Weight Change Trajectory in Patients With Locally Advanced Nasopharyngeal Carcinoma During the Peri–Radiation Therapy Period
Objectives: To analyze the weight change trajectory in patients with locally advanced nasopharyngeal carcinoma (LANPC) before, during, and after radiation therapy for a time span of 40 weeks.
Sample & Setting: 147 patients from a university-affiliated medical center in China were included.
Methods & Variables: Body weight was measured weekly during intensive treatment and biweekly after radiation therapy.
Results: All 147 patients experienced critical weight loss during the peri–radiation therapy period. Overall, body weight remained basically unchanged during induction chemotherapy, followed by a sharp and severe decrease during radiation therapy. At 20 weeks after radiation therapy, body weight had increased only slightly from the lowest point.
Implications for Nursing: A time-tailored intervention based on the weight change trajectory is necessary for patients with LANPC. According to the weight change trajectory, relevant interventions for maintaining body weight should be initiated as early as the second week of radiation therapy and no later than the fourth week of radiation therapy, and these interventions should continue for at least four weeks after radiation therapy.
Jump to a section
According to the International Agency for Research on Cancer, about 129,000 new cases of nasopharyngeal carcinoma (NPC) were diagnosed in 2018 (Bray et al., 2018; Tang, Chen, et al., 2016). Unlike other head and neck cancers, NPC is highly sensitive to radiation therapy; because of the distinct anatomical location for NPC, radiation therapy is the mainstay treatment modality (Chen et al., 2019; Chow, 2020; Chua et al., 2019; Colevas et al., 2018; He, Wu, et al., 2017; Sun et al., 2020). For early-stage NPC, radiation therapy has achieved a local control rate of 80%–90% (Chen et al., 2016; Chow, 2020; Colevas et al., 2018). For locally advanced NPC (LANPC), radiation therapy, combined with chemotherapy, is the standard treatment (Chen et al., 2019; Chow, 2020; Chua et al., 2019; Colevas et al., 2018; He, Wu, et al., 2017; Sun et al., 2020). Once NPC is diagnosed, anticancer treatment is intensive and continuous, generally bringing about systemic reactions. Several symptom clusters have been identified in patients with NPC during radiation therapy, including symptoms with general, gastrointestinal, nutritional, and social interaction effects (Langius et al., 2013; Qiu et al., 2011; Xiao et al., 2017). All symptom clusters were correlated with weight loss, and patients who lost more body weight experienced more severe symptom clusters (Langius et al., 2013; Xiao et al., 2017). Unintentional weight loss within a certain time frame renders many adverse effects, induces local recurrence and distant metastasis (Li et al., 2016; OuYang et al., 2016; Qiu et al., 2011; Shen et al., 2013; Solheim et al., 2014; Tang, Li, et al., 2016; Zeng et al., 2016), decreases quality of life (Haberer-Guillerm et al., 2015; Langius et al., 2013; Qiu et al., 2011), impairs treatment tolerance (Ravasco et al., 2005; Xu et al., 2016), and produces setup errors for radiation therapy (Hou et al., 2016; Yan et al., 2013). Therefore, more attention should be paid to weight change in patients with NPC receiving radiation therapy.
Because of the special status of radiation therapy in NPC treatment, the treatment period mainly focuses on radiation therapy, which is divided into three stages: before, during, and after radiation therapy. Although some studies have explored weight change in NPC treatment, most studies, whether in early or advanced disease, have focused on the radiation therapy treatment period, which is usually a time period of seven to eight weeks (Hou et al., 2016; Langius et al., 2013; Li et al., 2016, 2017; OuYang et al., 2016; Qiu et al., 2011; Shen et al., 2013; Xiao et al., 2017; Zeng et al., 2016). There is a lack of information about weight change before and after radiation therapy. The limited data associated with weight change before and after radiation therapy were available from two studies conducted in 2004 and 2011, which showed that 56% of patients with NPC had a weight loss of 5% in the three months before radiation therapy (Qiu et al., 2011), and the recovery in body weight lagged behind recovery of dietary intake until six months after radiation therapy (Ng et al., 2004).
