Depressive Symptoms and Quality of Life Associated With the Use of Monoclonal Antibodies in Breast Cancer Treatment
Objectives: To assess the relationship between (a) chemotherapy and monoclonal antibody (mAb) treatments and (b) depressive symptoms and quality of life (QOL) in patients with breast cancer.
Sample & Setting: 182 women with breast cancer in Spain who were undergoing chemotherapy with or without mAbs.
Methods & Variables: An observational, cross-sectional study was carried out. The European Organisation for Research and Treatment of Cancer (EORTC) QOL Questionnaire–Core 30 and the EORTC QOL Questionnaire–Breast Cancer were used to assess QOL. Patients were screened for depressive symptoms using the Beck Depression Inventory-II.
Results: No relationship was found between the use of mAbs with chemotherapy and QOL, except for incidence of diarrhea. However, depressive symptoms had a negative and highly significant influence on the majority of the QOL parameters.
Implications for Nursing: The presence of depressive symptoms negatively affects QOL. Used concurrently, mAbs and chemotherapy do not negatively influence QOL, but some adverse effects, such as diarrhea, are common.
Jump to a section
Breast cancer is the most common malignancy diagnosed in women worldwide (Sung et al., 2021). Treatment with chemotherapy has had an important impact on the prognosis of individuals diagnosed with breast cancer, significantly improving their overall survival and disease-free survival (Rossi et al., 2015) and quality of life (QOL) (So et al., 2010). Nevertheless, about 30% of women who are initially diagnosed with early-stage disease still eventually develop metastatic disease (Redig & McAllister, 2013). The emergence of cancer immunotherapies and other strategies for treating tumor cells has been promising in the treatment of breast cancer, particularly when compared to conventional treatments, which lack tumor selectivity and cause more side effects (García-Aranda & Redondo, 2019). Immunotherapies in the form of monoclonal antibodies (mAbs), such as trastuzumab and pertuzumab, have had an impact on human epidermal growth factor receptor 2 (HER2)–positive breast cancer treatment through antibody-dependent cellular cytotoxicity.
Treatment options for relapsing breast cancer are determined based on time to relapse, histologic subtypes and tumor burden, patient and tumor characteristics, aggressiveness of the disease, response to previous therapies, time since last exposure, agents used in the previous line of treatment, and cumulative doses (Jones, 2008). Breast cancer classification is based on the expression or lack of expression of protein receptors, including estrogen receptor, progesterone receptor, and HER2 (Bertucci et al., 2008). HER2 is overexpressed and/or amplified in about 15%–20% of cases of early-stage breast cancer at the time of first diagnosis and is correlated with a worse prognosis (Wuerstlein & Harbeck, 2017). The introduction of trastuzumab, a humanized mAb that binds to the HER2 receptor, in chemotherapy has significantly increased chances of survival in individuals with metastatic and early-stage HER2-positive breast cancer (Slamon et al., 2001, 2011).
Pertuzumab is another mAb that synergizes with the mechanism of action of trastuzumab. It was tested in the metastatic setting in combination with trastuzumab and showed substantial clinical activity with a reasonable toxicity profile (Eiger et al., 2019). Ado-trastuzumab emtansine (T-DM1) is an antibody–drug conjugate that links a microtubule toxin (emtansine) with trastuzumab. In early-stage HER2-positive breast cancer, neoadjuvant treatment with T-DM1, lapatinib, and nab-paclitaxel was more effective than the standard treatment (Patel et al., 2019). These new pharmacologic agents do not lack adverse reactions, and nursing practitioners responsible for administering therapeutic drugs must be aware of both the benefits and the risks involved in new treatments (Cáceres et al., 2017, 2019). Some mAbs, such as trastuzumab, are associated with depression as a common adverse reaction (Spanish Agency for Medicines and Health Products, 2020), which can affect the QOL of individuals treated with these drugs.
These new treatments have shown promise in terms of greater life expectancy for individuals diagnosed with breast cancer, but it is also important to determine the impact they may have on QOL. Individuals diagnosed with cancer can develop depression or anxiety related to their diagnosis, treatment, self-image, and the future (Christopher et al., 2014; Grabsch et al., 2006; Kim et al., 2019). In addition, chemotherapy may reduce individuals’ physical and cognitive functioning, leading to increased psychological distress (Gaston-Johansson et al., 1999). Depression is one of the major causes of psychiatric morbidity among those with cancer (Miovic & Block, 2007). Rates of depression in individuals receiving chemotherapy for breast cancer have been reported as being between 25% and 53% (Bower et al., 2011; Lee et al., 2020; Whisenant et al., 2020).
