Systematic Review of Cognitive Impairment in Colorectal Cancer Survivors Who Received Chemotherapy
Problem Identification: Cognitive impairment is a common and troublesome side effect experienced by many cancer survivors. It can have a significant impact on survivors’ ability to function and enjoy a high quality of life. However, most cognitive impairment research has focused on breast cancer survivors, despite the high rates of colorectal cancer and the toxicity of treatment agents in some colorectal cancer chemotherapeutic regimens, which have been linked to cognitive impairment. This review provides a novel synthesis of what is known about cognitive impairment in colorectal cancer survivors.
Literature Search: CINAHL®, Cochrane Library, Embase®, PsycINFO®, and PubMed® were systematically searched by a health sciences librarian.
Data Evaluation: Data were extracted across studies; findings about the prevalence, severity, and correlates of cognitive impairment were synthesized.
Synthesis: Across findings from 26 articles representing 24 independent studies, 13%–57% of participants had cognitive impairment. Potential demographic, physiologic, and psychological correlates of cognitive impairment were identified.
Implications for Practice: Findings indicate a need to focus research and patient assessments on early identification of risk factors, assessing for existing cognitive deficits and testing interventions to decrease cognitive impairment in colorectal cancer survivors.
Jump to a section
Cognitive impairment is experienced by as many as 75% of cancer survivors who have received chemotherapy (Janelsins et al., 2014). It is a complex treatment-related side effect experienced by cancer survivors both during chemotherapy (Hess et al., 2015; Moore et al., 2019) and more than 20 years after chemotherapy (Koppelmans et al., 2012; Stouten-Kemperman et al., 2015; Von Ah & Tallman, 2015). Cognitive impairment affects several domains, including attention/concentration, executive function, visuospatial ability, verbal/language skills, and memory (Kanaskie, 2012). These deficits are problematic because they may affect individuals’ abilities to carry out daily activities, experience social connectedness (Selamat et al., 2014), adhere to treatment plans (Bender et al., 2014), and achieve a high quality of life (Lycke et al., 2019). Cognitive impairment may be measured using self-report (e.g., questionnaires used to assess cancer survivors’ perceptions of their own cognitive function, including the Functional Assessment of Cancer Therapy–Cognitive Function [FACT-Cog]) (Wagner et al., 2004) and objective measures (a battery of neuropsychological assessments is the gold standard for assessing cognitive impairment in cancer survivors) (Wefel et al., 2011).
Although survivors across various cancer types report cognitive impairment (Lindner et al., 2014), previous studies focus primarily on breast cancer survivors (Bray et al., 2018). Similar to breast cancer, colorectal cancer has a high survival rate, and a large percentage of survivors receive chemotherapy as part of their treatment. Colorectal cancer is the third most common cancer worldwide, with 149,500 newly diagnosed individuals anticipated in 2021 (Siegel et al., 2021). Colorectal cancer survivors may receive surgery, radiation therapy, immunotherapy, targeted therapy, and/or chemotherapy (National Comprehensive Cancer Network, 2021a, 2021b). Specifically, colorectal cancer survivors with lymph node involvement or metastasis (stages III and IV), high-risk stage II colon cancer, and stage II rectal cancer (National Comprehensive Cancer Network, 2021a, 2021b) account for about 22%–57% of colorectal cancer diagnoses (National Cancer Institute Surveillance, Epidemology, and End Results Program, n.d.). These survivors typically receive chemotherapy, specifically 5-fluorouracil–based or platinum-based regimens (National Comprehensive Cancer Network, 2021a, 2021b), which are linked to cognitive impairment (McDougall et al., 2014). Because little research focuses on the cognitive impairment experience in colorectal cancer survivors, a detailed understanding of cognitive impairment in this population is needed to prevent and treat this deleterious side effect. This systematic review synthesizes what is known about cognitive impairment in colorectal cancer survivors.
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Moher et al., 2009) was used to guide this systematic review. This systematic review protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO #CRD42020152759).
Search
A health sciences librarian (J.L.C.) searched five databases (CINAHL®, Cochrane Library, Embase®, PsycINFO®, and PubMed®) from their dates of inception through October 3, 2019. The search combined subject headings and keywords for three main concepts: colorectal cancer, chemotherapy, and cognition. No limits, with the exception of Embase, were applied to the search. In Embase, the search was limited to articles only. Results were imported into EndNote, duplicates were removed, and the remaining studies were placed into Covidence, a systematic review software, to complete the review process.