Appropriate nutritional intervention has been demonstrated to improve the nutritional status and prognosis of patients with cancer (Jin et al., 2017; Meng et al., 2019; OuYang et al., 2016; Schuetz et al., 2019; Tanaka et al., 2018; Zhang et al., 2017). Body weight is a crucial indicator to assess nutrition, and it is an obligatory item in all nutrition guidelines to diagnose malnutrition (Cederholm et al., 2015; Mueller et al., 2011; Weimann et al., 2017). To assess and diagnose malnutrition, documentation of unintentional weight loss should be mandatory, either weight loss plus reduced body mass index (BMI) or weight loss plus reduced fat-free mass index, to diagnose malnutrition (Cederholm et al., 2015). Nurses can improve patients’ nutritional status and achieve nutritional goals by using appropriate materials to enhance patients’ understanding of nutrition knowledge, nutrition screening, monitoring patients’ body weight, and facilitating and reinforcing nutritional plans and interventions (McClinchy et al., 2015; Vasiloglou et al., 2019; Xu et al., 2017). An overall understanding of the weight change trajectory in patients with NPC during the peri–radiation therapy period is important for nurses to guide appropriate symptom self-management and clinical interventions. Because of the presence of silent, mild, and nonspecific symptoms in NPC, early detection remains difficult; 60%–90% of patients present with LANPC at diagnosis (Chow, 2020; Du, Tang, Chen, et al., 2015; Sun et al., 2020). For patients with LANPC, the addition of induction chemotherapy to radiation therapy is the standard regimen, which strongly predisposes patients to critical weight loss (Du, Tang, Mao, et al., 2015; Qiu et al., 2011; Vangelov et al., 2017; Zeng et al., 2016; Zhao et al., 2015). However, the weight change trajectory in patients with LANPC during the peri–radiation therapy period is unknown. As a result, there is little evidence to support a precise and time-tailored intervention in maintaining body weight for patients with LANPC.
The focus of precision medicine and precision nursing was essentially aimed at enabling personalized treatment and nursing to each individual by using genomics, laboratory testing, and analyses of symptoms and lifestyle and environmental factors (Collins & Varmus, 2015; Dorsey & Pickler, 2019; Hickey et al., 2019). The period of LANPC treatment is very long, taking at least five to seven months just for intensive anticancer treatment. More focused, clearly defined, and measurable objectives would increase the effectiveness of the intervention and be more cost-effective (Hickey et al., 2019; Vasiloglou et al., 2019). The current authors hypothesized that the body weight of patients with LANPC would change in a certain trajectory alone with advancement of anticancer treatment. Therefore, the purpose of this study was to analyze weight change trajectory in patients with LANPC during the peri–radiation therapy period, which is the period before, during, and after radiation therapy. In the present study, follow-up lasted 40 weeks, which consisted of 12 weeks before radiation therapy, 8 weeks during radiation therapy, and 20 weeks after radiation therapy completion. The results of this study can help to provide more precise management of weight loss in patients with LANPC during the peri–radiation therapy period.
Methods
This is a prospective, longitudinal, and observational study conducted at West China Hospital, a 4,500-bed university-affiliated medical center in Chengdu City, Sichuan Province, China. The study was approved by the ethics committee of the hospital, and written informed consent was obtained from all patients for research purposes.
Participants
Patients who were diagnosed with stage III to IVA LANPC based on the seventh edition of the American Joint Committee on Cancer staging system and admitted to the cancer center for radiation therapy were recruited. Patients who were aged 18–80 years; planned to receive radical radiation therapy plus induction chemotherapy, with or without concurrent or adjuvant chemotherapy; were without physical or radiographic-confirmed edema and body cavity effusion; had an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 2 or less; and had an estimated survival time of more than one year were eligible for inclusion.
Patients experiencing comorbidities or nutritional disorders that might have an effect on body weight, such as hyperthyroidism, hypothyroidism, or chronic kidney or heart disease, were excluded.
If the following situations happened during the observational period, the patient was eliminated from the study analysis: edema and effusion in the body cavity confirmed by physical examination or radiographic examinations or loss of two consecutive body weight records for any reasons.
Treatment Regimen
Radiation therapy: Intensity-modulated radiation therapy is the primary radiation therapy modality for patients with LANPC. The primary gross tumor volume of nasopharynx (GTVnx) and involved lymph nodes (GTVnd) included all known gross disease that was determined by clinical, imaging, and nasopharyngoscope examination. Clinical target volume–1 (CTV-1) was defined as the high-risk area that includes the GTV plus a 5 mm to 10 mm margin. CTV-2 was defined as the potentially involved area that included CTV-1 plus a 5 mm to 10 mm margin. In principle, the planned doses of radiation therapy for GTVnx, GTVnd, CTV-1, and CTV-2 were 66–70 Gy, 60–70 Gy, 60–66 Gy, and 54–58 Gy, respectively, in 30–35 fractions. Radiation therapy was delivered at one fraction per day, five days per week.
Chemotherapy: Docetaxel, cisplatin, and 5-fluorouracil (TPF) and gemcitabine and cisplatin (GP) delivered every 21 days per cycle were the mainstay regimens for induction chemotherapy. During chemotherapy, dexamethasone was routinely prescribed at 8 mg per day and taken orally the day before, on the day of, and the day after docetaxel administration. Generally, patients received three cycles of induction chemotherapy, and cisplatin-based chemotherapy (every 21 days per cycle) was prescribed concurrently during radiation therapy.
