Caregiver Characteristics Associated With Cognitive Complaints in Women With Breast Cancer
Objectives: To explore whether caregiver characteristics were associated with cognitive complaints reported by women with breast cancer undergoing chemotherapy.
Sample & Setting: 61 dyads of women with breast cancer and their caregivers were recruited at Duke Women’s Cancer Care Raleigh in North Carolina.
Methods & Variables: An exploratory, cross-sectional design was used. Data were obtained on patients and caregivers. Patient cognitive complaints were represented by cognitive impairment (CI) and cognitive ability (CA).
Results: Two significant associations were found: between patient CI and caregiver mental health, general health, and burden; and between patient CA and caregiver self-confidence and burden. Caregiver burden, which showed correlations with both patient CI and CA, was found to influence patient CA. Also, patient depression played a mediator role in the association between caregiver general health and patient CI.
Implications for Nursing: Healthcare providers should consider caregivers when assessing and managing patients’ cognitive symptoms. This study suggests the value of including caregivers when establishing interventions for patients who have cognitive complaints.
Jump to a section
As many as 75% of women with breast cancer complain of changes in their cognitive function (hereafter called cognitive complaints) during chemotherapy (Janelsins et al., 2018). Patients with cognitive complaints experience frequent forgetfulness (such as of names, dates, or telephone numbers); slower processing speeds; and difficulties in concentration, multitasking, and word retrieval (Asher, 2011; Ganz et al., 2013; Janelsins et al., 2014). Although such complaints may not be observed by others, patients themselves are aware that they are not functioning well compared with their prediagnosis baseline (Myers, 2013). For these reasons, many studies of patients with breast cancer acknowledge the value of patient reports of cognitive complaints (Myers, 2013; Savard & Ganz, 2016; Tannock et al., 2004). In addition, researchers have emphasized self-reported cognitive complaints as an important indicator of patients’ daily functioning (Ahles et al., 2002; Kohli et al., 2007; Von Ah et al., 2013).
Despite their high prevalence, knowledge about cognitive complaints during chemotherapy has not been consistent. Reviews of the empirical literature revealed that (a) the adverse effects of cancer and cancer treatments on the central nervous system and/or (b) co-occurring symptoms with chemotherapy (e.g., anxiety, depression, fatigue, sleep disturbance) can primarily influence patients’ cognitive complaints (Janelsins et al., 2014; Merriman et al., 2013). However, these two factors do not sufficiently explain the variabilities in cognitive complaints during chemotherapy. Some patients have complained that cognitive changes occurred during chemotherapy and persisted for more than one year following cancer treatment (Ng et al., 2018). Inconsistent manifestations of cognitive complaints heighten the need to investigate other potential factors, whether internal or external, that can explain these clinical variabilities.
Caregiver characteristics may provide a different perspective on the clinical variability of cognitive complaints reported by women with breast cancer. Caregiver characteristics can be defined as how the caregiver appraises and copes with the patient’s illness (Lyons & Lee, 2018), which includes burden, caregiver coping styles, health (e.g., physical, mental, and general health), and self-confidence in caregiving. Caregivers, chosen by patients as their main support person, possess diverse characteristics that they use to provide care (Nijboer et al., 1999; Wagner et al., 2011). Several studies have suggested that caregiver characteristics are associated with the quality of patient care and, as a result, influence health outcomes, such as physical and psychological distress (Ferrell et al., 1995; Litzelman et al., 2016; Milbury et al., 2013).
Despite the reported association between caregiver characteristics and patient health outcomes, only a few studies have explored their associations with patient cognitive complaints. A study by Saria et al. (2016) demonstrated that patients with brain metastasis reported fewer memory problems when caregivers showed an accepting attitude toward the care situation. Consistent with this finding, a study by some of the current authors (Yang et al., 2019) found that greater concentration problems were reported by patients with cancer when their caregivers experienced a high burden of care. In addition, a review by Yang et al. (2020) involving adults with cancer showed that patients’ cognitive function is associated with caregiver characteristics like mental health, burden, and coping. The study by Yang et al. (2019) also found that patient emotional distress influences patient concentration problems via caregiver burden; in other words, patient emotional distress increases caregiver burden, and such increased burden, in turn, influences patient concentration problems. Additional studies have reported opposite associations: that caregiver burden increases patient emotional distress (An et al., 2019; Milbury et al., 2013). These findings suggest that there are possible relationships among caregiver characteristics, patient cognitive complaints, and their co-occurring symptoms; however, the directionality among these three variables is unclear.