Selection of Studies
After completing the initial search, the research team (A.L.B., A.P., R.H., T.C.G., and Y.-N.C.) performed article screening. Two of the reviewers (Y.-N.C. and T.C.G.) independently performed the title/abstract screening and full-text screening for articles that met inclusion criteria. Discrepancies were resolved by a third reviewer (A.L.B., A.P., and R.H.) not involved in the respective discrepancy. Inclusion criteria included the following:
• Published in English
• Observational study
• Participants aged 18 years or older
• Participants with a colon, rectal, or colorectal cancer diagnosis
• Assessment of cognitive function during or after completing chemotherapy
Articles that included any assessment method of cognitive impairment were included. Studies with only a baseline measure of cognitive function prior to the initiation of chemotherapy were excluded. Interventional studies, qualitative studies, case reports, and published abstracts were also excluded.
Data Extraction
The data were extracted into a Microsoft® Word® template to capture research purpose, study location, design, sample characteristics, sample size, instruments used to measure cognition, data collection time points, cognitive function outcomes, conclusions, and study strengths and weaknesses. For studies sampling colorectal cancer and other cancer diagnoses, data were extracted solely from colorectal cancer survivors. In cases where results included a mixed sample of colorectal cancer survivors who received chemotherapy and those who did not, results were extracted of colorectal cancer survivors who received chemotherapy. In addition, if studies compared cognitive impairment between colorectal cancer survivors who received chemotherapy and those who did not, these between-group comparison results were extracted. One reviewer (Y.-N.C.) extracted all data; 100% of extracted data were reviewed by a second reviewer (T.C.G.) for accuracy.
Data Analysis
The authors reviewed and discussed data across studies to synthesize findings. Data specific to impaired cognitive function prevalence, severity, and correlates emerged as distinct reporting categories across studies, and thus became the structure to organize and synthesize findings. Because of the diverse cognitive impairment measures used across studies, the results were first categorized based on the type of measure (patient-reported versus neuropsychological assessment) to understand how cognitive impairment was defined and operationalized. The data were further categorized by the time points cognitive impairment was measured in relation to when the patient had chemotherapy (prechemotherapy versus during chemotherapy versus postchemotherapy). In terms of correlates of cognitive impairment, all correlates were identified across studies and further categorized into demographic, physiologic, and psychological correlates.
Quality Assessment