Mood disturbance in individuals with cancer may result in treatment-impaired QOL (Gold et al., 2016). Many studies have explored the cluster of symptoms experienced by individuals with breast cancer, including depressive symptoms (Crane et al., 2019; Lee et al., 2020), and some have referred to their possible relationship with treatment (Kim et al., 2008, 2012). So et al. (2021) conducted a systematic review on this symptom cluster, observing that depression and other psychological symptoms have a detrimental effect on QOL and that they are present at all stages of cancer treatment. These symptoms are likely the result of both the disease and its treatment because they are present before, during, and/or after treatment (Chow et al., 2019; Li et al., 2020). In a systematic review, So et al. (2021) reported heterogeneity both in the groups of symptoms and in the analyses of the studies reviewed, to which they propose separately examining the symptoms that occur with breast cancer.
Several studies have reported contradictory results in terms of depressive disorders among individuals with breast cancer receiving various drug regimens. For example, Chang et al. (2015) demonstrated that both chemotherapy and trastuzumab treatment, among others, were independent risk factors for developing depressive disorders; however, their relationship with QOL was not studied. Schwartzberg et al. (2019) studied health-related QOL in patients receiving eribulin with and without trastuzumab and found no deterioration in QOL. Only Trinca et al. (2019) studied the impact of depressive symptoms on the QOL of individuals with breast cancer undergoing chemotherapy and mAb treatment, showing that depressive symptoms in individuals with breast cancer receiving chemotherapy together with mAb treatment detrimentally affected certain aspects of QOL. However, the study by Trinca et al. (2019) had a small sample.
In summary, most studies have analyzed depression as part of a set of symptoms or have only studied the possible relationship between depressive symptoms and treatments in general, not by treatment group, such as mAb. Others have only studied the impact of treatments on QOL. However, there are no studies, to date, that analyze the three variables together (depressive symptoms, treatment with mAbs, and QOL) and have a large sample size. In addition, no data have been found on their impact on the Spanish population with breast cancer. As a result, the main aim of the current study was to evaluate depressive symptoms and QOL of individuals with breast cancer receiving chemotherapy together with mAb treatment.
Methods
Sample and Setting
The authors performed an observational, cross-sectional study between January and December 2017 at Badajoz University Hospital in Spain. A total of 182 individuals with breast cancer who were undergoing chemotherapy with or without mAbs in the recruitment period were included in the study. All individuals were enrolled and invited to take part in the study during chemotherapy and mAb treatment scheduled in the hospital’s oncology unit. Participants were selected through a nonprobabilistic, intentional, or convenience analysis. All fulfilled the inclusion and exclusion criteria.
Exclusion criteria were being aged younger than 18 years; having a neurologic disorder or cognitive impairment that would impede them from participating in the evaluation; having previously received treatment for other types of primary cancers; having recurrent illnesses, secondary cancer, or a previous malignant illness; and having a previously diagnosed psychiatric disorder. All documents were completed face-to-face with a nurse in the oncology unit. Participants’ clinical data were collected from their electronic health record.
All participants provided informed consent. Permission was obtained from the Ethics in Clinical Investigation Committee of Badajoz. Confidentiality was maintained in accordance with the Declaration of Helsinki and current legislation.
Instruments
Participants completed the European Organisation for Research and Treatment of Cancer QOL Questionnaire–Core 30 (EORTC QLQ-C30) (Aaronson et al., 1993) and the EORTC QLQ–Breast Cancer (QLQ-BR23) (Sprangers et al., 1996), which had been translated into Spanish (Cull et al., 2002) and validated for use in Spain (Arraras et al., 2001, 2002). Participants were screened for depressive symptoms using the Beck Depression Inventory–II (BDI) (Beck et al., 1996).
The EORTC QLQ-C30 is a 30-item questionnaire assessing function and symptoms that affect QOL in individuals with cancer. It is subdivided into three subscales: global health status and QOL, functional, and symptom (Fayers et al., 2001). All subscales range in score from 0 to 100. Higher scores for functional subscale items represent a higher or healthier level of functioning, and higher scores for global health status and QOL represent higher QOL. However, higher scores for symptom subscale items represent a higher level of symptomatology and problems (Fayers & Bottomley, 2002).
The EORTC QLQ-BR23 is a supplemental questionnaire to the EORTC QLQ-C30 created specifically for individuals with breast cancer. It consists of 23 questions, which are subdivided into two subscales: functional (body image, future perspective, sexual enjoyment, and sexual functioning) and symptom (arm symptoms, breast symptoms, systemic therapy side effects, and upset by hair loss) (Fayers et al., 2001). All subscales range in score from 0 to 100. Higher scores for functional subscale items represent a higher or healthier level of functioning, whereas higher scores for symptom subscale items represent a higher level of symptomatology and problems.