It is also unclear how caregiver characteristics are associated with patient cognitive complaints. Therefore, in this study, the authors explored the association between caregiver characteristics and cognitive complaints in women with breast cancer and whether this association is mediated by the patients’ co-occurring symptoms. The findings from this study may help to explain the phenomenon of cognitive complaints reported by patients with cancer and inform the development of future interventions for patients with cognitive concerns.
Methods
Conceptual Framework
A modified version of Bronfenbrenner’s ecological model, developed by the Centers for Disease Control and Prevention (CDC, 2018), was used as a guiding framework to explore factors associated with patient cognitive complaints. The CDC’s social-ecological model (SEM) consists of four levels, and the factors posited at each level interplay with each other. The individual level refers to the internal characteristics of a person, including their biologic and personal attributes. The relationship level refers to interpersonal relationships, including family and caregivers. The community level includes organizations and institutions, such as schools, workplaces, and neighborhoods. The societal level, the outer level of SEM, includes social and cultural norms that influence health behavior and individual experience.
The modified CDC SEM primarily focuses on the individual level and the relationship level. The individual level is called the patient level in the current study and includes patient co-occurring symptoms of anxiety, depression, fatigue, and sleep disturbance. Factors posited at the patient level (i.e., patient co-occurring symptoms), called patient factors in this study, are known factors that contribute to patient cognitive complaints. The relationship level is called the caregiver level in the current study and includes caregiver characteristics, such as caregiver coping styles, burden, health (e.g., physical, mental, and general health), and self-confidence in caregiving.
The purpose of the current study was to explore whether caregiver characteristics were associated with cognitive complaints reported by women with breast cancer undergoing chemotherapy. Three specific aims were explored: (a) whether patient factors are associated with patient cognitive complaints, (b) whether caregiver characteristics are associated with patient cognitive complaints, and (c) whether this association of caregiver characteristics with patient cognitive complaints is mediated by patient factors. Based on the conceptual model, the authors also created the analytic model to guide data analyses for the three specific aims.
Design, Sample, and Setting
This was an exploratory, cross-sectional study conducted at Duke Women’s Cancer Care Raleigh in North Carolina. Recruitment of study participants occurred over 10 months using face-to-face strategies. A total of 61 dyads of patients and caregivers were recruited for the study. Inclusion criteria for patients were as follows:
• Aged 18 years or older
• Newly diagnosed with breast cancer (stages I–IIIC)
• Undergoing either anthracycline- or taxane-based chemotherapy
• Able to speak, read, and write in English
Patients who had completed at least two cycles of chemotherapy were recruited because patients were likely to demonstrate cognitive complaints at the end of the second cycle (Cheung et al., 2015; Ng et al., 2018) Patients were excluded if they had a previous cancer diagnosis, a history of receiving chemotherapy and/or radiation therapy, or a history of neurodegenerative illness and reported hospitalization for psychiatric illness within the past two years. Caregivers, identified by the patient as the person who provided the majority of support, were also recruited for this study. Inclusion criteria for caregivers were as follows:
• Provision of unpaid assistance to the patient
• Aged 18 years or older
• English fluency
Data Collection
Data were collected from patients and caregivers (dyads) at the clinic on the same day that written informed consent was obtained. The institutional review board of the study site approved this study.