The quality of the included articles was evaluated separately by reviewers (Y.-N.C., T.C.G., R.H., and A.L.B.) using the Mixed Methods Appraisal Tool (MMAT), version 2011 (Pluye et al., 2011). The MMAT’s criteria assess methodology quality, such as research question, data collection and sampling strategy, sample representation, measures, and response rate (Pluye et al., 2011). A study’s quality was determined by calculating the percentage of MMAT criteria each study met (Pluye et al., 2011). Discrepancies were discussed to reach consensus.
Results
A total of 2,217 articles were identified in the initial search from the five databases (see Figure 1). After removing duplicates, 1,728 articles were screened for title and abstract. Following initial screening, 98 articles were retrieved for full-text review. Ultimately, 26 articles representing 24 independent studies met the eligibility criteria for inclusion in this review (see Table 1). The articles were published between 2006 and 2019 in 12 countries. Across studies, sample sizes ranged from 22 to 72,374. Most studies’ samples were more than 50% male. The mean age of participants ranged from 51 to 75 years, and the median age ranged from 53 to 78 years. Most studies did not report participants’ race and ethnicity; of those that did, most participants were White. In 14 articles, researchers employed a longitudinal design; 10 used a cross-sectional design, and 2 used a cohort design. One study used the National Cancer Institute Surveillance, Epidemiology and End Results Program cancer registries and Medicare-linked databases; as a result, International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to determine cognitive impairment (Du et al., 2013). Study quality ranged between 25% and 100% using the MMAT. 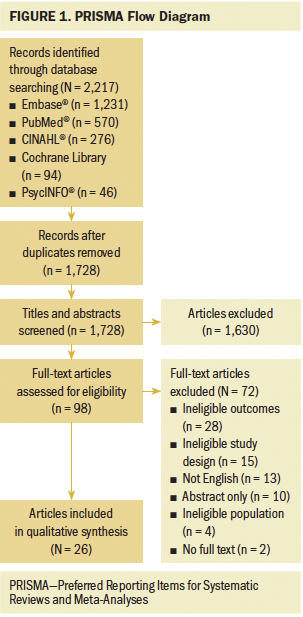
Prevalence of Cognitive Impairment
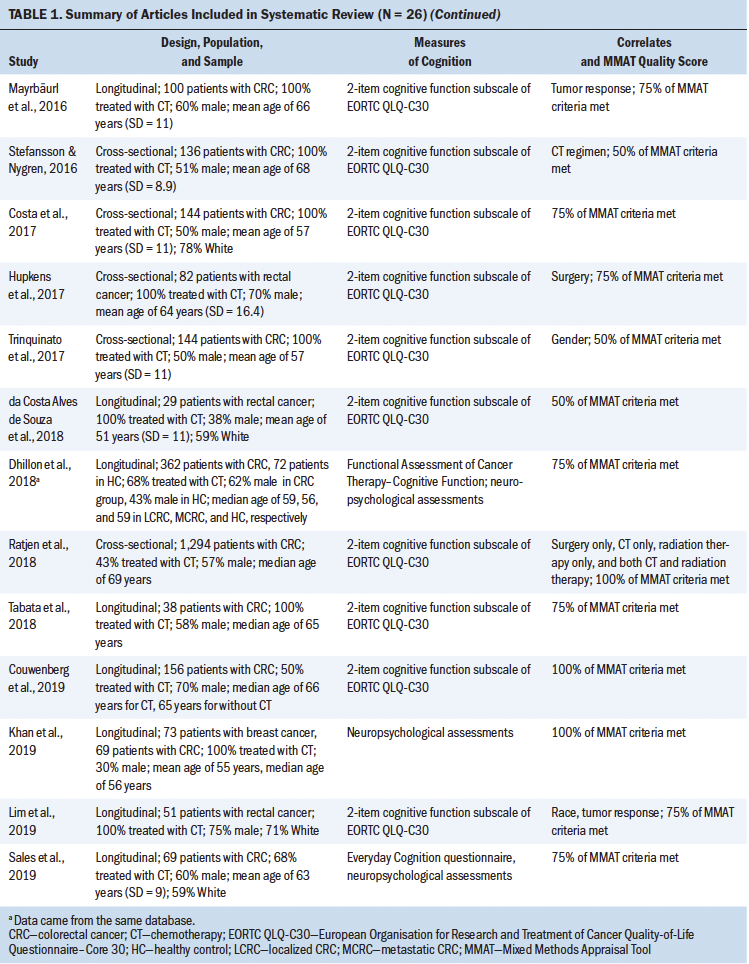
To determine the effects of chemotherapy on cognitive impairment in colorectal cancer survivors, researchers assessed the percentage of the study sample experiencing cognitive impairment at various times across the treatment continuum. Six studies measured the prevalence of cognitive impairment ranging from before beginning chemotherapy to as long as two years after its initiation (Aaldriks et al., 2013; Cruzado et al., 2014; Dhillon et al., 2018; Galica et al., 2012; Khan et al., 2019; Vardy et al., 2015). See Table 2 for details of cognitive impairment definitions, prevalence, and time points as measured in each study. Prior to chemotherapy, the prevalence of cognitive impairment was as low as 8% and as high as 66%. After the initiation of chemotherapy, the prevalence ranged from 13.3% to 57% at different time points during treatment. 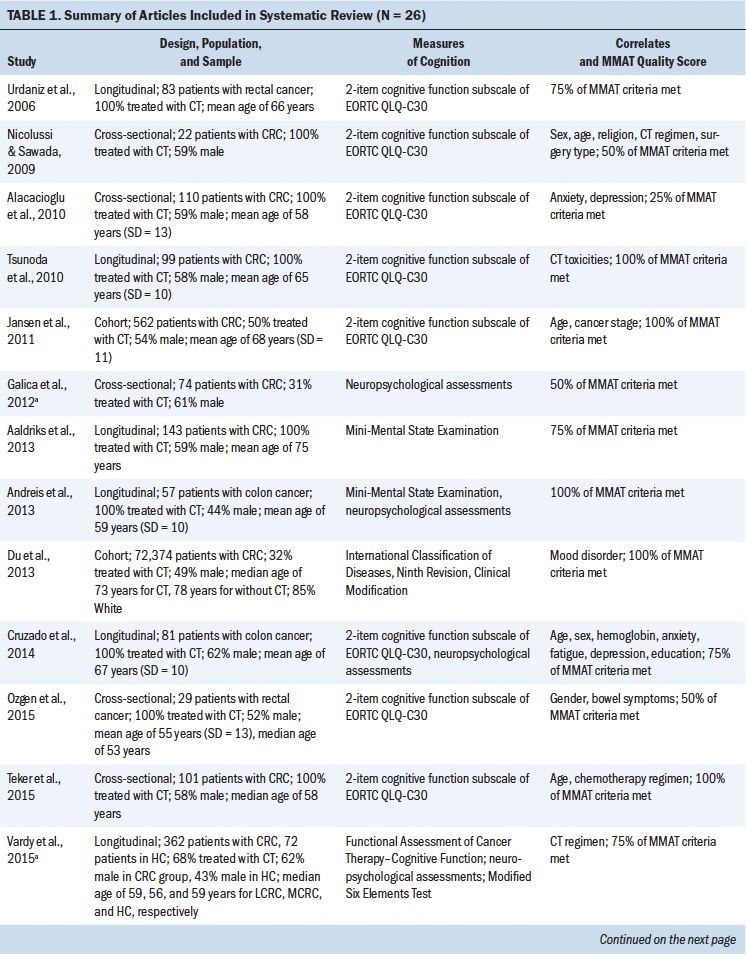
The prevalence of cognitive impairment has also been examined among survivors diagnosed with different stages of colorectal cancer. Two studies included a sample with mixed cancer stages (Aaldriks et al., 2013; Khan et al., 2019), and two other studies recruited only patients with localized colorectal cancer (stage II or III) (Cruzado et al., 2014; Galica et al., 2012). 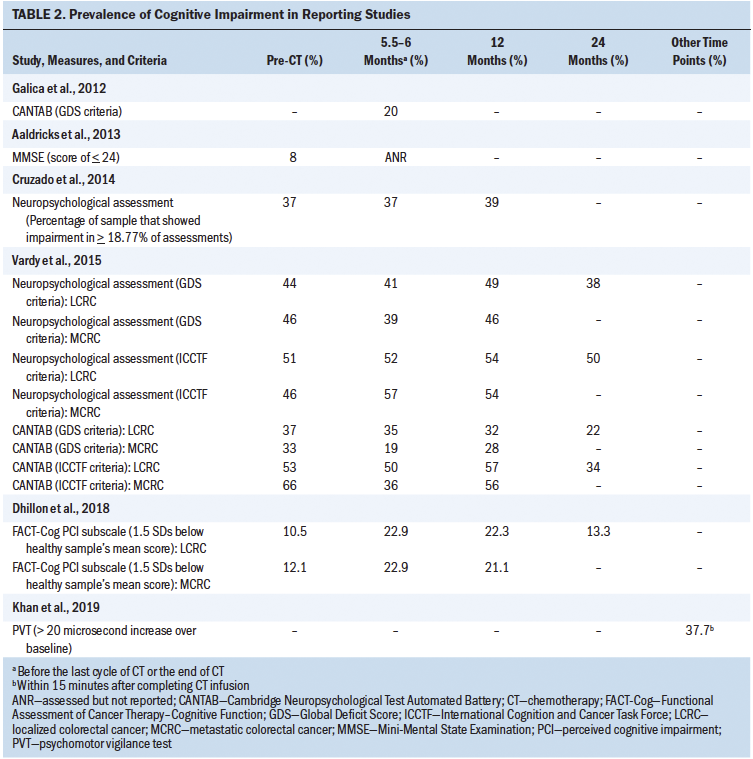