The BDI (Beck et al., 1996) was translated into Spanish (Sanz et al., 2003); this tool assesses an individual’s symptoms of depression in the previous two weeks. Scores of 0–13 indicate no or minimal depression; scores of 14–63 indicate mild, moderate, or severe depression (Beck et al., 1996).
Statistical Analysis
The sociodemographic and clinical characteristics of the total number of enrolled participants were analyzed with descriptive statistics. Chi-square tests were used to assess the relationship between qualitative variables. A two-way multivariate analysis of variance (MANOVA) was carried out to analyze the joint influence of mAb treatment and depression on QOL. In addition, a one-way MANOVA was performed to study the influence of tumor staging on QOL. Both analyses were performed using the corresponding univariate methods (two-way ANOVA and one-way ANOVA, respectively) to study each item separately; Bonferroni post hoc analysis was used in the case of tumor staging. All statistical analyses were performed using IBM SPSS Statistics, version 22.0, and jamovi, version 1.2. For all analyses, the alpha level was set at p ≤ 0.05.
Results
Of the 182 individuals with breast cancer included in the study, 38 (all with HER2-positive disease) received treatment with mAbs in combination with a type of chemotherapy drug. Participant characteristics related to social, demographic, and clinical variables are presented in Table 1. By comparing two groups with 38 participants and 144 participants in the study of mAb treatment, and with 54 participants and 128 participants in the study of depression, a power of 0.777 and a power of 0.867, respectively, can be ensured to detect an effect size (Cohen’s d) of 0.5. 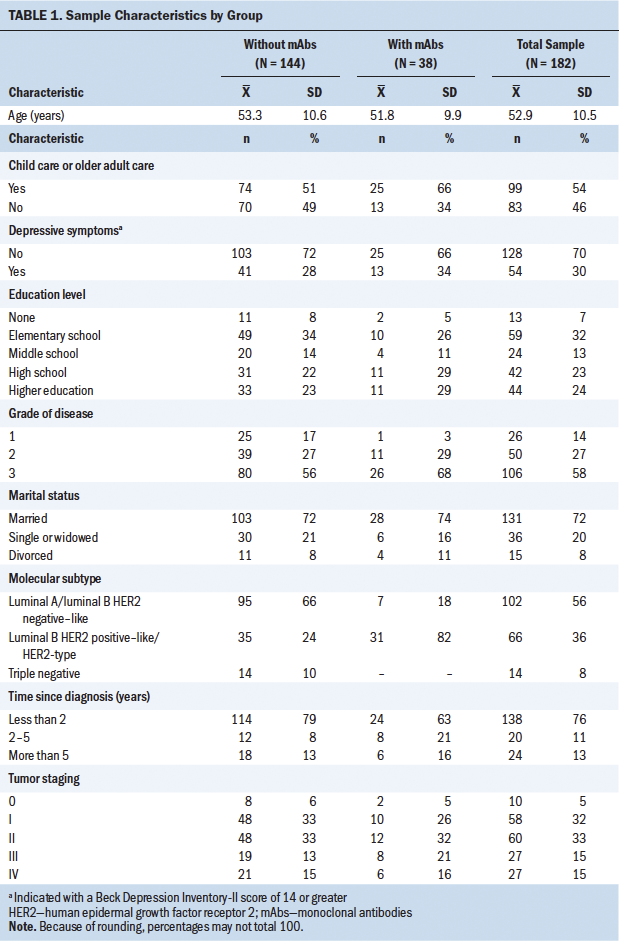
Trastuzumab (n = 33), pertuzumab (n = 18), and T-DM1 (n = 3) were the most prescribed mAbs of the sample, as well as denosumab (n = 2) in the case of bone metastasis. Chemotherapy treatment is based on the use of combination treatment regimens of taxanes, anthracyclines, alkylating agents, antimetabolites, and other antineoplastic agents, such as carboplatin.
The mean values on the BDI were 10.49 (SD = 8.6) for the group without mAbs and 10.66 (SD = 8.39) for the group with mAbs (p = 0.916). The mean values of the BDI were 10.66 (SD = 8.66) in participants without trastuzumab treatment and 9.91 (SD = 8.02) in participants with trastuzumab treatment (p = 0.647). No difference was found in BDI results related to child care or older adult care, or on number of children. There were also no differences found in terms of breast cancer staging, grade, or degree.
