = 25) or highly (n = 39) satisfied with the IVR daily reminder calls, and nine participants refused to answer this question on the survey. Participants were able to choose the frequency for reminders after the first four weeks of the study (daily reminders, every-other-day reminders, or no reminders). Participants were either very likely or highly likely to recommend the IVR daily calling system to promote adherence to family members/friends (n = 66) or their oncologist or nurse (n = 61).
Satisfaction With the Symptom Management Toolkit
Participants also reported their satisfaction with the symptom management toolkit, which they were referred to if their symptoms were rated a 4 or higher on a nine-point scale (higher scores indicated worse symptoms). Only 40% of participants reported using the symptom management toolkit during the last four weeks of the study. Participants rated their satisfaction with the toolkit as not at all (n = 5), somewhat (n = 19), very much (n = 32), and highly (n = 31) satisfied. Participants also rated their satisfaction with the adherence section of the symptom management toolkit as not at all (n = 6), somewhat (n = 23), very much (n = 36), and highly (n = 20) satisfied.
Discussion
IVR systems generate new ways to support self-management of cancer care and treatment in response to the changing cancer care model. The state of the science of technologies, such as IVR for patients receiving OAAs, is scarce, and, to the authors’ knowledge, this is the first study to report findings on patient satisfaction with an IVR system to promote adherence and symptom self-management in patients receiving OAAs. In this study, participants were satisfied with the IVR system to promote adherence and symptom management overall, which supports previous research that studied satisfaction with an IVR intervention to improve symptoms of pain in patients with cancer (Besse et al., 2016).
Some areas of the IVR intervention can be improved in the future. Participants noted that there were issues with the IVR system, such as the system calling more than scheduled, advancing to questions without enabling the participants to finish answering the previous question, and not giving participants the ability to change their answer once entered. The ability of the system to record patient-specific concerns could also be improved. Future research may move toward more interactive interventions using mobile health (mHealth) technologies that allow for capabilities, such as providing education and promoting facilitated communication with oncology healthcare professionals. Neither communication nor facilitated communication were included in the current study’s IVR intervention.
The symptom management toolkit was used by only 40% of the participants during the last four weeks of the study. This could have been because the intervention was started within one week of initiating an OAA and lasted for only 12 weeks, leaving little time for severe symptoms to develop. Patients also may have experienced symptoms early on in their treatment and used the symptom management toolkit more during the first eight weeks of the study. Other reasons could be that participants did not report severe enough symptoms or simply did not use the toolkit when it was available to them. The toolkit was provided in paper format and was quite lengthy; future interventions may consider digitizing the toolkit so that patients can have information readily available whenever they travel or are away from home. In addition, digitizing the toolkit would allow for specific algorithms to be incorporated to guide patients to additional care, if warranted.
This study is important to ongoing advances in technology-based interventions. Patients with cancer have voiced the need for better communication with oncology healthcare providers (Meade, 2018; Puts et al., 2017). Although the IVR intervention in the current study did not include a feature for communication, future technologies may incorporate additional IVR capabilities or other technologies, such as mHealth, to allow for facilitated communication with oncology healthcare professionals.
The mean age for participants in this study was more than 60 years, which was expected because the highest incidence of most solid tumor cancers occurs in older adults aged 60 years or older (Given & Given, 2016). It is estimated that 80% of adults aged 65 years or older own a cell phone, and more than 40% own a smartphone (Anderson & Perrin, 2017). The current study did not use IVR specific to mobile phones, and only about 30% of participants accessed the intervention on their mobile phone. Besse et al. (2016) reported on the ease of use for pain interventions delivered via mobile phones in adults ranging in age from 27 to 75 years, with a mean age of 58 years. Participants in the Besse et al. (2016) study also had an average of 3.86 conditions treated with other medications; this estimate may be lower in younger populations. These statistics may offer a promising extension of technology-based interventions via mobile phones for older adults, who are often prescribed OAAs. Future research should include the ability to track potential drug–drug interactions between OAAs and other prescriptions or over-the-counter medications to promote safety.
Adherence in the parent RCT was high, and the IVR intervention was not found to have an effect on patient adherence (Sikorskii et al., 2018). The sample for the RCT mainly included patients with stage IV cancer, which may have influenced adherence for those on last lines of therapy after other cancer treatments had been exhausted. This could explain why adherence reminders had the lowest satisfaction scores on the 12-week patient satisfaction survey because many participants felt they took their medication as prescribed and did not need reminders or believed that calls were too frequent. However, after the first four weeks of the intervention, participants were given the option to continue with daily reminders, decrease the frequency of reminders to every other day, or stop the reminder calls. It is promising that 78% of participants decided to either keep reminder calls daily or every other day. Future interventions should include options to individualize calls to meet patients’ specific needs or to opt out of reminder calls if not needed. The RCT lasted for only 12 weeks, which is a relatively short period of time to assess outcomes such as adherence, considering the chronic nature of cancer and treatment with OAAs.