Notably, another two studies reported differences in cognitive impairment prevalence between participants with localized disease and recurrent or metastatic disease (Dhillon et al., 2018; Vardy et al., 2015); at a given time point, these differences varied from as little as 0% at 6 months (Dhillon et al., 2018) and 12 months postbaseline (Vardy et al., 2015) to as much as 16% at 6 months postbaseline (Vardy et al., 2015).
Comparisons between cancer survivors treated and not treated with chemotherapy are critical in cognitive impairment research because, although it was initially thought that cognitive impairment resulted from chemotherapy, it is evident that people not treated with chemotherapy also experience cognitive impairment. Two studies found no significant difference in the prevalence of reported cognitive impairment between survivors treated and not treated with chemotherapy at prechemotherapy and 24 months post-treatment (Dhillon et al., 2018; Vardy et al., 2015). However, Dhillon et al. (2018) found that at six months postinitiation of treatment, significantly more participants who had received chemotherapy (22.9%) experienced cognitive impairment compared to those who had not received chemotherapy (10.1%) (p = 0.019). Similarly, Vardy et al. (2015) found that at 12 months postinitiation of treatment, significantly more participants who had received chemotherapy (57%) reported experiencing cognitive impairment than those who had not received chemotherapy (40%, p = 0.027). Taken together, these results indicate that survivors who do and who do not receive chemotherapy may experience cognitive impairment; however, during treatment, cognitive impairment may be worse among those who do receive chemotherapy.
Cognitive Impairment Severity
In addition to assessing whether colorectal cancer survivors experience cognitive impairment, examinations of cognitive impairment severity (i.e., the extent of impairment) were found in the existing literature. All but one study included in this review did not measure cognitive impairment severity (Du et al., 2013). Of note, cognitive impairment severity has been assessed both subjectively, through self-report measures, and objectively, through neuropsychological assessments.
Self-report measures: Self-report measures varied across studies, including the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (EORTC QLQ-C30), FACT-Cog, and Everyday Cognition questionnaire, complicating synthesis. Eighteen of twenty-six studies (69%) used the two-item cognitive function subscale from the EORTC QLQ-C30. The EORTC QLQ-C30 cognitive function subscale does not contain a cut point that would identify someone as cognitively impaired. Rather, this measure has cognitive function subscales that range from 0 to 100; higher scores indicate higher cognitive function. Across studies, the mean range of EORTC QLQ-C30 cognitive function subscale scores was 71.9–96.8 prechemotherapy, 76–88.3 during chemotherapy, 70.2–93.9 at the end of chemotherapy, and 41.5–96.5 postchemotherapy.
