Genitourinary Symptoms in Breast Cancer Survivors: Prevalence, Correlates, and Relationship With Sexual Functioning
Objectives: To evaluate (a) the prevalence of genitourinary symptoms, (b) which demographic and clinical factors predict genitourinary symptoms, and (c) the association between genitourinary symptoms and sexual functioning in breast cancer survivors.
Sample & Setting: A secondary analysis of cross-sectional, patient-reported outcomes data from 1,085 breast cancer survivors was conducted.
Methods & Variables: Prevalence and correlations with demographics, clinical factors, and sexual functioning were identified using descriptive analysis, multivariable logistic regression analysis, chi-square tests, t tests, and Pearson correlation coefficients.
Results: Symptoms included vaginal/vulvar irritation, pelvic discomfort, problems with urinary control, vaginal infection, and vaginal bleeding. Younger age, more comorbidities, and taking treatment for menopausal symptoms were significantly related to reporting genitourinary symptoms. Experiencing more symptoms was associated with lower sexual functioning.
Implications for Nursing: The prevalence, correlates, and relationship of genitourinary symptoms with sexual functioning supports the assessment and treatment of these symptoms as part of routine care for breast cancer survivors.
Jump to a section
About 70% of postmenopausal women experience genitourinary symptoms (Gandhi et al., 2016; Moral et al., 2018; North American Menopause Society, 2020). Although genitourinary symptoms are usually caused by naturally occurring postmenopausal estrogen loss, they can also be caused by chemotherapy and/or endocrine therapy for breast cancer (Mac Bride et al., 2010; North American Menopause Society, 2020). Many breast cancer survivors experience genitourinary symptoms, yet they are underreported (Baumgart et al., 2011), underassessed, and undertreated (Biglia et al., 2020; Cook et al., 2018; Lester & Bernhard, 2009; Moral et al., 2018). Genitourinary symptoms, also called genitourinary syndrome of menopause (Portman & Gass, 2014), are defined as unpleasant symptoms that include urologic and genital symptoms. Urologic symptoms indicate problems with urinary control, such as stress/urge incontinence (Chung et al., 2020), urgency, frequency, dysuria or burning, recurrent urinary tract infections, and dryness (Cook et al., 2018). Genital symptoms include vaginal dryness, vaginal/vulvar irritation, soreness and burning, itching, bleeding, infection, and vaginal/pelvic pain (Nappi & Kokot-Kierepa, 2012; Portman & Gass, 2014). Genitourinary symptoms are associated with lower quality of life among breast cancer survivors and can interfere with sexual functioning (Gandhi et al., 2016; Lester & Bernhard, 2009).
A comprehensive assessment of genitourinary symptoms in breast cancer survivors has not been previously reported (Cook et al., 2018; Lester & Bernhard, 2009). Most studies have focused on a single symptom or the most common symptoms, such as vaginal dryness, vaginal/vulva irritation, or problems with urinary control (North American Menopause Society, 2020). In addition, the prevalence of genitourinary symptoms varies because of differences in symptom assessments. In previous studies, 32%–75% of breast cancer survivors reported at least one genitourinary symptom, such as vaginal discharge, irritation, bleeding, dryness, or urine control problems (Baumgart et al., 2011; Biglia et al., 2020; Chin et al., 2009; Lester et al., 2012). Many of these studies suggested that studies with larger sample sizes are needed in groups of women who have reported any genitourinary symptoms rather than specific symptoms.
Although cancer treatments are known to increase the risk of genitourinary symptoms in breast cancer survivors (North American Menopause Society, 2020), little is known about which demographic and clinical factors are related to these symptoms in breast cancer survivors. In cancer survivors with diagnoses other than breast cancer, factors influencing symptom burden include age, number of comorbidities, income, cancer site (Bubis et al., 2018), education, and ongoing cancer treatment (Shi et al., 2011). In women without cancer, factors associated with genitourinary symptoms include body mass index (BMI) (Geng et al., 2018), menopause, ovarian failure, and vaginal childbirth (Gandhi et al., 2016). Because demographic and clinical factors related to genitourinary symptoms in breast cancer survivors have not been well studied, additional research exploring their relationship is needed to better manage survivors’ symptoms.
Sexual functioning has been reported as a significant problem in breast cancer survivors, and genitourinary symptoms have been linked with sexual functioning (Champion et al., 2014). In breast cancer survivors, some studies have shown that genitourinary symptoms were associated with lower quality of sexual life (Gandhi et al., 2016; Lester & Bernhard, 2009; Moral et al., 2018; Shifren et al., 2019) because of decreases in sexual lubrication, and loss of or difficulties with libido, sexual arousal, orgasm, pleasure from sex, and sexual desire (Boquiren et al., 2016; Gupta et al., 2006). The correlation between genitourinary symptoms and sexual functioning varies based on the number and type of symptoms assessed. To date, information regarding the association between genitourinary symptoms and sexual functioning in breast cancer survivors is limited. Because individuals may be hesitant to talk about sexual functioning, both genitourinary symptoms and sexual functioning are often underassessed overall (Mac Bride et al., 2010).