Nursing protocol: Patients received nursing measures and health education formulated by the cancer center, which were primarily carried out by verbal education, handouts, and telephone follow-up. During periods of intensive anticancer treatment, the health education was conducted daily if the patients were hospitalized and weekly if they were discharged. Patients were encouraged to eat nutritious foods; obtain as much protein and calories as they could; eat enough fresh fruits and vegetables and drink enough water; and have multiple small meals. For patients with severe oropharyngeal pain during radiation therapy, lidocaine diluent was given orally before eating to reduce pain. For patients whose nutritional status was affecting their anticancer treatment, oncologists consulted nutritionists for nutritional prescriptions. However, nutritional intervention was a selective treatment rather than a regular measure. There was no unanimously recognized standard for nutritional consultation, and consultation was dependent upon the subjective judgment and clinical experiences of oncologists. Only the patients who had received nutritional consultation were likely to be prescribed additional nutritional intervention.
Procedures
Data collection: Patients were first approached after NPC diagnosis. Data were collected by four trained oncology nurses and an oncologist. Data were categorized into four types: demographic and disease characteristics, treatment information, blood examination, and body weight and BMI. Demographic and disease characteristics, including sex, age, ECOG Performance Status score, smoking history, and cancer stage, were collected once before induction chemotherapy. Treatment information and blood examination (i.e., chemotherapy regimen and dose of radiation) were gathered along with data on the treatment process; Epstein–Barr virus DNA, hemoglobin, serum total protein, and albumin were recorded 12 weeks before radiation therapy and at the beginning and ending weeks of radiation therapy.
Body weight assessment: Body weight assess-ments started from NPC diagnosis and lasted for 20 weeks after radiation therapy completion. Body weight was recorded weekly during intensive anticancer treatment (i.e., during the induction chemotherapy and radiation therapy period). Considering that weight loss is severe during radiation therapy, body weight was measured one day before radiation therapy as the baseline for radiation therapy. According to the current authors’ preliminary study (n = 10), the trend of weight loss lasted for two weeks after radiation therapy. Therefore, weekly measurement of body weight needed to be continued until the second week after radiation therapy completion. After that, body weight was recorded every two weeks.
For patients who received induction chemotherapy for more than 12 weeks, even if the body weight was measured for more than 12 weeks, only the weight measurements for the 12 weeks before radiation therapy were analyzed. All patients were given a predesigned booklet to record their body weight and any special events that patients considered to be related to their treatment.
Body weight was collected by oncology nurses biweekly either by telephone calls or in person if patients were hospitalized. For any week in which no body weight data were recorded, the average weight value from the week before and the week after was estimated as the weight for that week. BMI was defined as weight (kg) divided by the square of height (m).
Body weight was assessed on a calibrated digital scale, with an accuracy of 0.01 kg. Patients were required to measure their body weight on an empty stomach after urination in the morning, after taking off shoes and thick coats, and while wearing light indoor clothes. Body weight was measured twice, and the average value was recorded to ensure accuracy.
Critical weight loss criteria: According to the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines and consensus statement, unintentional weight loss greater than 5% during the past three months (to account for acute illnesses) or greater than 10% during any period of time (considered to be relevant for chronic conditions) in adults was defined as critical weight loss (Cederholm et al., 2015; Weimann et al., 2017).
Statistical analysis: IBM SPSS Statistics, version 21.0, was used to save and analyze data. Categorical variables were analyzed by frequency, cumulative rate, and proportion. Continuous variables were analyzed by average, standard deviation, and 95% confidence interval. Cumulative incidence of critical weight loss during radiation therapy was estimated by the Kaplan–Meier method, with one day before radiation therapy as the original point.
Results
Sample Characteristics
One hundred and eighty patients were enrolled from February 2018 to March 2020. Among those, 147 patients completed follow-up and were analyzed. The major reasons for exclusion included the loss of two consecutive body weight records and non-response to telephone calls. The study process is shown in Figure 1.