The combined influence of mAb treatment and depression on QOL was studied via two-way MANOVA. The results showed a significant interaction (p = 0.032) between both factors. After separately analyzing various QOL items (see Tables 2 and 3), it was observed that this interaction was related to the diarrhea item (p = 0.008). The use of mAbs together with depression was associated with particularly high scores for this item. Apart from this detail, no significant influence of mAb treatment was observed on QOL, with the global MANOVA result for this factor being p = 0.209. In the study of each variable, only a clearly significant partial influence was observed for the diarrhea variable (p = 0.001). 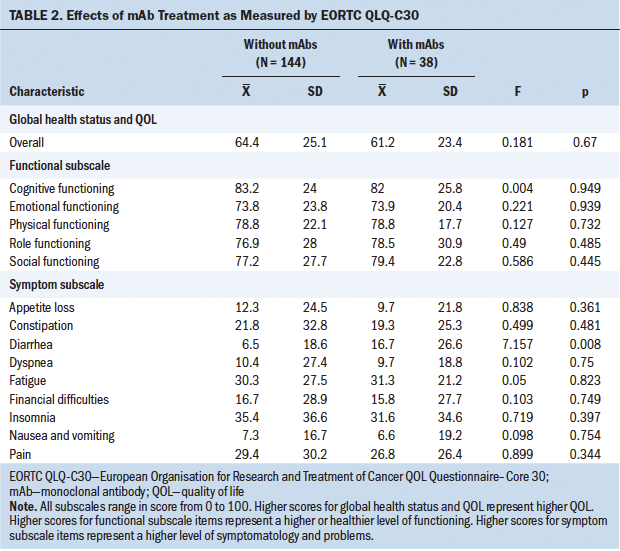
The opposite occurred with depression, whose influence was clearly significant (p < 0.001). In light of this result, the influence of depression on QOL was studied in more detail and separately for each item; these results are shown in Tables 4 and 5, where it can be seen that depressive symptoms have a negative and significant (p < 0 .001) influence on the majority of the QOL parameters studied. 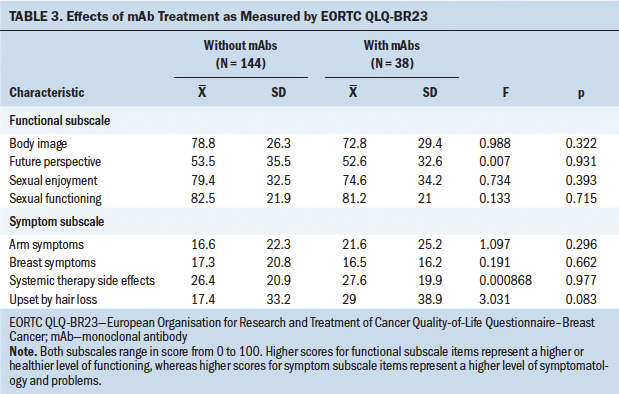
To determine the influence of tumor staging on QOL, a one-way MANOVA was performed, with a significant result (p = 0.023). Nevertheless, in the detailed study of the different items, significant values were obtained only for nausea and vomiting (p = 0.006) and future perspective (p = 0.042). Because post hoc comparisons (Bonferroni) for these two variables gave no significant results, they were not considered to be conclusive. 
In sum, the current authors found no clear partial influence of mAb treatment on QOL, except in the diarrhea variable, where both an interaction with depression and a partial influence on QOL were observed. The influence of depression is obvious and should not be attributed to tumor staging, the effects of which were not clear. 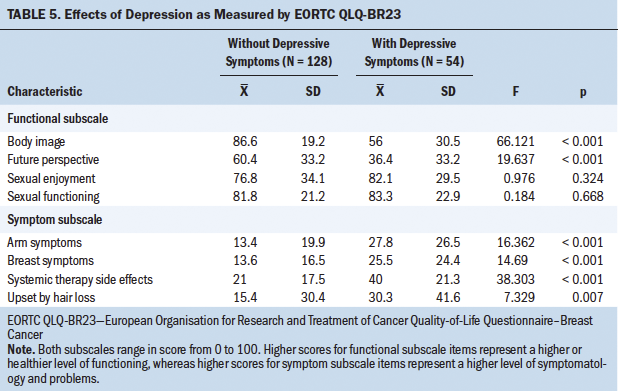
Discussion
The use of mAbs in individuals with breast cancer in the current study follows national and international recommendations for the treatment of this disease (European Society of Medical Oncology, n.d.; National Comprehensive Cancer Network, n.d.; Spanish Society of Medical Oncology, 2020).