The purpose of this study was to better understand the prevalence of genitourinary symptoms, factors related to those symptoms, and their relationship with sexual functioning in breast cancer survivors. In particular, the study aims were to (a) evaluate the prevalence of five genitourinary symptoms (vaginal/vulvar irritation, pelvic discomfort, problems with urinary control, vaginal infection, and vaginal bleeding), (b) explore which demographic and clinical factors predict genitourinary symptoms, and (c) determine the association between genitourinary symptoms and sexual functioning in breast cancer survivors.
Conceptual Model
The theory of unpleasant symptoms illustrates reciprocal interactions among three main components: influential factors, symptoms, and performance outcomes (Lenz et al., 1997; Lenz & Pugh, 2003). According to this theory, influential factors (e.g., physiologic, psychological, situational) can affect the intensity, timing, level of perceived distress, and quality of symptoms, which, in turn, affect performance outcomes (e.g., functional, cognitive, physical). The symptoms also interact with each other. Physiologic, psychological, and situational factors influence symptoms and performance, and symptoms and performance also influence these physiologic, psychological, and situational factors. For example, depression (psychological factor) affects pain experience (symptom), the intensity of pain influences the treatment of depression, and both depression and pain impair the ability to work (performance outcome). Conversely, the intensity of pain and the inability to work affect depression management (Bair et al., 2003).
Based on the theory of unpleasant symptoms, the authors developed a situation-specific model of genitourinary symptoms in breast cancer survivors (see Figure 1). Because of the limitations of the dataset used for this study, psychological factors and the intensity, timing, perceived distress level, and quality of symptoms were removed from the model. With this revised model, two types of factors were relevant to the current study. The first type of factor was physiologic, which included estrogen deprivation because of chemotherapy and/or endocrine therapy, such as selective estrogen receptor modulators (SERMs) like tamoxifen or aromatase inhibitors (AIs) like letrozole. The genitourinary tract (vulva, vagina, and lower urinary tract) and supported pelvic structure contain epithelial tissue that is sensitive to estrogen deprivation (North American Menopause Society, 2020). Estrogen deprivation results in a thinner vaginal and urethral epithelium, loss of fat, narrow vaginal opening, and shrinkage of the urethra and clitoral prepuce. This, in turn, can lead to atrophy in the vulva, vagina, pelvic floor, and urinary tract, which results in a variety of genitourinary symptoms, including vaginal soreness and bleeding, vaginal dryness, infection, itching and irritation, discomfort, urinary incontinence, and difficult or painful intercourse (Chin et al., 2009; Lester et al., 2015). 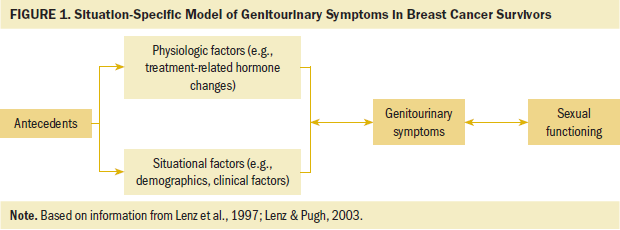
The second type of factor was situational, which included demographic and clinical factors, such as age, marital status, education, race and ethnicity, income, BMI, age at first menses, years since breast cancer diagnosis, non–breast cancer-related comorbidities, menopausal status, the use of treatment to alleviate genitourinary symptoms (e.g., treatments for menopause), and the use of endocrine therapy. The performance outcome was sexual functioning. Sexual functioning is defined as how the body responds to sexual stimulation that is expressed by sexual activity, desire, lubrication, orgasm, frequency, satisfaction, and pain (Rosen et al., 2000). Lastly, the symptom variable in the model was genitourinary. Genitourinary symptoms can negatively affect sexual interest, lubrication, arousal, orgasm during sexual activity, and sexual satisfaction. Therefore, genitourinary symptoms can be related to decreased sexual functioning (Burich & DeGregorio, 2011; Melisko et al., 2010; Nappi et al., 2013).