The general patient information, including age, sex, performance status score, cancer stage, radiation therapy and chemotherapy modalities, blood examination, and body weight during the peri–radiation therapy periods are shown in Tables 1 and 2. 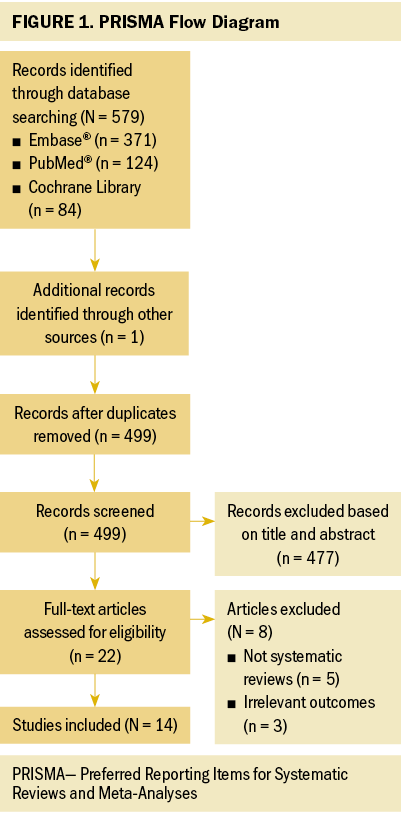
Weight Change Trajectory During the Peri–Radiation Therapy Period
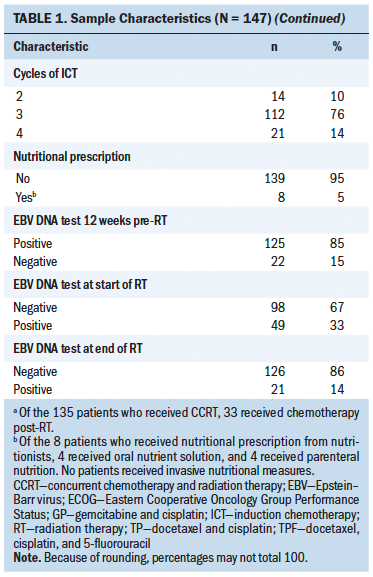
All 147 patients experienced weight loss during the peri–radiation therapy period, and there were different weight change trajectories in the three stages. In the induction chemotherapy period, the average body weight was 64.95 kg (SD = 10.95) (BMI = 23.95 [SD = 3.05]) at the beginning and 64.43 kg (SD = 10.72) (BMI = 23.76 [SD = 2.99]) at the end, presenting an average decrease of 0.52 kg (SD = 2.16) (BMI loss of 0.19 [SD = 0.82], weight loss percentage = 0.71% [SD = 3.46]). During radiation therapy, the average initial body weight was 64.43 kg (SD = 10.72) (BMI = 23.76 [SD = 2.99]), and the average final body weight was 57.45 kg (SD = 9.89) (BMI = 21.18 [SD = 2.79]), with a significant decrease of 6.99 kg (SD = 2.99) (BMI loss of 2.58 [SD = 1.08], weight loss percentage = 10.81% [SD = 4.33]). As long as 20 weeks after radiation therapy, average body weight increased by 0.9 kg from the lowest point, and there was a 7.24 kg (SD = 4.47) weight loss as compared to the baseline body weight at the beginning of therapy. In an overall review of the weight change trajectory, body weight remained basically unchanged during induction chemotherapy, sharply declined during radiation therapy, and then increased to just slightly more than the lowest point of body weight after radiation therapy (see Figures 2 and 3). 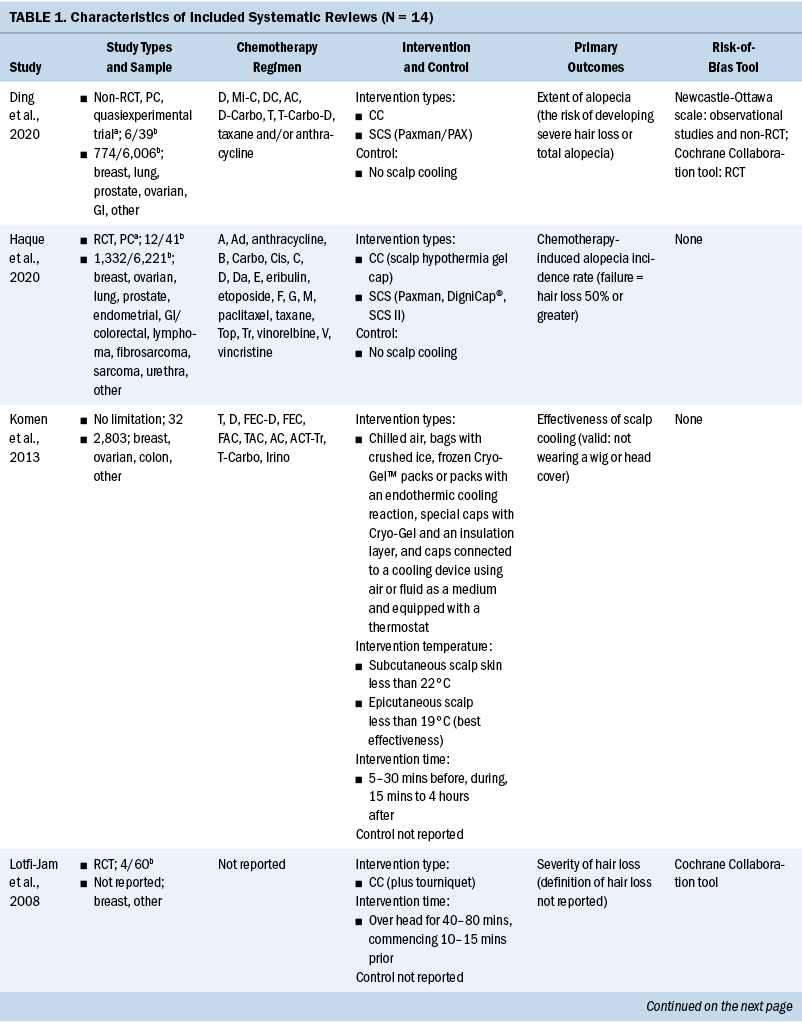
The majority of patients completed radiation therapy in the seventh to eighth week. The lowest body weight (56.81 kg [SD = 9.61], weight loss percentage = 11.72% [SD = 4.79]) was not in the week of radiation therapy completion, but rather in the second week after radiation therapy. Body weight remained at this low point for four weeks (below but close to 57 kg). Thereafter, body weight began to rise very slowly; by the end of the 20th week after radiation therapy, the weight increased no more than 1 kg as compared to the lowest point. 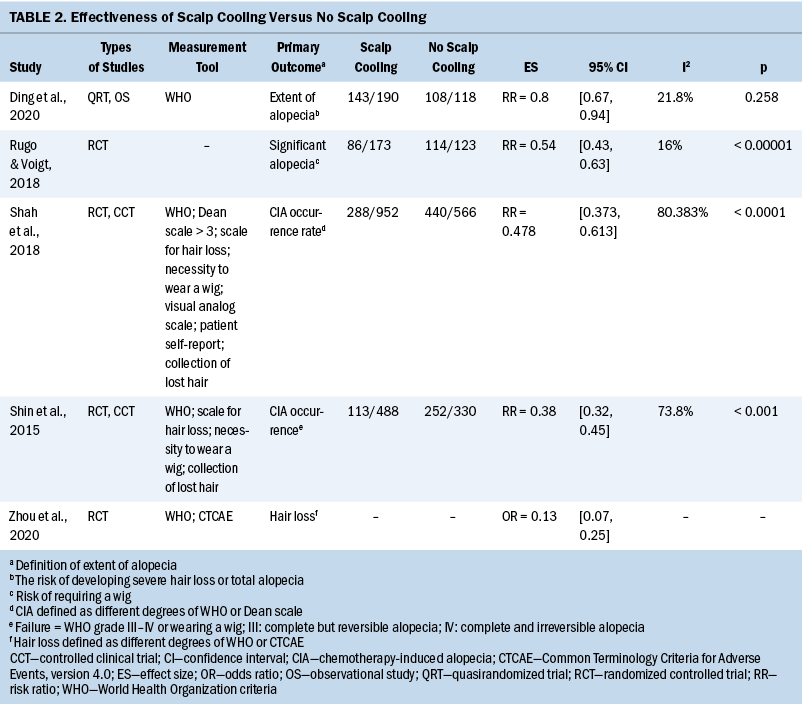
Weight Change Trajectory and Critical Weight Loss During Radiation Therapy