Patients: Instruments for patients were a demographic questionnaire, the Functional Assessment of Cancer Therapy–Cognitive Function (FACT-Cog), and the Patient-Reported Outcomes Measurement Information System-43 Profile (PROMIS-43), version 2.1, of anxiety, depression, fatigue, and sleep disturbance. The demographic questionnaire included patient age, marital status, education level, race/ethnicity, and employment status. The FACT-Cog was used to assess the degree of cognitive complaints in patients with cancer (Von Ah & Tallman, 2015). Patients were asked to rate the frequency of 37 statements based on their experiences using a five-point Likert-type scale. The FACT-Cog assesses the following four subscales: (a) perceived cognitive impairment (CI), (b) perceived cognitive abilities (CAs), (c) impact on quality of life, and (d) perception and comments from others. In the current study, the two subscale scores of CI and CA were used to represent patient cognitive complaints, following Lai et al.’s (2009) report that patient cognitive complaints were comprised of patient-reported CA and CI. A higher score for each subscale (CA range = 0–36, CI range = 0–80) denotes fewer cognitive complaints. Cronbach’s alphas calculated using this study sample were 0.95 for CI and 0.96 for CA. Patients’ anxiety, depression, fatigue, and sleep disturbances were assessed by the PROMIS-43. Patients were asked to respond to six questions for each domain using a five-point Likert-type scale. These questions are a subset of items from a larger item bank that has demonstrated high content validity and reliability (Cella et al., 2010; Reeve et al., 2007). Higher scores for each domain reflect higher levels of symptoms (range = 6–30). Cronbach’s alphas were 0.92 for anxiety, depression, and fatigue, and 0.87 for sleep disturbance.
Additional information, such as type of surgery (mastectomy, lumpectomy), stage of breast cancer, chemotherapy regimens, concurrent administration of hormones, and National Comprehensive Cancer Network (NCCN) Distress Thermometer score, was recorded using clinical notes. The NCCN Distress Thermometer consists of a single-item 11-point Likert-type scale representing a global screener of distress (ranging from 0 [no distress] to 10 [extreme distress]). The associated NCCN Problem List helps identify potential sources of distress related to patients’ practical (e.g., housing, transportation), family (e.g., family health issues), emotional (e.g., depression, nervousness), physical (e.g., breathing,, eating), and spiritual/religious concerns. Patients responded to the Problem List items using a binary response scale (yes/no) (Cutillo et al., 2017).
Caregivers: Instruments for caregivers were a demographic questionnaire, the Brief COPE, the Caregiver Reaction Assessment (CRA), and the PROMIS Global Health, version 1.2. The demographic questionnaire included caregiver age, gender, marital status, employment status, race/ethnicity, hours of interaction with the patient, co-residence with the patient, and type of relationship with the patient (e.g., spouse, adult child).
The Brief COPE was used to assess different ways that caregivers cope with the patient’s disease (Carver, 1997). This instrument is comprised of 14 types of coping strategies, and each strategy is assessed using two items. Responses for each item use a four-point Likert-type scale ranging from 1 (“I haven’t done this at all”) to 4 (“I have been doing this a lot”). The sum of item scores for each coping strategy ranges from 2 to 8, with higher scores indicating greater use of that coping strategy. In this study, 14 coping strategies were collapsed into 4 strategies: seeking social support, positive thinking, avoidance, and problem solving. This four-factor structure of the Brief COPE showed satisfactory internal consistency (Cronbach’s alpha greater than 0.6) (Baumstarck et al., 2017). The Cronbach’s alpha of each coping strategy in this study ranged from 0.76 to 0.83.
The CRA is a 24-item scale used to assess the degree of caregiver reactions toward the care situation, positive (e.g., self-confidence) or negative (e.g., burden), using a five-point Likert-type scale (Given et al., 1992). The CRA consists of five dimensions: self-confidence, lack of family support, impact on finances, impact on schedule, and impact on health; caregiver burden can be assessed with the combined scores of four dimensions (lack of family support, impact on finances, impact on schedule, and impact on health). The Cronbach’s alpha for caregiver burden was 0.78 and 0.73 for self-confidence in this study.