In the current study, the QOL of individuals with breast cancer and depressive symptoms undergoing adjuvant therapy was explored. Almost 30% of the participants enrolled in the study and undergoing chemotherapy for breast cancer presented with symptoms of depressive disorders. In the group that was undergoing both chemotherapy and mAb treatment (23%), the percentage of depressive symptoms was 34%. The fact that participants were receiving both chemotherapy and mAb treatment did not imply an increase in the symptoms of depression. In the package insert for trastuzumab, the most frequently employed mAb (87%) in the current study, depression is reported as a common collateral side effect occurring in 1 or more of 10 patients (Spanish Agency for Medicines and Health Products, 2020).
Previous studies have reported conflicting results in regard to depressive disorders in breast cancer survivors who received differing adjuvant therapies. Chang et al. (2015) found that of 36,586 individuals with breast cancer, 1,342 (4%) were found to have depressive disorders. The use of trastuzumab was considered to be a risk factor for developing depressive disorders (Chang et al., 2015). Some studies show that chemotherapy worsens depression, as well as anxiety and quality of sleep. This leads to deterioration of QOL (Kim et al., 2019; Reich et al., 2008; Trinca et al., 2019). In a study by Trinca et al. (2019) that evaluated the impact of depressive symptoms on QOL, 36% of individuals with breast cancer presented with depressive symptoms and 16% with a major depressive episode.
Quality of Life
The global health status of the whole sample (N = 182) shows normal results, with a mean of 63.7 (SD = 24.7). No differences were found in QOL related to the type of treatment participants were undergoing; there were no differences between chemotherapy alone or chemotherapy plus mAbs. Only the diarrhea item was statistically significant in the group of participants undergoing mAb treatment. Diarrhea is a frequent adverse effect during treatment with trastuzumab, pertuzumab, and T-DM1. This relationship has been reported in the literature, as well as with the addition of nausea, vomiting, pain, dyspnea, insomnia, loss of appetite, and constipation (Schwartzberg et al., 2019).
The current study did not find a significant influence of mAb treatment on QOL; however, with the depression factor, its influence was clearly significant. Almost no studies address the relationship between chemotherapy use with or without mAbs, depressive symptoms, and QOL. In the study by Trinca et al. (2019), scores on various subscales of the EORTC QLQ-C30 and the EORTC QLQ-BR23 were also better in the group of patients on regimens containing mAbs, as compared to the group of patients undergoing chemotherapy treatment without mAbs.
The relationship between the use of several antineoplastic and immunomodulatory agents and QOL has also been examined in the literature. Schwartzberg et al. (2019) determined that most patients experience stable or improved health-related QOL scores (measured with the EORTC QLQ-C30 and -BR23) when receiving eribulin mesylate and trastuzumab as first-line therapy for locally recurrent or metastatic breast cancer. In the current study, the relationship between QOL and depressive symptoms during treatment with trastuzumab was analyzed. No differences were found in QOL or depression disorders in participants with or without trastuzumab treatment, which aligns with findings from Trinca et al. (2019). Nevertheless, others have reported improved QOL items in those treated with trastuzumab (Rugo et al., 2010; Schwartzberg et al., 2019).
Limitations
The current study is not without limitations. For example, the study lacked a representative sample because of its small size. In addition, the study did not compare QOL of the same individuals at several time points, but rather compared different participants with various times elapsed since diagnosis. The cross-sectional study design could have introduced some selection bias into the study population. However, every effort was made to include as many patients being seen in the study hospital as possible during the recruitment period. In future studies, the authors intend to expand the cohort, as well as to carry out longitudinal follow-up of the patients.
Implications for Practice
Nursing practitioners, as those responsible for administering therapeutic drugs, must know the benefits and the risks of new treatments. For example, mAbs can produce serious adverse reactions, which requires close monitoring of individuals who are on these therapeutic regimens.
In addition, nursing practitioners should be aware that mAbs are not contraindicated in individuals with breast cancer if depression is the concern. Although mAbs do not appear to increase depressive symptoms in individuals with breast cancer, some adverse effects, such as diarrhea, are common. Therefore, patients should be informed about their likelihood, and the appropriate recommendations should be made for their care. Overall, mAbs are a breakthrough in the treatment of breast cancer, improving prognosis and QOL. 
Conclusion
Results from the current study show that depression is closely related to QOL, but that the use of mAbs together with chemotherapy does not imply a loss in global QOL, although a higher incidence of symptoms such as diarrhea may occur. Taking into account the possible association between the use of mAbs and the appearance of depressive disorders as an adverse event, the authors did not find any important associations with these kinds of depressive symptoms in any of the study groups. No association was found between a greater incidence of depressive symptoms in the group of participants undergoing chemotherapy plus mAb treatment. Despite the small sample size, this may indicate that depressive symptoms in individuals with breast cancer receiving chemotherapy together with mAb treatment do not detrimentally affect QOL.