Methods
This is a secondary analysis of data from a large cohort study of quality of life in younger (aged 45 years or younger) versus older (aged 55–70 years) breast cancer survivors. The parent study collected data from younger (n = 505) and older (n = 622) breast cancer survivors, partners, and controls of women without breast cancer. The full eligibility criteria for the parent study are reported elsewhere (Champion et al., 2014). Briefly, women who were treated for breast cancer and diagnosed either when they were aged 45 years or younger or when they were aged 55–70 years were recruited. Participants had completed breast cancer treatment trials from the Eastern Cooperative Oncology Group–American College of Radiation Imaging Network (ECOG-ACRIN) and were identified from the ECOG-ACRIN statistical center database. Additional eligibility criteria included not having a recurrence, being three to eight years postdiagnosis, and receiving treatment with a similar chemotherapy regimen of adriamycin, paclitaxel, and cyclophosphamide. Informed consent was obtained from all participants (Champion et al., 2014).
Because the purpose of the current study was to assess a host of genitourinary symptoms and test associated variables rather than test differences by age group, the younger and older groups were combined. Only breast cancer survivors with complete data on the genitourinary symptoms were included in this analysis. The parent study consisted of 1,127 breast cancer survivors. The current study’s authors identified 1,085 breast cancer survivors from the parent study who had reported data for all five genitourinary symptoms (vaginal/vulvar irritation, pelvic discomfort, problems with urinary control, vaginal infection, and vaginal bleeding) and were included in this secondary analysis. Ages of survivors included in the current study ranged from 28 to 78 years.
Measures
Situational and physiologic factors were selected based on the conceptual model. Demographic and clinical factors were collected using an investigator-designed questionnaire and health record reviews. Demographic information included age, marital status, education, race, ethnicity, income, and BMI. Clinical factors included age at first menses, years since breast cancer diagnosis, number of reported non–breast cancer-related comorbidities (e.g., heart disease, hypertension, diabetes, depression), menopausal status, treatments for menopausal symptoms (e.g., vitamin E, herbs, vaginal creams/lubricants, vaginal estrogen, antidepressants), and use of endocrine therapy (e.g., SERM, such as tamoxifen, raloxifene, or toremifene; AI, such as letrozole, anastrozole, or exemestane). Menopausal status was measured by asking whether women had a menstrual period during the past 3 months, with no change in regularity (premenopausal); had a menstrual period during the past 3 months, with cycles that were less regular, or had a menstrual period during the past 12 months but not in the past 3 months (perimenopausal); or had not had a menstrual period during the past 12 months (postmenopausal). Reasons for postmenopausal status included natural aging (natural); a hysterectomy or the removal of ovaries (surgical); or breast cancer treatment, the use of medicine not related to breast cancer, or other or unsure (artificial because of ovarian failure secondary to drugs, radiation therapy or chemicals, or medications) (Geerlings et al., 2001).
The dataset included self-reported genitourinary symptoms. Women were asked if they had ever been diagnosed with or had a problem with urinary control. Response options were yes or no. Pelvic and vaginal symptoms were assessed by asking whether the women had experienced pelvic discomfort, vaginal bleeding (between periods), a vaginal infection, or vaginal/vulvar irritation during the past month. Response options for these symptoms were rated on a scale ranging from 1 (not at all) to 5 (very much). Responses of “not at all” were considered as “symptom not present,” and the remaining responses were collapsed into “symptom present.” In this way, all genitourinary symptoms could be consistently reported to match the dichotomous response option for “problem with urinary control.”
Sexual functioning, which was also self-reported in the dataset, was assessed using a seven-item sexual index scale ranging from 1 (strongly agree) to 5 (strongly disagree). Items were summed to create a total score and two subscale scores (sexual enjoyment and sexual difficulty). After reverse scoring relevant items, total scores ranged from 7 to 35, with higher scores indicating better functioning. Enjoyment scores ranged from 4 to 20, with higher scores indicating more enjoyment; sexual difficulty scores ranged from 3 to 15, with higher scores indicating more difficulty. Sexual enjoyment items consisted of interest, enjoyment, satisfaction, and thoughts of sexual activity (e.g., “I am able to relax and enjoy sexual activity”). Sexual difficulty items consisted of problems with becoming aroused, lubricating, and achieving orgasm during sexual activity (e.g., “I have difficulty becoming sexually aroused”). The sexual index scale was a modified version of an existing scale (Hudson et al., 1981), with a Cronbach’s alpha of 0.79 (Champion et al., 2013).