Changes in patient-reported cognitive function throughout chemotherapy treatment were assessed in five studies (Cruzado et al., 2014; Lim et al., 2019; Mayrbäurl et al., 2016; Tabata et al., 2018; Tsunoda et al., 2010); one study found that cognitive function decreased significantly after chemotherapy (Mayrbäurl et al., 2016), one found significant decreases during chemotherapy (Lim et al., 2019), and the remaining three observed nonsignificant differences (Cruzado et al., 2014; Tabata et al., 2018; Tsunoda et al., 2010).
Findings are also mixed in regard to differences in cognitive impairment severity between survivors who do or do not receive chemotherapy. For example, two studies found no significant differences in cognitive function that would indicate differences in cognitive impairment severity (Jansen et al., 2011; Ratjen et al., 2018). Another study reported no differences at 3, 12, 18, and 24 months post–treatment initiation; however, this study found worse cognitive function among those receiving chemotherapy at 6 months post–treatment initiation (Couwenberg et al., 2019). Similarly, Dhillon et al. (2018) reported that although cognitive function was similar between groups prior to treatment, cognitive impairment was significantly worse at 6, 12, and 24 months post–treatment initiation among those who had received chemotherapy compared to those who had not (p < 0.001, p = 0.038, p = 0.045). In summary, findings across studies indicate that, although cognitive impairment may be experienced by survivors who do and do not receive chemotherapy, it may be more severe among those who receive chemotherapy.
Neuropsychological assessments: Eight studies used objective neuropsychological assessments, such as the Mini-Mental State Examination, to assess cognitive impairment. These assessments are administered by a trained professional, with some requiring the assessors to possess certain credentials, education, and training and to follow a formalized assessment procedure. The various assessments involve different tasks, which are evaluated with a specialized scoring criterion. Two studies used neuropsychological assessments to assess whether cognitive function changed during the course of cancer treatment; no significant changes were found (Aaldriks et al., 2013; Andreis et al., 2013). In addition, three studies administered neuropsychological assessments to compare cognitive impairment differences between participants who had received chemotherapy and those who had not; again, no significant differences were noted (Galica et al., 2012; Sales et al., 2019; Vardy et al., 2015). No significant findings arose when using objective neuropsychological assessments, whereas mixed findings resulted from using patient-reported questionnaires. These findings indicate that, although no changes were identified in objective assessments, cancer survivors might actually be experiencing cognitive impairment, highlighting a need for nurses to use self-report measures.
Domains of cognitive impairment: Various neuropsychological assessments (e.g., psychomotor vigilance test, Trail Making Test, clock-drawing test, digit symbol test, Rey Auditory Verbal Learning Test) have been used to measure specific domains of cognitive function. Domains of cognitive function have been classified as attention/concentration, executive function, visuospatial ability, and memory, based on Lezak et al. (2004) and McGinty et al. (2014). It is helpful to examine each domain because, although changes in overall cognitive function may not be measurable, significant changes in specific domains of cognitive function may be identified. For example, two studies found significant decreases in attention/concentration from before, to during, to after chemotherapy (Andreis et al., 2013; Khan et al., 2019). Cruzado et al. (2014) identified significant declines in immediate and delayed memory from prechemotherapy, to during chemotherapy, to postchemotherapy; another study observed significant improvements in immediate memory from prechemotherapy, to the end of chemotherapy, to postchemotherapy (Andreis et al., 2013). Results from one study indicated a significant decline in executive function among participants who had received chemotherapy compared to those who had not (Sales et al., 2019). Cruzado et al. (2014) identified significant improvements in executive function from prechemotherapy to during chemotherapy. No changes in visuospatial ability and verbal/language skills were identified in any of the studies reviewed. Neuropsychological assessments of overall cognitive function did not identify any changes in colorectal cancer survivors who had received chemotherapy; however, across studies, some changes in the specific domains of attention/concentration, executive function, and memory have been identified and warrant a more detailed understanding.
Correlates of Cognitive Impairment
Identifying correlates of cognitive impairment may lead to better and earlier assessment, identification of high-risk individuals with colorectal cancer, and more opportunities for prevention and treatment. Various demographic, physiologic, and psychological factors have been explored as potential correlates of cognitive impairment.
Demographic correlates: Conflicting findings were found in regard to sex/gender. Two studies reported that females had significantly worse cognitive function compared to males after receiving chemotherapy (p = 0.001, p = 0.002) (Nicolussi & Sawada, 2009; Trinquinato et al., 2017), whereas two other studies found no association between sex/gender and cognitive function (Cruzado et al., 2014; Ozgen et al., 2015).