The authors gratefully acknowledge the oncology unit of Badajoz University Hospital for their collaboration in carrying out this study.
About the Author(s)
Macarena C. Cáceres, PhD, is an associate professor in the Department of Nursing at the University of Extremadura, Demetrio Pérez-Civantos, MD, PhD, EDIC, is an adjunct professor in critical care medicine in the Department of Biomedicine at the University of Extremadura and a consultant in intensive care medicine at Badajoz University Hospital, Jorge Guerrero-Martín, PhD, is an associate professor in the Department of Nursing at the University of Extremadura, Marta Nadal Delgado, MS, is a PhD student in the Psychology Unit of the Extremadura Oncology Association, Casimiro López-Jurado, RN, is an assistant professor in the Department of Nursing at the University of Extremadura, and Noelia Durán-Gómez, PhD, is an associate professor in the Department of Nursing at the University of Extremadura, all in Badajoz, Spain. This work was supported, in part, by grants from the Junta de Extremadura and the European Regional Development Fund (IB18101 and GR18045). Cáceres and Durán-Gómez contributed to the conceptualization and design and provided statistical support. Guerrero-Martín, Nadal Delgado, and Durán-Gómez completed the data collection. Cáceres, Pérez-Civantos, and Durán-Gómez provided the analysis. Cáceres, Pérez-Civantos, Nadal Delgado, López Jurado, and Durán-Gómez contributed to the manuscript preparation. Cáceres can be reached at mcaceres@unex.es, with copy to ONFEditor@ons.org. (Submitted November 2020. Accepted May 1, 2021.)
References
Aaronson, N.K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N.J., . . . Takeda, F. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(5), 365–376. https://doi.org/10.1093/jnci/85.5.365
Arraras, J.I., Arias, F., Tejedor, M., Pruja, E., Marcos, M., Martínez, E., & Valerdi, J. (2002). The EORTC QLQ-C30 (version 3.0) quality of life questionnaire: Validation study for Spain with head and neck cancer patients. Psycho-Oncology, 11(3), 249–256. https://doi.org/10.1002/pon.555
Arraras, J.I., Tejedor, M., Illaramendi, J.J., Vera, R., Pruja, E., Marcos, M., . . . Valerdi, J.J. (2001). The EORTC Breast Cancer Quality Questionnaire (QLQ-BR23): A psychometric study with Spanish patients. Psicología Conductual, 9(1), 81–97.
Beck, A.T., Steer, R.A., & Brown, G. (1996). Beck Depression Inventory–II. APA PsycTests. https://doi.org/10.1037/t00742-000
Bertucci, F., Finetti, P., Cervera, N., Esterni, B., Hermitte, F., Viens, P., & Birnbaum, D. (2008). How basal are triple-negative breast cancers? International Journal of Cancer, 123(1), 236–240. https://doi.org/10.1002/ijc.23518
Bower, J.E., Ganz, P.A., Irwin, M.R., Kwan, L., Breen, E.C., & Cole, S.W. (2011). Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29(26), 3517–3522. https://doi.org/10.1200/jco.2011.36.1154
Cáceres, M.C., Durán Gómez, N., Guerrero Martín, J., Pérez Civantos, D.V., Lemus, C., & Postigo Mota, S. (2017). Monoclonal antibodies: A new revolutionary therapy. Revista Rol de Enfermería, 40(7–8), 524–530.
Cáceres, M.C., Guerrero-Martín, J., Pérez-Civantos, D., Palomo-López, P., Delgado-Mingorance, J.I., & Durán-Gómez, N. (2019). The importance of early identification of infusion-related reactions to monoclonal antibodies. Therapeutics and Clinical Risk Management, 15, 965–977. https://doi.org/10.2147/TCRM.S204909
Chang, C.-H., Chen, S.-J., & Liu, C.-Y. (2015). Adjuvant treatments of breast cancer increase the risk of depressive disorders: A population-based study. Journal of Affective Disorders, 182, 44–49. https://doi.org/10.1016/j.jad.2015.04.027
Christopher, T., Gradishar, W.J., O’Shaughnessy, J.A., Bramsen, B., & Lurie, R.H. (2014). Clinical roundtable monograph: Effective management of quality of life in metastatic breast cancer. Clinical Advances in Hematology and Oncology, 12(2, Suppl. 4), 1–14.