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics, version 26.0. For aim 1, descriptive statistics were used to generate the percentage of women with each of the five genitourinary symptoms (present, not present). For aim 2, demographic and clinical factors were reported as means and standard deviations for continuous variables or n values and percentages for categorical variables. Chi-square tests and t tests were used to compare these factors between asymptomatic (no symptoms reported) and symptomatic (at least one symptom reported) women. Chi-square and t tests were not performed for individual symptoms (present, not present) because several symptoms were not frequently reported. All factors that were significant at p < 0.2 were entered into a multivariable logistic regression model (Hosmer et al., 2013). Experiencing at least one genitourinary symptom was the dichotomous dependent variable. The correlates were age, non–breast cancer-related comorbidities, marital status, menopausal status, race, and treatment for menopausal symptoms. For aim 3, t tests were used to compare sexual functioning, sexual enjoyment, and sexual difficulty between the presence or absence of individual symptoms, as well as between asymptomatic (no symptoms) and symptomatic women (at least one symptom or two or more symptoms). Pearson correlation coefficients were reported to assess the relationship between the number of genitourinary symptoms and the sexual functioning variables.
Results
The sample demographic and clinical variables for symptomatic and asymptomatic breast cancer survivors are presented in Table 1. Several significant results were indicated. The average age of symptomatic survivors at the time of the study was slightly younger than 55 years, whereas the average age of asymptomatic survivors was 58 years (p = 0.01). Symptomatic survivors reported slightly more comorbidities (2.2) than asymptomatic survivors (1.9) (p = 0.024). More symptomatic survivors were more likely to be pre- or perimenopausal (p < 0.001) or taking treatment for their menopausal symptoms (p < 0.001) than asymptomatic survivors. The average age at first menses for the sample was about 12 years, and the average time since the initial breast cancer diagnosis was almost 6 years. At the time of the study, the entire sample had an average of two non–breast cancer-related comorbidities. The majority of the sample was overweight or obese, was non-Hispanic and Caucasian, had attained at least a two-year college education, and had incomes more than $30,000 at the time of the study. The sample was primarily married or in a long-term committed relationship (287 symptomatic survivors and 518 asymptomatic survivors). Most of the sample was postmenopausal (naturally, surgically, or artificially), and slightly less than half reported taking a SERM or AI at the time of the study. 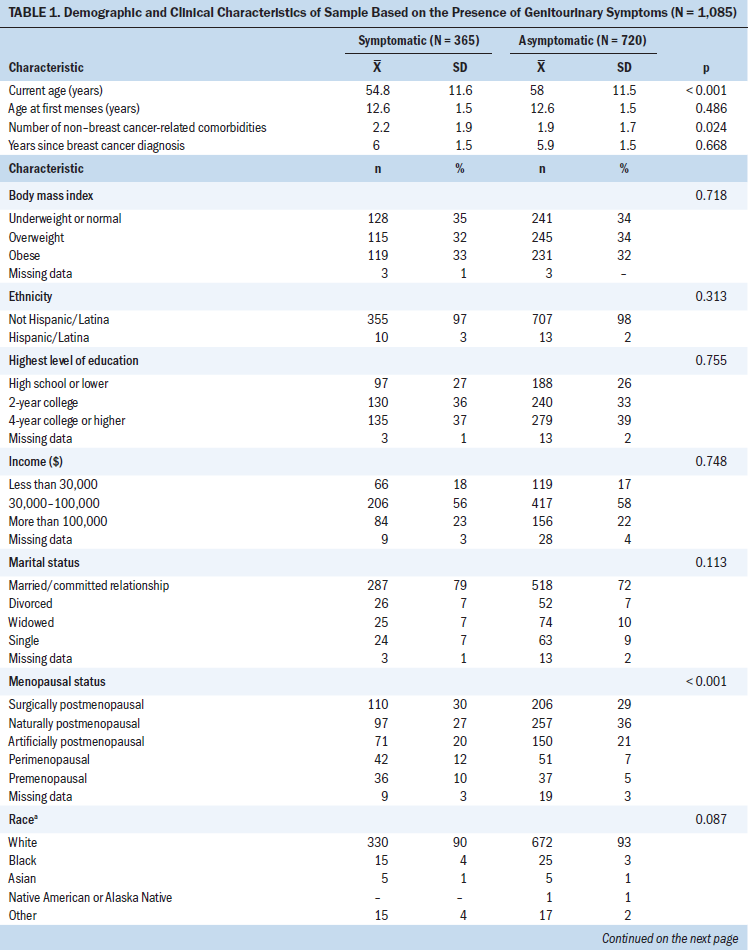
Aim 1: Symptom Prevalence
Prevalence rates of the five symptoms for the sample are reported in Table 2. The most common symptom was vaginal/vulvar irritation (n = 163), followed by pelvic discomfort (n = 162), problems with urinary control (n = 99), vaginal infection (n = 46), and vaginal bleeding (n = 44). Thirty-four percent of the sample had at least one genitourinary symptom, and 10% had two or more symptoms. Only one breast cancer survivor reported having all five symptoms. 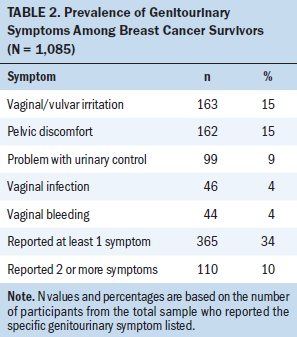
Aim 2: Demographic and Clinical Factors and Symptoms
The bivariate analyses of demographic and clinical factors consisted of 365 symptomatic and 720 asymptomatic survivors. The analyses indicated that breast cancer survivors with at least one genitourinary symptom were more likely to be younger; had a greater number of non–breast cancer-related comorbidities; and were of an underrepresented race (i.e., Black, Hawaiian Native or Pacific Islander, Asian, Native American or Alaska Native, or other), married, pre- or perimenopausal, and taking treatment for menopausal symptoms (p < 0.2 for all variables). There was no difference between asymptomatic and symptomatic women on age at first menses, years since breast cancer diagnosis, BMI, ethnicity, education, income, and treatment with a SERM or AI (p > 0.05 for all variables).