All patients but one experienced weight loss during radiation therapy, with an average loss of 0.87 kg (SD = 0.37) (weight loss percentage = 1.35% [SD = 0.54]) weekly. The fastest speed of weight loss occurred between the fourth and seventh weeks of radiation therapy, presenting a decrease of about 2% weekly as compared to the previous week. In the fifth to sixth weeks, when body weight decreased most sharply, body weight decreased by 2.28% (SD = 1.33) and 2.31% (SD = 1.44) weekly, respectively. By the fifth week of radiation therapy, the average weight loss was 5.91% (SD = 2.8), which can be considered critical weight loss; by the week of radiation therapy completion, weight loss reached 10.81% (SD = 4.33). Among patients who experienced the most severe weight loss, seven patients lost weight greater than 5% within one week. 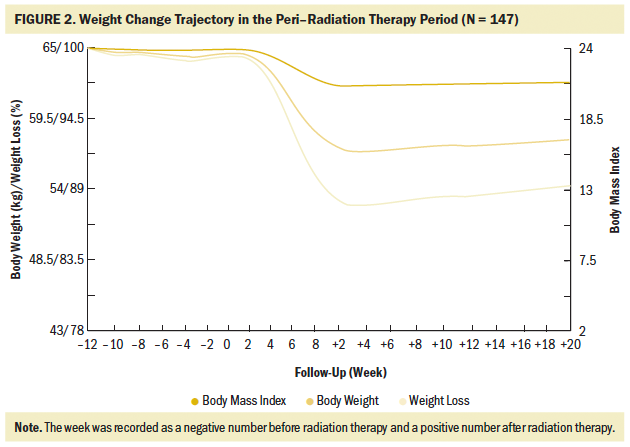
The first two patients experienced critical weight loss in the second week of radiation therapy. By the eighth week of radiation therapy, during which the majority of the patients finished radiation therapy, 134 (91%) patients experienced critical weight loss of greater than 5%, and 88 (60%) experienced critical weight loss of greater than 10%. Two weeks after radiation therapy completion, when the body weight dropped to the lowest point, an additional 10 patients experienced critical weight loss of greater than 10% (N = 98). During the entire radiation therapy period, weeks 4 to 6 were the periods when most patients had critical weight loss of greater than 5%, with 30, 54, and 34 patients experiencing critical weight loss in weeks 4, 5, and 6, respectively, accounting for 88% (n = 118 of 134) of the total critical weight loss. The weight change trajectory during radiation therapy exhibited a slight decline in the first three weeks, followed by a sharp decline in the middle three weeks, and a relatively gentle decrease in the last two weeks (see Figure 4). 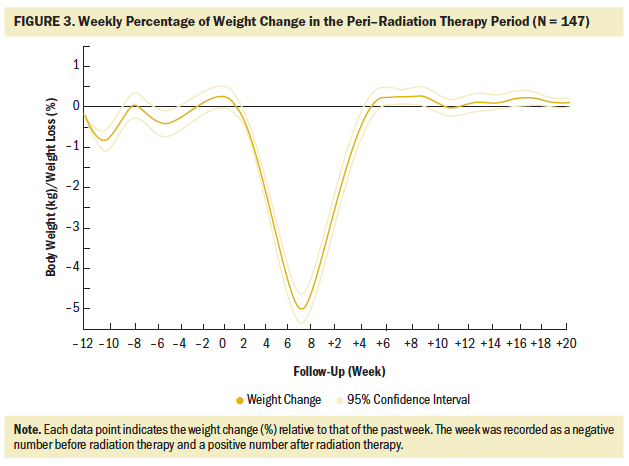
Discussion
Radiation therapy combined with induction chemotherapy is the standard treatment for LANPC (Chen et al., 2019; Chow, 2020; Chua et al., 2019; Colevas et al., 2018; He, Wu, et al., 2017). To the authors’ knowledge, this is the first study that analyzed the weight change trajectory in patients with LANPC before, during, and after radiation therapy over a span of 40 weeks. The current study showed that 91% of patients with LANPC experienced critical weight loss (greater than 5%) in the peri–radiation therapy period, with an average weight loss of 7.24 kg (SD = 4.47), an average BMI decrease of 2.66 (SD = 1.59), and an average weight loss percentage of 10.85% (SD = 6.04). Overall, body weight remained basically unchanged during induction chemotherapy, followed by a sharp and severe decrease during radiation therapy and for as long as 20 weeks after radiation therapy completion, with only a slight increase in body weight from the lowest point. 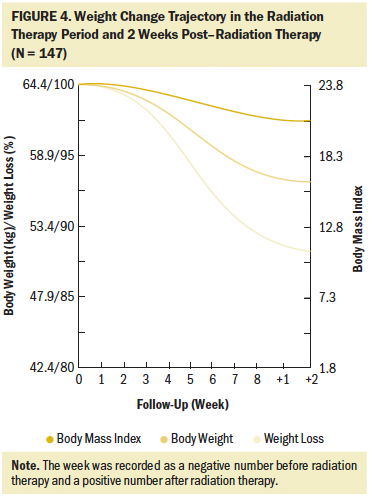