Chow, S., Wan, B.A., Pidduck, W., Zhang, L., DeAngelis, C., Chan, S., . . . Chow, E. (2019). Symptom clusters in patients with breast cancer receiving radiation therapy. European Journal of Oncology Nursing, 42, 14–20. https://doi.org/10.1016/j.ejon.2019.07.004
Crane, T.E., Badger, T.A., Sikorskii, A., Segrin, C., Hsu, C.-H., & Rosenfeld, A.G. (2019). Trajectories of depression and anxiety in Latina breast cancer survivors. Oncology Nursing Forum, 46(2), 217–227. https://doi.org/10.1188/19.ONF.217-227
Cull, A., Sprangers, M., Bjordal, K., Aaronson, N., West, K., & Bottomley, A. (2002). EORTC quality of life group translation procedure (2nd ed). European Organisation for Research and Treatment of Cancer.
Eiger, D., Pondé, N.F., & de Azambuja, E. (2019). Pertuzumab in HER2-positive early breast cancer: Current use and perspectives. Future Oncology, 15(16), 1823–1843. https://doi.org/10.2217/fon-2018-0896
European Society of Medical Oncology. (n.d.). ESMO guidelines. https://www.esmo.org/guidelines
Fayers, P., Aaronson, N.K., Bjordal, K., Groenvold, M., Curran, D., & Bottomley, A. (2001). EORTC QLQ-C30 scoring manual. European Organisation for Research and Treatment of Cancer.
Fayers, P., & Bottomley, A. (2002). Quality of life research within the EORTC—The EORTC QLQ-C30. European Journal of Cancer, 38(Suppl. 4), 125–133. https://doi.org/10.1016/S0959-8049(01)00448-8
García-Aranda, M., & Redondo, M. (2019). Immunotherapy: A challenge of breast cancer treatment. Cancers, 11(12), 1822. https://doi.org/10.3390/cancers11121822
Gaston-Johansson, F., Fall-Dickson, J.M., Bakos, A.B., & Kennedy, M.J. (1999). Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Practice, 7(5), 240–247. https://doi.org/10.1046/j.1523-5394.1999.75008.x
Gold, M., Dunn, L.B., Phoenix, B., Paul, S.M., Hamolsky, D., Levine, J.D., & Miaskowski, C. (2016). Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. European Journal of Oncology Nursing, 20, 97–105. https://doi.org/10.1016/j.ejon.2015.06.003
Grabsch, B., Clarke, D.M., Love, A., McKenzie, D.P., Snyder, R.D., Bloch, S., . . . Kissane, D.W. (2006). Psychological morbidity and quality of life in women with advanced breast cancer: A cross-sectional survey. Palliative and Supportive Care, 4(1), 47–56. https://doi.org/10.1017/s1478951506060068
Jones, S.E. (2008). Metastatic breast cancer: The treatment challenge. Clinical Breast Cancer, 8(3), 224–233. https://doi.org/10.3816/CBC.2008.n.025
Kim, H.-J., Barsevick, A.M., Beck, S.L., & Dudley, W.N. (2012). Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: A secondary analysis. Oncology Nursing Forum, 39(1), E20–E30. https://doi.org/10.1188/12.ONF.E20-E30
Kim, H.-J., Barsevick, A.M., Tulman, L., & McDermott, P.A. (2008). Treatment-related symptom clusters in breast cancer: A secondary analysis. Journal of Pain and Symptom Management, 36(5), 468–479. https://doi.org/10.1016/j.jpainsymman.2007.11.011
Kim, J.H., Paik, H.-J., Jung, Y.J., Kim, D.-I., Jo, H.J., Lee, S., & Kim, H.Y. (2019). A prospective longitudinal study about change of sleep, anxiety, depression, and quality of life in each step of breast cancer patients. Oncology, 97(4), 245–253. https://doi.org/10.1159/000500724
Lee, L.J., Ross, A., Griffith, K., Jensen, R.E., & Wallen, G.R. (2020). Symptom clusters in breast cancer survivors: A latent class profile analysis. Oncology Nursing Forum, 47(1), 89–100. https://doi.org/10.1188/20.ONF.89-100
Li, H., Sereika, S.M., Marsland, A.L., Conley, Y.P., & Bender, C.M. (2020). Symptom clusters in women with breast cancer during the first 18 months of adjuvant therapy. Journal of Pain and Symptom Management, 59(2), 233–241. https://doi.org/10.1016/j.jpainsymman.2019.10.002
Miovic, M., & Block, S. (2007). Psychiatric disorders in advanced cancer. Cancer, 110(8), 1665–1676. https://doi.org/10.1002/cncr.22980
National Comprehensive Cancer Network. (n.d.). NCCN Guidelines. https://www.nccn.org/professionals/physician_gls/default.aspx