The logistic regression model showed that having at least one genitourinary symptom was associated with being younger (odds ratio [OR] = 0.98, 95% confidence interval [CI] [0.96, 0.99], p = 0.01), a greater number of non–breast cancer-related comorbidities (OR = 1.19, 95% CI [1.09, 1.29], p < 0.001), and taking treatment for menopausal symptoms (OR = 1.73, 95% CI [1.31, 2.3], p < 0.001) (see Table 3). In addition, survivors who were artificially postmenopausal were less likely to report genitourinary symptoms when compared to premenopausal women (OR = 0.5, 95% CI [0.28, 0.89], p = 0.018). 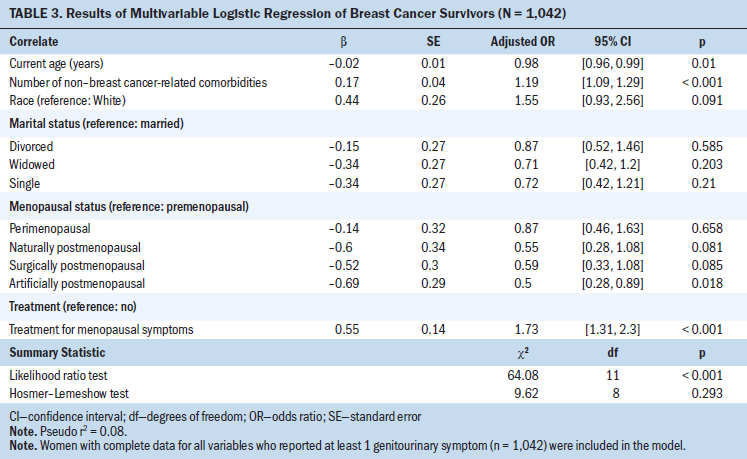
Aim 3: Genitourinary Symptoms and Sexual Functioning
Women reporting at least one or two or more genitourinary symptoms were associated with lower sexual functioning, which was characterized by less sexual enjoyment and more sexual difficulty (p < 0.001 for all variables). Specific symptoms were also associated with lower sexual functioning, with a few exceptions (see Table 4). Vaginal bleeding was not associated with any aspect of sexual functioning, pelvic discomfort was not associated with sexual difficulty, and vaginal infection was only marginally (p = 0.096) associated with sexual enjoyment. The total number of genitourinary symptoms was weakly correlated with total sexual functioning scores and scores on the sexual enjoyment and sexual difficulty subscales at –0.16, –0.15, and 0.15 (p < 0.001 for all variables), respectively. 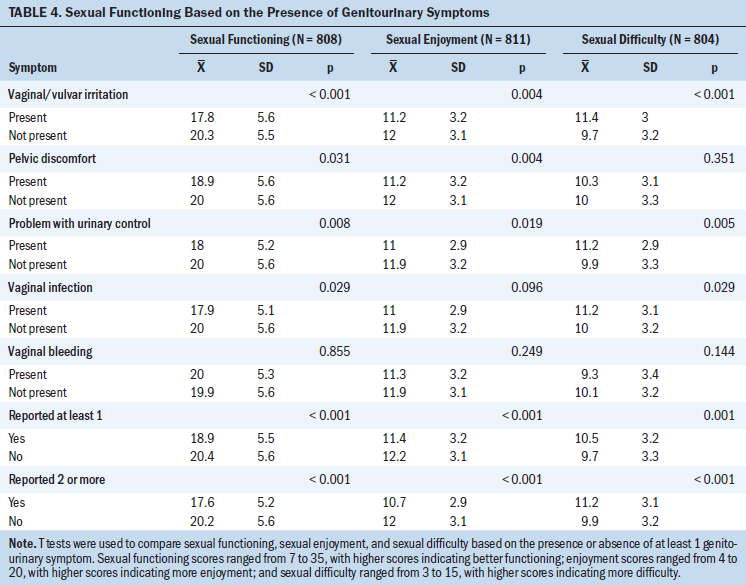
Discussion
To the authors’ knowledge, this is the first study to evaluate the prevalence of multiple genitourinary symptoms, the relationship of these symptoms with demographic and clinical factors, and the relationship with sexual functioning in a large cohort of long-term breast cancer survivors. The results of this study add to the literature on the prevalence of genitourinary symptoms in this population. Unlike previous studies that examined single symptoms or selected participants based on specific symptoms, the dataset for the current study included five genitourinary symptoms (vaginal/vulvar irritation, pelvic discomfort, problems with urinary control, vaginal infection, and vaginal bleeding).