The PROMIS Global Health consists of 10 items that provide a general perception of caregiver health, including physical, mental, general, and social health. Four items are used to assess physical health. Of these, three items concerning physical status, physical activities, and fatigue are rated from 1 to 5, with higher scores indicating better physical health (total range = 3–15; Cronbach’s alpha for this study = 0.74); one item asks the caregiver to rate pain from 0 to 10, with higher scores indicating worse pain. Four items concern quality of health, mental status, social activities, and emotional problems, which are rated from 1 to 5, with higher scores indicating better mental health (total range = 4–20; Cronbach’s alpha for this study = 0.82). In addition, a single item asks for a general rating of health on a scale ranging from 1 to 5, with higher scores indicating better caregiver general health condition (range = 1–4).
Data Analysis
Three analyses were conducted using SAS, version 9.4. First, descriptive statistics were calculated to summarize the dyads’ demographic and clinical information. Pearson correlations were used to estimate the bivariate relationships between caregiver characteristics and patient cognitive complaints (CI and CA). Second, hierarchical multiple regression was used to assess whether patient factors and/or caregiver characteristics influenced the variance in patient cognitive complaints, after controlling for the effects of the predictors previously entered (study aims 1 and 2) (Cohen et al., 2002). In stage 1 of the hierarchical multiple regression, patient demographic information was entered into the model, followed by patient factors in stage 2, and then by caregiver characteristics in stage 3. Third, a backward regression was performed to find statistically significant predictors of patient cognitive complaints among caregiver characteristics and patient factors. Finally, using the principles proposed by Baron and Kenny (1986), a three-step regression approach was further implemented to determine whether the influence of caregiver characteristics on patient cognitive complaints was mediated by patient factors (study aim 3). When performing the three-step regressions, patient age and level of education (as a proxy indicator of a cognitive reserve) (Jung et al., 2017) were controlled for their confounding effects on patient cognitive complaints. Therefore, to address study aim 3, three regression equations were estimated, controlling for patient age and education: (a) the influence of caregiver characteristics on patient factors, (b) the influence of caregiver characteristics on patient cognitive complaints, and (c) the influence of patient factors and caregiver characteristics on patient cognitive complaints.
Results
Sample Characteristics
A total of 61 dyads of patients and caregivers participated in this study. There are no missing values in the obtained data.
Patients: The majority of patients were married (n = 36) and White (n = 36), and had a mean age of 54.28 years (SD = 11.85). In addition, most had completed at least some college, and 22 were working full-time. Patients who participated in this study were primarily postmenopausal (n = 34), had been diagnosed with stage IA cancer (n = 25), and had not had any surgeries for breast cancer removal (lumpectomy, mastectomy) (n = 26). Patients reported no problems in terms of performance, as measured with the Eastern Cooperative Oncology Group Scale of Performance Status (all scores of 0), and the mean for distress, measured with the NCCN Distress Thermometer, was 2.26 (SD = 2.83). Detailed demographic and clinical information of patients is summarized in Table 1. 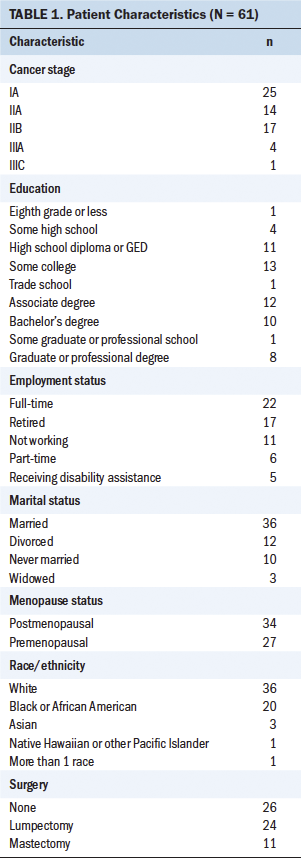