Previous studies demonstrated that chemotherapy could cause severe treatment-related weight loss; the reasons for this included cancer consumption, pain, nausea, vomiting, diarrhea, and oral and gastrointestinal mucositis (Johansson et al., 2010; Morio et al., 2016; Ock et al., 2016; Takayoshi et al., 2017; Tang et al., 2018; Wong et al., 2019). In chemotherapy regimens using cisplatin and/or 5-fluorouracil, weight loss was more serious, with the rate of loss ranging from 2.3% to greater than 3% early in treatment (Morio et al., 2016; Ock et al., 2016; Takayoshi et al., 2017). In the current cohort, more than two-thirds of the patients (n = 112, 76%) received the standard three cycles of induction chemotherapy within 12 weeks. Despite the intensive and radical regimens used, body weight only decreased by 0.52 kg (SD = 2.16) (weight loss percentage = 0.71% [SD = 3.46]), indicating that induction chemotherapy did not cause significant weight loss. This finding was inconsistent with that of other studies in which patients with cancer received similar chemotherapy regimens (Argiles et al., 2010; Carroll et al., 2018; Kang et al., 2015; Sarcev et al., 2008). Dexamethasone has been proven effective in preventing nausea, vomiting, and appetite decreases in various chemotherapy regimens, particularly in hyperemetic regimens (Argiles et al., 2010; Carroll et al., 2018; Kang et al., 2015; Sarcev et al., 2008). In the current study, TPF and GP were the dominant chemotherapy regimens, and dexamethasone was routinely prescribed for three days. In addition, TPF and GP regimes were administered every 21 days per cycle, and the relatively long interval was helpful to body weight recovery, which could be partially proven by the wavy curve in weight change during induction chemotherapy. A study by Qiu et al. (2011) focusing on weight loss and its predictors during radiation therapy demonstrated that 56% (n = 89 of 159) of patients with NPC had a weight loss of 5% in the last three months before radiation therapy. Because Qiu et al. (2011) did not report treatment of patients before radiation therapy, the current authors are unable to provide more information on this result. However, by analyzing the baseline characteristics of patients in the current study and Qiu et al.’s (2011) study, it is notable that patients included in the current study seemed to be in better general condition as compared to that of patients included in Qiu et al.’s (2011) study, with 90% (n = 132 of 147) of patients having a performance score of 0 and no patients with comorbidities that potentially affected body weight. In Qiu et al.’s (2011) study, 26% (n = 41 of 159) of patients had Karnofsky Performance Status scores of 70 or less, and 43% (n = 69 of 159) of patients had at least one comorbidity. All of these factors contributed to maintaining body weight and help explain why the weight remained stable in the current study.