Regarding prevalence, 34% of breast cancer survivors in the current study reported experiencing at least one of the five genitourinary symptoms, which is lower than other previously published studies that found prevalence rates of 50%–75% (Baumgart et al., 2011; Chin et al., 2009; Sousa et al., 2018). Baumgart et al. (2011) found that about 58% of postmenopausal breast cancer survivors receiving an AI and 32% of those receiving tamoxifen had at least one of eight genitourinary or menopausal symptoms: vaginal bleeding, vaginal discharge, vaginal irritation, vaginal dryness, dyspareunia, hot flashes, cold sweats, and night sweats. Plausible explanations for variations in prevalence include differences in menopausal status, cancer-related endocrine therapy, and the number and types of genitourinary or menopausal symptoms assessed. The current study included breast cancer survivors of pre-, peri- and postmenopausal status, with most survivors being postmenopausal and less than half of survivors (44% symptomatic, 46% asymptomatic) taking SERMs or AIs. Previous studies included only breast cancer survivors who were postmenopausal (Baumgart et al., 2011; Chin et al., 2009) or were receiving adjuvant endocrine therapy (Sousa et al., 2018). The lower prevalence rate in the current study could be because survivors in the sample were not selected based on experiencing genitourinary symptoms or receiving treatment for these symptoms.
Consistent with the situation-specific model of genitourinary symptoms in breast cancer survivors, correlations were found between demographic and clinical factors and genitourinary symptoms. The results indicate that demographic and clinical factors predicted genitourinary symptoms, which provides some evidence of individuals who are more likely to report symptoms. In the current study, symptomatic survivors were younger overall. The parent study found that younger survivors reported worse anxiety, marital satisfaction, and fear of recurrence than older survivors (Champion et al., 2014). There is some support for the belief that these younger survivors were more aware of genitourinary symptoms. For example, loss of fertility because of premature menopause may make younger survivors more likely to report genitourinary symptoms (Ben Charif et al., 2015; Su et al., 2020). In addition, in a study by Bubis et al. (2018) based on Cancer Care Ontario’s Symptom Management Reporting Database, which did not specifically study breast cancer survivors and genitourinary symptoms, younger age was a predictor for symptom burden for survivors. Bubis et al. (2018) reported that among 120,745 participants, cancer survivors (breast [30% of total survivors], gastrointestinal, or genitourinary) younger than age 50 years were more likely to report ongoing symptoms (e.g., pain, anxiety, tiredness) than survivors aged 51–60 years. The current study also found that survivors with more non–breast cancer-related comorbidities were more likely to be symptomatic, which is consistent with previous studies (Constantine et al., 2014; Moyneur et al., 2020). Other studies also found that survivors are more likely to report symptoms, such as pain, anxiety, or urine control problems, as comorbidities increase (Bubis et al., 2018; Shi et al., 2011).
The results regarding menopausal status were counterintuitive. Women who were artificially postmenopausal were less likely to be symptomatic than premenopausal women, independent of their current age. Previous studies found that being postmenopausal was a main risk factor for genitourinary symptoms (Gandhi et al., 2016; Geng et al., 2018), and a systematic review by Parish et al. (2013) found that 50% of postmenopausal women reported genitourinary symptoms. It is possible that premenopausal women were more sexually active, younger, and more aware of the genitourinary symptoms. In addition, the current study found that survivors taking treatment for menopausal symptoms were more likely to report genitourinary symptoms, which are considered a subset of menopausal symptoms (Calleja-Agius & Brincat, 2015; Moral et al., 2018). Therefore, it is possible that women who were symptomatic enough to take medication for other menopausal symptoms would also report more genitourinary symptoms.