Inconsistent findings were also reported in regard to the relationship between age and cognitive function. Two studies reported no relationships between age and cognitive function (Nicolussi & Sawada, 2009; Teker et al., 2015); one study found that older age was correlated with worse cognitive function (p = 0.001) (Cruzado et al., 2014), and another reported that participants aged younger than 70 years who had received chemotherapy had worse cognitive function than participants who had not received chemotherapy, but these differences were not present in participants aged older than 70 years (Jansen et al., 2011).
One study identified that lower education levels were associated with worse cognitive function (p = 0.001) (Cruzado et al., 2014). Related to race, one study reported significantly lower cognition scores for Asian individuals compared to White individual during the third week (p = 0.015) and at the end of chemotherapy and radiation therapy (p = 0.032); however, these differences were not observed during the first week of chemotherapy and radiation therapy (Lim et al., 2019). One study examined the relationships between religion and cognitive function and reported no association (Nicolussi & Sawada, 2009).
Findings are mixed in regard to sex/gender and cognitive function, and age and cognitive function. In addition, no relationship was found between religion and cognitive function. Only two studies tested sociodemographic variables (i.e., education, Asian race) (Cruzado et al., 2014; Lim et al., 2019); consequently, the results should be interpreted conservatively.
Physiologic correlates: Three studies compared cognitive function between participants receiving different various 5-fluorouracil–based chemotherapy regimens (Nicolussi & Sawada, 2009; Stefansson & Nygren, 2016; Teker et al., 2015). No significant differences were found, except that cognitive function was significantly worse in participants treated with FOLFOX (leucovorin, 5-fluorouracil, and oxaliplatin) as compared to those treated with 5-fluorouracil only, FUFA (5-fluorouracil and leucovorin), or 5-fluorouracil and cisplatin (p = 0.001) (Nicolussi & Sawada, 2009). Another study compared participants who received 5-fluorouracil to those who received 5-fluorouracil or capecitabine only and an oxaliplatin regimen, finding a significantly higher prevalence of cognitive impairment among those treated with 5-fluorouracil at 12 and 24 months (p = 0.017) (Vardy et al., 2015). When comparing cognitive function with the treatment intent of individuals with colorectal cancer, one study observed significantly lower cognitive function in participants undergoing adjuvant, or curative, chemotherapy as compared to those undergoing palliative chemotherapy (p = 0.011) (Teker et al., 2015). Another study found that participants who had received both chemotherapy and radiation therapy had nonsignificant higher odds of reporting lower cognitive function as compared to those who had not (Ratjen et al., 2018). In regard to surgical intervention, one study found significantly worse cognitive function in participants who underwent total mesorectal excision rather than a watch-and-wait policy, following neoadjuvant chemotherapy and radiation therapy (p = 0.02) (Hupkens et al., 2017). Another study found no significant differences between participants who underwent different types of surgery (Nicolussi & Sawada, 2009).
Mixed results were found in regard to the relationship between tumor response and cognitive function. Lim et al. (2019) found no difference in cognitive function between good and poor tumor responses across the treatment continuum. In contrast, another study reported significantly worse cognitive function in participants with progressive disease than those with stable disease or (partial) remission (p = 0.017) (Mayrbäurl et al., 2016). In regard to cancer stage, one study found no significant differences in cognitive function between participants with stage II disease as compared to those with stage III disease (Jansen et al., 2011). One study examined the association between cognitive function and hemoglobin, finding no significant association (Cruzado et al., 2014).
One study found that cognitive function significantly increased from prechemotherapy to postchemotherapy in participants with grade 0 and 1 toxicities, as classified by the National Cancer Institute (1999) Common Toxicity Criteria; however, participants with grade 2 and 3 toxicities did not see an increase in cognitive function (p < 0.0001) (Tsunoda et al., 2010). Other studies examined cognitive function in relation to bowel urgency symptoms, fecal soiling, anorectal manometry, and fatigue and reported no significant relationships (Cruzado et al., 2014; Ozgen et al., 2015).
Taken together, findings indicate that cognitive impairment may be worse among patients who are treated with FOLOX, those treated with 5-fluorouracil compared to capecitabine only, those undergoing adjuvant chemotherapy, those who receive chemotherapy and radiation therapy, those who undergo total mesorectal excision as compared to those who take a watch-and-wait approach, and those who have higher grades of toxicities. However, none of these findings were prominent across studies; thus, these correlates may serve only as potential variables for nurses to assess, better understand, and further explore in future research.