Limitations
This study is one of the first to report on satisfaction with an IVR system used in an RCT to promote adherence and symptom management among patients initiating a new OAA. However, there were some limitations. First, this is a secondary analysis that was restricted to the parent study’s postintervention satisfaction survey on specific intervention components. The satisfaction survey was researcher developed, and reliability and validity were not evaluated. Notably, some data are lacking that may have been helpful to collect in the parent RCT. For example, satisfaction with the symptom management toolkit was specific to the last four weeks of the study (weeks 9–12), but it would have been important to understand how satisfied participants were in the early weeks of treatment when they may have experienced new or threating symptoms after starting a new OAA. For example, 87 participants reported overall satisfaction with the symptom management toolkit on the survey, but only 41 participants reported using the toolkit during the last four weeks of the study, so the opportunity to obtain data for the entire 12-week period was lost. Secondly, satisfaction scores were included only for participants in the experimental arm. The reason for this was that the IVR intervention differed for participants in the experimental arm in that the intervention included daily adherence reminder calls and referral to the symptom management toolkit as outlined in the study protocols. The control arm simply received weekly symptom assessments via the IVR system with no intervention and were only given access to the symptom management toolkit after completing the study. However, satisfaction results in the control group were comparable to satisfaction results in the experimental group.
Adherence was found to be high, which may have negatively affected satisfaction with the intervention in improving adherence. Adherence reminder calls received the lowest satisfaction ratings, with only 58% of participants reporting that they were very or highly satisfied. Many participants reported that the reason for not being satisfied was that they did not need reminders to adhere to their OAA; however, a majority did find the reminder calls useful. This underscores the need for individualized interventions because supportive needs can differ among patients. About 30% of participants in the parent study used their mobile phone for IVR calls as opposed to a landline telephone, which limits the ability to generalize findings to other mHealth interventions. Lastly, the sample was White and mainly of non-Hispanic or non-Latino ethnicity. Non-minorities and those with higher levels of education may have more access to technology and, therefore, may be more accepting of technology-based interventions (Gordon & Hornbrook, 2016; Smith, 2014).
Implications for Nursing
The paradigm shift in care from oncology clinics to the home environment coupled with the introduction of more OAAs has required nurses to respond to changing delivery care models. Nurse-led interventions involving supportive technologies are limited (Pereira-Salgado et al., 2017; Spoelstra et al., 2013). Although oncologists are involved in prescribing and making changes to the medication regimen, nurse practitioners are well embedded in the care of patients receiving OAAs, and nurses often triage patient-reported issues. Therefore, as cancer care shifts to the home setting, IVR systems and other technology-based interventions that address adherence to OAAs and symptom self-management are crucial. Nurses are well positioned to lead such interventions and to be actively involved in the development of IVR systems and other mHealth technologies through research and practice experience, but more research is needed (ClinicalTrials.gov, 2013; Spolestra et al., 2013).
One such area in which nurses can contribute to the development of technology-based interventions is patient education. Patient education can take place during a time of heightened stress and anxiety for patients, particularly when initiating a new OAA treatment regimen. Such stress and anxiety can negatively affect patients’ retention and cognitive processing of information. Nurses can help to develop patient education that can be integrated into technology-based interventions so that patients with cancer and their caregivers can have access to trusted information at the touch of a button.
An IVR system can also help nurses to track adherence and symptom trends, which are important in oncology assessments and ongoing clinical care. Two previous studies involving an IVR system intervention in the supportive care of patients receiving OAAs have been nurse led (ClinicalTrials.gov, 2013; Spoelstra et al., 2013). In addition, oncology nurses can be vital to building algorithms within IVR- or technology-based intervention platforms specific to symptom management. Such algorithms could refer patients experiencing symptoms to the symptom management toolkit or prompt patients to seek medical care if the patient-reported symptom is severe enough.
Another critical contribution of nurses is implementation of technology-based interventions into the clinical setting. Nurses understand the clinical operations of their oncology centers and are important stakeholders in determining the usability, acceptability, and feasibility of technology-based interventions. Oncology nurses may also assess and evaluate high-risk patients who may benefit from technology-based supportive care interventions to improve adherence and symptom management. Using digitized symptom management toolkits can allow patients to have evidence-based education regarding symptoms, educate patients on how to manage symptoms at home, and alert patients about when to contact an oncology healthcare professional. Such resources can help supplement and refresh standard patient education that occurs in the oncology clinic. 
Conclusion
Patients in this study reported favorable satisfaction with an IVR system and symptom management toolkit for adherence to OAAs. Adherence needs may vary among patients, and interventions should be individualized and targeted toward patient needs. Future technology-based interventions should include more interactive capabilities that allow patients to play an active part in their self-management, such as integration of interactive smartphone algorithms that are individualized to patient-entered data.
About the Author(s)
Victoria K. Marshall, PhD, RN, is an assistant professor in the College of Nursing at the University of South Florida in Tampa, and Barbara A. Given, PhD, RN, FAAN, is a university distinguished professor and associate dean emeritus in the College of Nursing at Michigan State University in East Lansing. No financial relationships to disclose. Given contributed to the conceptualization and design. Marshall completed the statistical support. Both authors completed the data collection, provided the analysis, and contributed to the manuscript preparation. Marshall can be reached at vkmarshall@usf.edu, with copy to ONFEditor@ons.org. (Submitted March 2020. Accepted June 9, 2020.)