Although other studies have shown that education, BMI, and treatment with a SERM or AI are related to genitourinary symptoms, the results of the current study were not consistent with these findings. In a study of 1,071 breast cancer survivors with an average age of 50 years, Aydin et al. (2014) found that lower education status and higher BMI were negatively associated with genitourinary symptoms. Geng et al. (2018) also found that BMI was negatively associated with genitourinary symptoms in a study of 1,294 women, including 607 premenopausal and 687 postmenopausal women, with an average age of 52 years. Although no significant differences in genitourinary symptoms were found between survivors who were receiving treatment with a SERM or AI versus those who were not, more than 44% of breast cancer survivors being treated with a SERM or AI in the current study experienced at least one genitourinary symptom. This finding is consistent with the results of a study by Baumgart et al. (2011), which reported genitourinary symptom rates of 58% among breast cancer survivors receiving AIs and 32% among breast cancer survivors receiving tamoxifen.
Results on the relationship between genitourinary symptoms and sexual functioning were weak but consistent with those of previous studies (Baumgart et al., 2013; Biglia et al., 2017; Boquiren et al., 2016; Gupta et al., 2006; Lester & Bernhard, 2009; Sousa et al., 2017, 2018). Except for vaginal bleeding, each symptom was associated with either a lower total sexual functioning score, less sexual enjoyment, more sexual difficulty, or a decrease in all three of the sexual functioning variables. However, the magnitude of the correlations was weak between the number of genitourinary symptoms and sexual functioning, sexual enjoyment, and sexual difficulty. Correlations may have differed because of differences in symptoms across studies. For example, vaginal dryness, which has a higher association with sexual problems compared to other genitourinary symptoms (Gupta et al., 2006; Lester et al., 2012), was not included in the current study’s dataset. Gupta et al. (2006) found that vaginal dryness and sexual problems were more highly correlated as compared to other symptoms (r = 0.58). In addition, Boquiren et al. (2016) found that vaginal dryness predicted sexual functioning (b = –0.5). The weak correlation may also be explained by differences in sexual functioning measures. Using a five-point Likert-type scale, Gupta et al. (2006) found that 60% of breast cancer survivors reported sexual problems. Boquiren et al. (2016) reported that 83% of breast cancer survivors with body image disturbance reported sexual dysfunction as measured using the Female Sex Function Index. Unlike previous studies (Baumgart et al., 2013; Biglia et al., 2017; Lester et al., 2012), the current study did not measure pain with intercourse. Lastly, weak correlations may be a result of different patient populations. The current study included younger and older breast cancer survivors. In a study by Bober and Varela (2012), younger survivors had higher rates of sexual dysfunction. Older survivors, who may not be as sexually active, may not be as concerned with sexual functioning as a part of their quality of life. Future studies should include a more comprehensive list of genitourinary symptoms and all dimensional aspects of sexual functioning.
Strengths and Limitations
This study included a large dataset of breast cancer survivors, with ages ranging from 28 to 78 years at the time of the study. Five genitourinary symptoms, all of which are part of the genitourinary syndrome of menopause, and their associations with sexual functioning, sexual enjoyment, and sexual difficulty were examined. Another strength of the current study is that it observed potential demographic and clinical factors related to genitourinary symptoms.
However, the cross-sectional study design limits the ability to explore a temporal relationship between genitourinary symptoms and demographic and clinical factors. This was a secondary data analysis, which limited the data available for this study. As a result, the full model of the theory of unpleasant symptoms could not be studied. This study also used a sexual survey measure that was modified from another scale (Hudson et al., 1981) and did not include the severity of sexual problems. Most participants were married, well educated, non-Hispanic and Caucasian, of relatively high income, and postmenopausal; therefore, the results may not be generalizable to other breast cancer survivors. An international survey found that more women in Finland reported that genitourinary symptoms affected their quality of life and social life as compared to women in other countries, including the United States, Sweden, and Canada (Nappi & Kokot-Kierepa, 2010). In addition, research has suggested that Hispanic women are more likely to discuss genitourinary symptoms with healthcare providers than women from other ethnic groups (Kingsberg et al., 2013). Future studies of diverse racial and ethnic groups are needed to further evaluate the potential for differences in symptom experience based on race or ethnicity.
Implications for Practice
Genitourinary symptoms are common in breast cancer survivors and influence their survivorship care (Gandhi et al., 2016; Moral et al., 2018). Understanding the association between genitourinary symptoms and demographic and clinical factors may help nurses to identify and refer survivors who are at higher risk for these symptoms. Because of the prevalence of genitourinary symptoms in this patient population, screening and assessing symptoms should be a part of routine care for breast cancer survivors. Considering the stigma associated with talking about these symptoms, communication between healthcare providers and survivors is critical for early identification of symptoms.