Psychological correlates: Alacacioglu et al. (2010) found significantly worse cognitive function in participants with anxiety or depression than those without anxiety or depression (p < 0.0001), whereas Cruzado et al. (2014) found no relationships between anxiety or depression and cognitive function. In another study, mood disorder was identified as significantly moderating the relationship between chemotherapy and drug-induced dementia, which was classified as a type of cognitive impairment (Du et al., 2013). Because of contradictory findings, no conclusions can be drawn about the associations of anxiety and depression with cognitive impairment from this body of literature.
Discussion
This review provides a novel synthesis of what is known about cognitive impairment in colorectal cancer survivors treated with chemotherapy. Numerous domains of cognitive function have been assessed using a variety of measures (objective and subjective) at several time points in the cancer treatment continuum. Across studies, 13%–57% of colorectal cancer survivors experienced cognitive impairment after the initiation of chemotherapy. This is similar to research reporting a cognitive impairment prevalence of 17%–75% among survivors of various cancer types (Myers, 2009). The range of cognitive impairment was variable across studies, possibly because studies did not use consistent measures. For example, Vardy et al. (2015) applied two different scoring criteria (Global Deficit Score criteria and International Cognition and Cancer Task Force criteria) and found different prevalence rates using the same measures. In addition, Dhillon et al. (2018) defined cognitive impairment as 1.5 standard deviations below the healthy sample’s mean score, whereas Van Dyk et al. (2020) suggested a cutoff score for the 18-item and 20-item perceived cognitive impairment subscales after a sensitivity and specificity estimation. This review consequently illuminates the difficulties in accurately capturing cognitive impairment prevalence: lack of standardized measures, criteria, and cutoff scores.
Cognitive function may not be accurately captured because a majority of the included studies focused on assessing quality of life using the EORTC QLQ-C30. The EORTC QLQ-C30 includes a cognitive function subscale, which contains only two items referring to the attention and memory domains: item 20—“Have you had difficulty in concentrating on things, like reading a newspaper or watching television?”—and item 25—“Have you had difficulty remembering things?” (European Organisation for Research and Treatment of Cancer, n.d.). Because the EORTC QLQ-C30 cognitive function subscale measures a relatively narrow domain of cognitive function, the accuracy of the findings should be interpreted cautiously and may underestimate survivors’ experiences.
This review identifies that the prevalence of cognitive impairment, as assessed by subjective self-report measures, is relatively greater than that measured by objective measures. This inconsistency may be attributable to the use of cognitive function measures and the variation in cognitive impairment definitions and domains measured across studies. It highlights the necessity of including both self-report and objective measures when assessing cognitive impairment in cancer survivors. Survivors may score within range on a neuropsychological assessment, yet subjectively be experiencing cognitive impairment. The International Cognition and Cancer Task Force endorses using methods such as neuroimaging (Deprez et al., 2018) and neuropsychological assessments (Wefel et al., 2011) to assess cognitive impairment. Neuroimaging has been used in cognitive neuroscience to understand the etiologies of other forms of cognitive impairment (e.g., attention-deficit/hyperactivity disorder, Alzheimer disease, schizophrenia). In addition, prior studies incorporated this method in cognitive impairment studies of patients with breast cancer. However, to the best of the current authors’ knowledge, neuroimaging has been used in only one cognitive impairment study involving colorectal cancer survivors. The study used diffusion tensor imaging and found no significant differences in frontal subcortical white matter between survivors who had and had not received chemotherapy (Sales et al., 2019).
Because of the diverse measures employed across studies, methods of previous research were used in this review to organize the instruments into five cognitive function domains (McGinty et al., 2014). The findings indicated mixed results in changes related to attention/concentration, executive function, and memory, whereas no changes were found in visuospatial ability and verbal/language skills among survivors after initiating chemotherapy. These findings aligned with a meta-analysis by Hodgson et al. (2013) of 13 studies with a control group, which indicated that chemotherapy had a significantly negative impact on memory and no significant effect on visuospatial ability. However, different from the current review findings, Hodgson et al. (2013) found a significant negative effect of chemotherapy on executive function and verbal/language skills but no effect on attention/concentration. In addition, another meta-analysis of 44 studies found that impaired memory and attention were presented only in cross-sectional studies (Lindner et al., 2014). However, these studies included a more diverse sample, mainly focusing on breast cancer, testicular cancer, and lymphoma survivors (Hodgson et al., 2013; Lindner et al., 2014), whereas the current review focuses exclusively on colorectal cancer survivors. The heterogeneity in neuropsychological assessment tools and measures used in this review highlights a need for future studies to use common measures to better understand cognitive impairment in this population.