In the current study, weight loss primarily occurred during the eight weeks of radiation therapy, with an average weekly decline of 0.87 kg (SD = 0.37) (weight loss percentage = 1.35% [SD = 0.54]), a total decrease of 6.99 kg (SD = 2.99) (weight loss percentage = 10.81% [SD = 4.33]), a BMI decrease of 2.58 (SD = 1.08), and weight loss percentage of 10.81% (SD = 4.33). According to critical weight loss criteria based on the guidelines, of the 134 (91%) patients who lost greater than 5% of their body weight, 66% of patients (n = 88 of 134) lost greater than 10% in the final week of radiation therapy. Many studies showed prevalent weight loss in patients with NPC during radiation therapy, with an incidence reaching about 90% (Langius et al., 2013; Li et al., 2017; Ng et al., 2004). More than half of those lost 5% or greater of their body weight (Langius et al., 2013; Ng et al., 2004; Shen et al., 2013; Zeng et al., 2016), with weight loss ranging from 4.33 kg to 6.97 kg (Li et al., 2017; Qiu et al., 2011; Shen et al., 2013). As compared to previous studies, patients with LANPC in the current study had weight loss with a higher incidence, at an earlier time, and to a more severe degree; more than 90% of the patients could be diagnosed with nutritional risk requiring nutritional support. Induction chemotherapy, concurrent chemotherapy and radiation therapy, and advanced cancer stage are the top three strongest predictors of critical weight loss during radiation therapy (Du, Tang, Mao, et al., 2015; Qiu et al., 2011; Vangelov et al., 2017; Zeng et al., 2016; Zhao et al., 2015). Although induction chemotherapy was not demonstrated to cause significant weight loss in the current study, all of the included patients possessed the other two negative predictors, which account for the critical weight loss characteristics during radiation therapy. Considering the weight change trajectory, it is suggested that weight management should be conducted as early as in the second week and no later than the fourth week of radiation therapy in patients with LANPC.
Previous studies have generally ended body weight observation in the last week of radiation therapy (i.e., the seventh or eighth week of radiation therapy) (Hou et al., 2016; Langius et al., 2013; Li et al., 2016, 2017; OuYang et al., 2016; Qiu et al., 2011; Shen et al., 2013; Xiao et al., 2017; Zeng et al., 2016), and very few data on weight change are available after radiation therapy completion. A study by Ng et al. (2004) with 37 participants implemented a follow-up of body weight for six months after radiation therapy, showing that the recovery of body weight significantly lagged behind the recovery of calorie intake. However, that study did not report the specific weight change trajectory in the post–radiation therapy period. The current authors’ research demonstrated that weight loss did not cease immediately after radiation therapy completion. In fact, weight loss dropped to the lowest point in the second week after radiation therapy completion and remained at this low point for four weeks. The long-term complications after radiation therapy were not evaluated in the current study because the study focused on body weight. However, the languished regain in body weight may be ascribed to factors that have been proven in other studies (He, Chen, et al., 2017; McLaughlin, 2013; McLaughlin & Mahon, 2014). Taste dysfunction, xerostomia, and trismus are common long-term treatment-related side effects in head and neck cancer survivors, particularly in patients with NPC with advanced-stage disease and radiation therapy completion within one year, predisposing patients to poor nutrition (He, Chen, et al., 2017; McLaughlin, 2013; McLaughlin & Mahon, 2014). In view of the weight change trajectory during the post–radiation therapy period, the current authors recommend that nutritional interventions should continue for at least four weeks after the completion of radiation therapy.
Strengths and Limitations
As compared to previous studies, the current study had several advantages. First, the authors fully considered the backbone status of radiation therapy in NPC treatment, combining the treatment before, during, and after radiation therapy as a whole treatment cycle. Based on that, the authors comprehensively investigated the weight change trajectory in patients with LANPC. Second, both absolute and relative values of weight change during the peri–radiation therapy period were assessed, which eliminated the incomparability caused by individual baseline values, providing an individual reference for each patient to conduct self-monitoring of their weight change when receiving radiation therapy. Third, for the weight change after radiation therapy completion, the sample size was nearly four times that of the similar study available so far, which guarantees the reliability of the results to a certain degree.