Caregivers: The majority of caregivers were White (n = 38), male (n = 35), and the spouses or partners of patients; had a mean age of 52.49 (SD = 15.32); worked full-time (n = 34); and lived with the patient (n = 32). About 21% of caregivers reported that they interacted with the patient 24 hours per day, and the mean number of hours of caregiver interaction with the patient was 10.9 (SD = 8.1). Detailed demographic information of caregivers is summarized in Table 2. 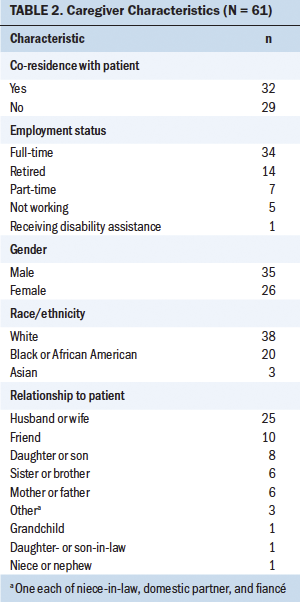
Bivariate Relationships Between Caregiver Characteristics and Patient Cognitive Complaints
Bivariate relationships between caregiver characteristics and patient cognitive complaints were examined. In this study, patient cognitive complaints were presented by subscales of CI and CA; therefore, the relationship between caregiver characteristics and each subscale was examined. The variance inflation factor values were all less than 4, which indicates that there were no concerns with multicollinearities among caregiver characteristics (O’Brien, 2007).
Patients showed less CI when caregivers reported better mental and general health (r = 0.3, p = 0.01; r = 0.32, p = 0.02; respectively) and less burden of care (r = –0.28, p = 0.03). In addition, better patient CA was found when caregivers were more confident (r = 0.29, p = 0.02) in caregiving and experienced less burden of care (r = –0.3, p = 0.02).
Predictors of Patient Cognitive Complaints
Cognitive impairment: In stage 1, patient demographic characteristics of age and education were entered into model 1 (see Table 3). None of these characteristics showed a significant relationship with patient CI. In stage 2, when patient factors were entered into model 2, patients’ anxiety, depression, and fatigue were significant for CI (beta = 1.29, p = 0.02; beta = –1.82, p = 0.001; beta = –0.85, p = 0.01; respectively). These factors also remained significant in model 3 in stage 3. Model 2 yielded an adjusted R2 value of 0.28 and explained significant variability in patient CI (p < 0.001). In model 3, when caregiver characteristics were entered, no significant predictors were found among caregiver characteristics. However, caregiver characteristics entered into model 3 accounted for a 10% increase in the adjusted R2, which was significant (p < 0.001). 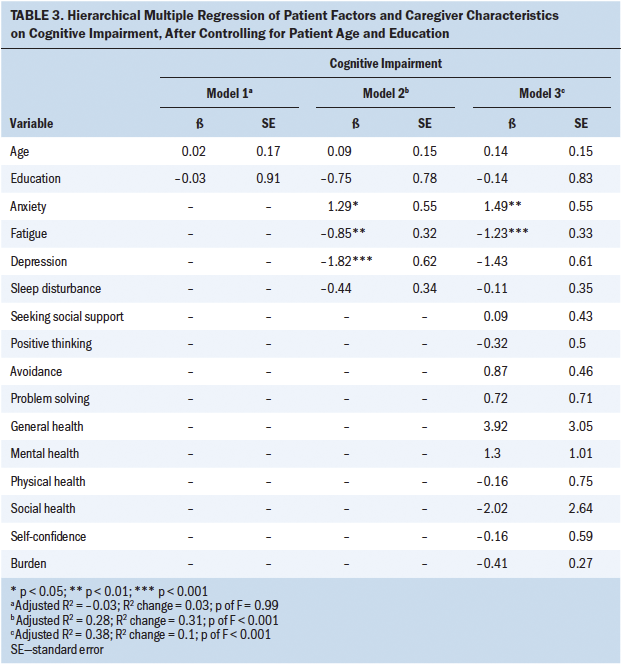
Cognitive ability: In stage 1, patient demographic characteristics of age and education were entered into model 1. The patient’s education level showed a significant relationship with patient CA (beta = 1.49, p < 0.001) (see Table 4). These demographics yielded an adjusted R2 value of 9% and explained significant variability in patient CA (p < 0.02). In stage 2, when patient factors were entered into model 2, none showed a significant relationship with CA. It showed a decrease in adjusted R2, which was not significant (p < 0.13). However, when caregiver characteristics were entered into model 3, caregiver burden showed a significant association with patient CA (beta = –0.53, p < 0.001). Caregiver characteristics entered into model 3 accounted for an increase of 18% in the adjusted R2, which was significant (p < 0.02). 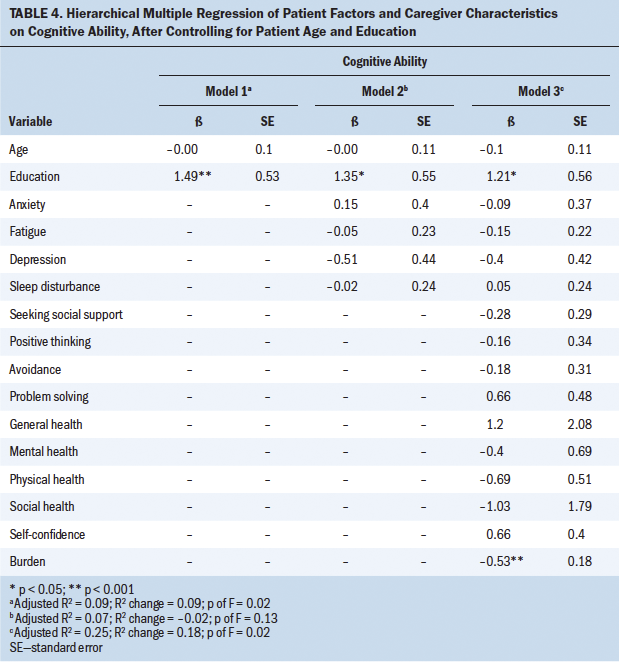