Patel, T.A., Ensor, J.E., Creamer, S.L., Boone, T., Rodriguez, A.A., Niravath, P.A., . . . Chang, J.C. (2019). A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Research, 21(1), 100. https://doi.org/10.1186/s13058-019-1186-0
Redig, A.J., & McAllister, S.S. (2013). Breast cancer as a systemic disease: A view of metastasis. Journal of Internal Medicine, 274(2), 113–126. https://doi.org/10.1111/joim.12084
Reich, M., Lesur, A., & Perdrizet-Chevallier, C. (2008). Depression, quality of life and breast cancer: A review of the literature. Breast Cancer Research and Treatment, 110(1), 9–17. https://doi.org/10.1007/s10549-007-9706-5
Rossi, L., Stevens, D., Pierga, J.-Y., Lerebours, F., Reyal, F., Robain, M., . . . Rouzier, R. (2015). Impact of adjuvant chemotherapy on breast cancer survival: A real-world population. PLOS ONE, 10(7), e0132853. https://doi.org/10.1371/journal.pone.0132853
Rugo, H., Brammer, M., Zhang, F., & Lalla, D. (2010). Effect of trastuzumab on health-related quality of life in patients with HER2-positive metastatic breast cancer: Data from three clinical trials. Clinical Breast Cancer, 10(4), 288–293. https://doi.org/10.3816/CBC.2010.n.037
Sanz, J., Perdigón, A.L., & Vázquez, C. (2003). The Spanish adaptation of Beck’s Depression Inventory-II (BDI-II): 2. Psychometric properties in the general population. Clínica y salud, 14(3), 249–280.
Schwartzberg, L., McIntyre, K., Wilks, S., Puhalla, S., O’Shaughnessy, J., Berrak, E., . . . Vahdat, L. (2019). Health-related quality of life in patients receiving first-line eribulin mesylate with or without trastuzumab for locally recurrent or metastatic breast cancer. BMC Cancer, 19(1), 578. https://doi.org/10.1186/s12885-019-5674-5
Slamon, D., Eiermann, W., Robert, N., Pienkowski, T., Martin, M., Press, M., . . . Crown, J. (2011). Adjuvant trastuzumab in HER2-positive breast cancer. New England Journal of Medicine, 365(14), 1273–1283. https://doi.org/10.1056/NEJMoa0910383
Slamon, D.J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., . . . Norton, L. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine, 344(11), 783–792. https://doi.org/10.1056/NEJM200103153441101
So, W.K.W., Law, B.M.H., Ng, M.S.N., He, X., Chan, D.N.S., Chan, C.W.H., & McCarthy, A.L. (2021). Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Medicine, 10(8), 2531–2565. https://doi.org/10.1002/cam4.3794
So, W.K.W., Marsh, G., Ling, W.M., Leung, F.Y., Lo, J.C.K., Yeung, M., & Li, G.K.H. (2010). Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. European Journal of Oncology Nursing, 14(1), 17–22. https://doi.org/10.1016/j.ejon.2009.07.005
Spanish Agency for Medicines and Health Products. (2020). Herceptin® [Package insert]. https://cima.aemps.es/cima/dochtml/ft/00145001/FT_00145001.html
Spanish Society of Medical Oncology. (2020). SEOM clinical guidelines. https://seom.org/publicaciones/guias-clinicas/105418-guias-clinicas-seom
Sprangers, M.A., Groenvold, M., Arraras, J.I., Franklin, J., te Velde, A., Muller, M., . . . Aaronson, N.K. (1996). The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. Journal of Clinical Oncology, 14(10), 2756–2768. https://doi.org/10.1200/JCO.1996.14.10.2756
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Trinca, F., Infante, P., Dinis, R., Inácio, M., Bravo, E., Caravana, J., . . . Marques, S. (2019). Depression and quality of life in patients with breast cancer undergoing chemotherapy and monoclonal antibodies. Ecancermedicalscience, 13, 937. https://doi.org/10.3332/ecancer.2019.937
Whisenant, M., Wong, B., Mitchell, S.A., Beck, S.L., & Mooney, K. (2020). Trajectories of depressed mood and anxiety during chemotherapy for breast cancer. Cancer Nursing, 43(1), 22–31. https://doi.org/10.1097/NCC.0000000000000670
Wuerstlein, R., & Harbeck, N. (2017). Neoadjuvant therapy for HER2-positive breast cancer. Reviews on Recent Clinical Trials, 12(2), 81–92. https://doi.org/10.2174/1574887112666170202165049