However, the current study still has limitations. First, a survivor bias might exist because the patients included had completed planned radiation therapy. Patients excluded from the analysis may have experienced more severe complications, such as mucositis, dysphagia, and bone marrow suppression, resulting in more serious weight loss. If all patients were included in the final analysis, the actual weight loss may have been more severe. Second, factors related to weight change, such as caloric intake, daily activities, and treatment-induced side effects, were not assessed. The primary reason for the lack of assessment was that these factors were complex and required professional instrument, which could have resulted in a high probability of inaccurate records during the long follow-up time of 40 weeks without guidance of clinical professionals. In addition, to make these records, patients need to be trained. Patients who fail to meet the training requirements, such as older adults or less educated people, are very likely to be excluded from the study, resulting in reduced representativeness of the sample and further limiting the generalization of the study results. Weight loss is the dominant symptom and a comprehensive indicator reflecting radiation therapy–related syndrome clusters among patients with NPC receiving radiation therapy, and is the most crucial item for evaluating and diagnosing nutritional risk (Cederholm et al., 2015; Langius et al., 2013; Mueller et al., 2011; Weimann et al., 2017; Xiao et al., 2017). Therefore, in terms of the stated research purposes, the authors believe that the stated limitations would not compromise the study results.
Implications for Nursing
NPC has an extremely uneven worldwide distribution, mainly occurring in eastern and southeastern Asia and North Africa (Bray et al., 2018; Tang, Chen, et al., 2016). The ESPEN consensus statement noted that, in a real clinical setting, access to body composition measurements is limited and complicated and that body weight is (a) easier to measure than BMI and fat-free mass index and (b) less affected by factors such as baseline body weight, catabolic diseases, and age (Cederholm et al., 2015).
It is important for patients to have an overview of the disease process to undertake self-monitoring, particularly under chronic disease conditions (Hickey et al., 2019; van Riel et al., 2019). The symptoms and signs of the disease, post-treatment rehabilitation, and time of recovery are of the biggest concern and interest to patients with head and neck cancer, and making the comprehensive information available for nurses and patients is helpful to better manage treatment-related symptoms (Dodd et al., 2001; Dorsey & Pickler, 2019; Lin et al., 2020; Saroa et al., 2018). Although weight loss indicates poor outcomes and prognosis, it is a symptom that can be alleviated by cooperation of the nurses and patients. Because it would take at least four to seven months just for intensive anticancer treatment in LANPC, early nutritional intervention is ambiguous in the literature associated with patients with NPC (Jin et al., 2017; Meng et al., 2019; Schuetz et al., 2019; Tanaka et al., 2018; Zhang et al., 2017) and without a widely recognized recommendation. Therefore, the results of the current study (a) provide evidence for a precise and time-tailored intervention with weight management in patients with LANPC, which would be particularly beneficial to nurses in developing countries where there is a high NPC incidence but limited health resources, and (b) offer a reference to patients for their self-monitoring of weight change during radiation therapy because of its prevalence and severity. If the patients know the weight change trajectory in advance, it could help to reduce the disease uncertainty and the fear of anticancer treatment. 
Conclusion
Patients with LANPC universally experienced weight loss in the peri–radiation therapy period, and there is a different weight change trajectory before, during, and after radiation therapy. More active measures should be adopted to reduce the degree and slow down the speed of weight loss in patients with LANPC. Nurses should use the most precise information to initiate health care (Dorsey et al., 2019). To improve treatment effectiveness and save medical resources, a time-tailored intervention based on weight change trajectory is necessary. According to the weight change trajectory, the relevant interventions should be initiated as early as the second week and no later than the fourth week of radiation therapy, and the intervention should be continued for at least four weeks after radiation therapy completion.
About the Author(s)
Xiaoxia Zhang, RN, MSN, is a PhD candidate in the West China School of Nursing and in the Department of Breast Surgery/Cancer Center; Juan Liu, RN, BSN, and Huaqin Yu, RN, BSN, are RNs in the Department of Head and Neck Oncology/Cancer Center; Xiaotian Su, RN, BSN, is an RN in the Department of Breast Surgery/Cancer Center; Hongxiu Chen, RN, MSN, is a PhD candidate in the West China School of Nursing; Yinbo He, MD, is the technical head of the Department of Radiation Oncology/Cancer Center; Zhe Liu, MD, is an oncologist in the Cancer Center; and Xiuying Hu, RN, PhD, is a professor in the Innovation Center of Nursing Research at the West China School of Medicine, all at the West China Hospital at Sichuan University in Chengdu City, Sichuan Province, China. This article was supported by a research grant (18ZD033) from the Health Commission of Sichuan Province, China. Zhang and Hu contributed to the conceptualization and design. Zhang, J. Liu, Yu, Su, He, and Z. Liu completed the data collection. Zhang and He provided statistical support. Zhang provided the analysis. Zhang and Chen contributed to the manuscript preparation. Hu can be reached at hxyyzxx@163.com, with copy to ONFEditor@ons.org. (Submitted May 2020. Accepted August 8, 2020.)