Mediating Effect of Patient Factors on Association Between Caregiver Characteristics and Patient Cognitive Complaints
Results of regression with backward selection found six predictors of patient CI: patient fatigue (p < 0.001), anxiety (p < 0.001), depression (p = 0.03), caregiver use of avoidance coping (p < 0.001), burden (p = 0.01), and general health condition (p < 0.001). To test whether the association between caregiver characteristics and patient cognitive complaints was mediated by patient factors, three sets of three-step regression equations were computed, resulting in nine combinations. From these nine tests, two relationships were found that constituted a mediation effect: (a) caregiver general health condition directly influenced patient CI, and (b) caregiver general health condition indirectly influenced CI via patient depression.
Specifically, in the first equation, the caregiver’s general health condition predicted patient depression (beta = –2.19, p < 0.001), which can be interpreted as patients showing a lower level of depression when they received care from caregivers who maintained overall good health (see Figure 1). The second equation showed that the caregiver’s general health condition predicted patient CI (beta = 5.41, p < 0.01). This shows that patients demonstrated less CI when they received care from caregivers reporting overall good health status. The third equation was performed to evaluate whether the strength of the association between caregiver general health condition and patient CI decreased when patient depression was included. When both caregiver general health and patient depression were entered as predictors of patient CI, controlling for patient age and education, the model accounted for 13% of the variance. In this equation, depression was a significant predictor of CI (beta = –1.12, p < 0.02). In addition, the beta estimate presenting the relationship between caregiver general health condition and patient CI decreased from the significant 5.41 (p < 0.01) to a nonsignificant 3.01 (p = 0.19) when patient depression was included in the equation. 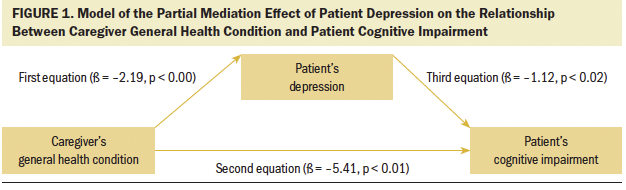
The Sobel test was used to verify the significance of the mediation effect of patient depression. To perform the Sobel test, the following values and formula were used (see Figure 2): z value = a × b / SQRT (b2 × sa2 + a2 × sb2), where a is the estimated path coefficient from the predictor to the mediator, b is the estimated path coefficient from the mediator to the outcome, Sa is the standard error of a, Sb is the standard error of b, and SQRT is the square root. Once the z value was obtained, it was entered into a z value to p value calculator to determine its significance. The calculation showed that patient depression was significant in the association between caregiver general health condition and patient CI (p = 0.04). In other words, the patient’s depression partially mediated the association between caregiver general health condition and patient CI, after controlling for the patient’s age and education. 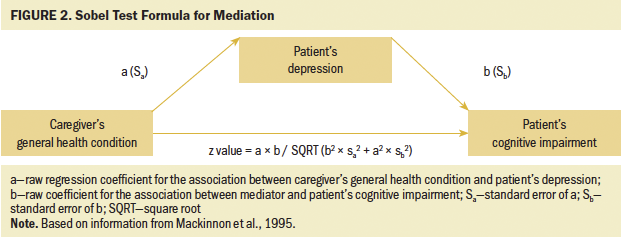
Another regression analysis with backward selection was used to find predictors of CA. Patient education and depression (p < 0.00 and p < 0.03, respectively) were significant predictors of CA. Caregiver burden, self-confidence, and physical health were significant predictors of CA (burden, p < 0.01; self-confidence, p < 0.03; physical health, p < 0.04). However, no mediation effects were found on patient CA.