Understanding factors related to symptoms can also help clinicians and researchers to develop targeted treatments for survivors who are at risk for experiencing symptoms. If genitourinary symptoms can be prevented and treated with early assessment and appropriate treatment (Sousa et al., 2017; Vincent, 2015), sexual functioning may improve (Gandhi et al., 2016). Vaginal estrogen treatment is contraindicated, although findings from a meta-analysis suggested low-dose vaginal estrogen was a safe and effective treatment (Pavlović et al., 2019). In a study by Melisko et al. (2017), women with early-stage breast cancer had improved genitourinary symptoms and sexual functioning after receiving 7.5 mcg of estradiol every 24 hours for 90 days. Other treatments, such as vaginal dehydroepiandrosterone and laser therapy, should be used with caution because of insufficient evidence of their safety and efficacy for breast cancer survivors (North American Menopause Society, 2020). Vaginal moisturizers and lubricants (nonhormone therapies) are available without a prescription, which may help to alleviate genitourinary symptoms in breast cancer survivors (Crean-Tate et al., 2020; Gandhi et al., 2016; North American Menopause Society, 2020; Sussman et al., 2019).
Nurses play a vital role in educating survivors and encouraging communication with oncologists about genitourinary symptoms, sexual functioning, and treatment choices. Early assessment, appropriate treatments, and communication between survivors and healthcare providers are important for reducing symptoms, improving sexual functioning, and enhancing survivors’ quality of life (Gandhi et al., 2016). 
Conclusion
This study identified the prevalence, correlation, and relationship of sexual functioning to five genitourinary symptoms in breast cancer survivors. The relationship between these symptoms and demographic and clinical factors provides preliminary information regarding individuals who may be at higher risk for experiencing genitourinary symptoms. The results from this study can be used to develop targeted interventions for genitourinary symptoms, which may improve sexual functioning.
About the Author(s)
Ying Sheng, PhD, RN, is a postdoctoral research fellow, Janet S. Carpenter, PhD, RN, FAAN, is a distinguished professor, the Audrey Geisel Chair of Innovation, and associate dean of research, Andrea A. Cohee, PhD, RN, and Susan Storey, PhD, RN, AOCNS®, are assistant professors, both in the Department of Community Health Systems in the Melvin and Bren Simon Comprehensive Cancer Center, all in the School of Nursing at Indiana University; Timothy E. Stump, MA, is a statistician in the Department of Biostatistics in the School of Medicine at Indiana University; and Patrick O. Monahan, PhD, is a professor in the Department of Biostatistics in the School of Medicine at Indiana University and the director of statistics and psychometrics for the Indiana University Cancer Prevention and Control Program, all in Indianapolis; David Cella, PhD, is the Ralph Seal Paffenbarger Professor and chair of the Department of Medical Social Sciences, both in the Department of Medical Social Sciences in the Feinberg School of Medicine, and the director of the Institute for Public Health and Medicine–Center for Patient-Centered Outcomes, all at Northwestern University in Chicago, IL; and Victoria L. Champion, PhD, RN, FAAN, is a distinguished professor, the Edward and Sarah Stam Cullipher Endowed Chair in the School of Nursing, and the associate director of community outreach and population at the Melvin and Bren Simon Comprehensive Cancer Center at Indiana University in Indianapolis. The data used in this article are from a study that was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (group co-chairs: Robert L. Comis, MD, and Mitchell D. Schnall, MD, PhD) and was supported, in part, by public health service grants (CA189828, CA180795, CA37403, CA35199, CA17145 and CA49883) from the National Cancer Institute, National Institutes of Health, and the U.S. Department of Health and Human Services. Sheng is supported as a postdoctoral fellow under 5T32CA117865 (principal investigator [PI]: V. Champion), Cohee is supported by the Indiana Clinical and Translational Sciences Institute KL2 Program (UL1TR002529; PI: A. Shekhar), and Storey is supported by a research grant (RE01) from the Oncology Nursing Foundation. Sheng, Carpenter, Cella, and Champion contributed to the conceptualization and design. Champion completed the data collection. Sheng, Carpenter, Cohee, Storey, Stump, Monahan, and Champion provided statistical support. Sheng, Carpenter, Stump, and Monahan provided the analysis. All authors contributed to the manuscript preparation. Sheng can be reached at shengyin@iu.edu, with copy to ONFEditor@ons.org. (Submitted August 2020. Accepted October 10, 2020.)