Discussion
This study explored factors associated with cognitive complaints (CI and CA) reported by women with breast cancer undergoing chemotherapy, guided by the CDC SEM. On the patient level, patient anxiety, fatigue, and depression were significant predictors of patient CI. Patients who had higher levels of anxiety, depression, and fatigue experienced greater CI. However, no association was found between patient CA and patient factors. This finding is consistent with the study conducted by Von Ah and Tallman (2015), who reported that patient negative affect (e.g., depression) is associated with patient CI, as assessed by the FACT-Cog, but not with CA.
On the caregiver level, patients demonstrated better CA and less CI when their caregivers achieved better mental and general health conditions and expressed higher confidence in caregiving and lower burden of care. This result is consistent with previous research showing that patient CA items capture general self-efficacy (Lai et al., 2009), suggesting that positive emotions may be involved in patients’ cognitive process. Despite the significant bivariate relationships between caregiver characteristics and patient cognitive complaints, only caregiver burden remained a significant predictor of patient CA in the final regression model. Although other caregiver characteristics (including confidence, mental health, and general health condition) lost their independent contribution to patient cognitive complaints, the combinations of entire caregiver characteristics used in this study accounted for the variance of cognitive complaints. This finding reinforces the need to consider caregivers when establishing and implementing strategies for patients who have cognitive complaints.
There were three significant predictors of patient CI from the patient level (fatigue, anxiety, and depression) and the caregiver level (avoidance coping, burden, and general health condition). The mediation analysis found a direct influence of caregiver general health condition on patient CI and an indirect influence through patient depression. In other words, poor caregiver general health leads to increased patient depression, which, in turn, increases the severity of patient CI. Concerning the influence of caregiver general health on patient depression, this result aligned with a review of empirical studies by Li and Loke (2014) that showed the reciprocal influence of distress outcomes in dyads of patients with cancer and caregivers.
Caregiver burden did not mediate the association between patient factors and cognitive complaints, and this is inconsistent with the previous study (Yang et al., 2019). This contradiction may be explained by the combining of variables of interest in previous studies, as compared to the method of exploration in the current study. In the previous study (Yang et al., 2019), one variable was created that encompassed diverse dimensions of emotional distress, including worry, irritability, nervousness, and sadness; however, in the current study, the authors created separate variables per each dimension of emotional distress. Also, in the same manner, the current authors assessed both CI and CA to represent patient cognitive complaints, but in the previous study, the authors assessed only concentration problems.
Although CI and CA appear similar, they are independent constructs that comprise patient cognitive complaints (Lai et al., 2009; Von Ah & Tallman, 2015). CA refers to the capacity for patients to perform tasks involving attention/concentration, verbal fluency, memory, and mental acuity, whereas CI is defined as deficits that patients perceive when performing such tasks (Costa et al., 2018). Several researchers have emphasized the importance of assessing these two constructs separately and recommend using the FACT-Cog as an assessment tool (Lai et al., 2009; Von Ah & Tallman, 2015). Consequently, it is important to assess both CI and CA when assessing patient cognitive complaints.
Limitations
This study has the limitation of making causal inference because it used a cross-sectional design. A cross-sectional design is useful in beginning to explore the relationships between factors on each level, but this does not provide ideas of how each factor influences patient cognitive complaints over time (Setia, 2016). Future studies should involve repeated observations over time, and a longitudinal study will help to determine cause and effect (Caruana et al., 2015). Also, this study was conducted in a single outpatient oncology center of a large academic medical center. The sample was relatively small and homogeneous in terms of sociodemographic and clinical diversity, which may impede the generalizability of the study findings. A large and heterogeneous sample is needed for future studies to be representative of all patient–caregiver dyads.
Implications for Nursing
This study provides a foundation of more precise and effective dyadic interventions that may improve both patient and caregiver outcomes in the context of cancer. For example, programs that help to decrease caregiver burden can be provided with the aim of improving patient cognitive function. These findings also reinforce the need for health professionals to assess caregiver characteristics. Systematic assessments of caregivers are not typically performed in clinical settings; as a result, healthcare professionals may be missing opportunities to offer supportive interventions to caregivers. If health professionals intervene to modify caregiver characteristics, this may contribute to improvements in caregiver well-being and in the cognitive function of the patient. 
Conclusion
Based on these exploratory findings, the authors have determined that caregiver characteristics are factors associated with cognitive complaints in women with breast cancer undergoing chemotherapy. More specifically, three caregiver characteristics—caregiver mental health, general health condition, and burden—were found as associated factors of patient CI; caregiver self-confidence and burden were associated with patient CA. Of those associated factors, the authors found that caregiver burden predicts patient CA. In addition, it was determined that patient depression mediates the association between caregiver general health and patient CI. For example, better caregiver general health decreases patient depression, which, in turn, improves patient CI. This study suggests that support for caregivers should be a component of interventions for patients who have cognitive complaints.
About the Author(s)
Yesol Yang, PhD, NP-C, is a T32 postdoctoral fellow at the Ohio State University Comprehensive Cancer Center–Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus; and Victoria Poillucci, Med, MSN, ACNP-BC, is a nurse practitioner and advanced practice team lead in medical oncology at Duke University Health System and the Duke Cancer Institute, Deborah “Hutch” Allen, PhD, RN, CNS, FNP-BC, AOCNP®, is the director of nursing research and evidence-based practice at Duke University Health System, Wei Pan, PhD, is an associate professor in the School of Nursing and the School of Medicine at Duke University, Eleanor McConnell, PhD, RN, is an associate professor and a core investigator in the School of Nursing at Duke University, the Durham VA Medical Center, and the Geriatric Research Education and Clinical Center of the Durham VA Health Care System, and Cristina C. Hendrix, DNS, GNP-BC, FAAN, is the division chair of Health Systems and Analytics in the School of Nursing at Duke University and a core investigator at the Geriatric Research Education and Clinical Center, all in Durham, NC. This study was supported by the Oncology Nursing Foundation Dissertation Research Grant/Genentech. Yang, Allen, McConnell, and Hendrix contributed to the conceptualization and design. Yang and Poillucci completed the data collection. Yang, Pan, and Hendrix provided statistical support. Yang, Allen, and Hendrix provided the analysis. Yang, Allen, Pan, McConnell, and Hendrix contributed to the manuscript preparation. Yang can be reached at yesol.yang@duke.edu, with copy to ONFEditor@ons.org. (Submitted January 2021. Accepted February 24, 2021.)