Cognitive impairment is hypothesized to be caused by inflammation, genetics, stress response, and/or central nervous system neurotoxicity of antineoplastic agents (Chung et al., 2018). Although etiology was beyond the scope of this project, previously studied correlates of cognitive impairment were thoroughly examined. This review provides evidence to further explore some potential correlates of cognitive impairment risk in survivors with colorectal cancer, including the following:
• Sociodemographic variables
• Higher level of treatment toxicity
• FOLFOX treatment regimen
• Surgery following chemotherapy
• Receiving chemotherapy and radiation therapy
In addition, the current review found mixed results on the correlation between older age and cognitive function. Similar results were found in a prior meta-analysis reporting no effects of age on cognitive impairment (Hodgson et al., 2013). However, considering the variance of prevalent age across cancer types and the research gap identified in older adults with cancer (Loh et al., 2016), the relationship between age and cognitive impairment needs to be further explored.
Mixed results between emotional distress and cognitive function emerged in this review. Consequently, it is not possible to make conclusions about the relationship between cognitive impairment and emotional distress among survivors of colorectal cancer. However, methodologic differences between studies—some used subjective measures and others used objective measures—may contribute to conflicting results. Hermelink et al. (2007) found that subjective cognitive function is significantly correlated with anxiety and depression. Additional research is needed to better understand the relationship between emotional distress and cognitive impairment. Identifying correlates will be a helpful step toward understanding risk factors, prevention, and treatment for cognitive impairment. In addition, it also highlights the necessity of including these correlates as covariates when studying cognitive impairment to precisely assess the extent of the cognitive impairment problem in the colorectal cancer population.
Cognitive impairment may affect various aspects of cancer survivors’ lives, such as daily and social activities, and personal and professional roles (Selamat et al., 2014). Although diverse results were found across studies, cognitive impairment does exist in colorectal cancer survivors receiving chemotherapy and beyond. However, no standardized neuropsychological assessment is conducted among cancer survivors planning to receive chemotherapy. Results from the current study raise an alert for recommending that healthcare providers regularly screen for cognitive impairment from the initiation of chemotherapy using screening questions, patient-reported questionnaires, and/or neuropsychological assessments; once these screenings are completed, healthcare providers should further consider whether a neurology referral is needed. Healthcare providers should heed patient-reported cognitive impairment because survivors may be struggling, even when objective assessments do not show deficits. Additional research is needed to identify standardized and consistent cognitive impairment measures to enable researchers to synthesize findings across studies and have a clearer understanding of cognitive impairment in the colorectal cancer population. Although this review is unable to provide specific and appropriate follow-up time intervals, cognitive function should be regularly assessed to detect these changes earlier. By doing so, effective interventions may be tested to prevent cognitive function from declining and further affecting daily activities and quality of life. 
Limitations
This review is not without its limitations. First, no gray literature was searched. Therefore, publication bias may be present because of nonsignificant results not being reported. In addition, methodologic inconsistencies across studies complicated synthesis and prohibited meta-analyses, preventing the ability to draw conclusions about correlates of cognitive impairment in this population. As such, reported findings are preliminary and warrant additional research.
Conclusion
Early identification and an understanding of the mechanisms of cognitive impairment and its change over time is critical for nurses to prevent and treat cognitive impairment—a life-altering side effect. This systematic review synthesized current literature on the prevalence, severity, and correlates of cognitive impairment in colorectal cancer survivors who received chemotherapy. Future research assessing cognitive impairment using both self-report and objective measures with well-established validity and sensitivity is needed to provide definitive results on cognitive impairment in this patient population.
About the Author(s)
Ya-Ning Chan, MSN, RN, is a PhD student in the School of Nursing, Ashley Leak Bryant, PhD, RN-BC, OCN®, FAAN, is an associate professor in the School of Nursing and the assistant director for cancer research career enhancement in the Lineberger Comprehensive Cancer Center, Jamie L. Conklin, MLIS, is a nursing librarian in the Health Sciences Library, and Tyra Claire Girdwood, BSN, RN, is a PhD student in the School of Nursing, all at the University of North Carolina at Chapel Hill; Aaron Piepmeier, PhD, is an assistant professor in the Department of Exercise Science at Elon University in North Carolina; and Rachel Hirschey, PhD, RN, is an assistant professor in the School of Nursing at the University of North Carolina at Chapel Hill and an associate member of the Lineberger Comprehensive Cancer Center in Durham, NC. Chan was supported by the Carol Ann Beerstecher Graduate Nursing Scholarship (2019–2020), the Class of 1967 Forever Fund Scholarship (2020–2021), and the Helen Watkins Umphlet Graduate Scholarship (2020–2021) from the School of Nursing at the University of North Carolina at Chapel Hill, as well as the Doctoral Degree Scholarship in Cancer Nursing from the American Cancer Society. Girdwood was supported by the Robert Wood Johnson Foundation. Chan, Bryant, Conklin, Piepmeier, and Hirschey contributed to the conceptualization and design. Chan, Bryant, Conklin, Girdwood, Piepmeier, and Hirschey completed the data collection. Chan, Bryant, Girdwood, Piepmeier, and Hirschey provided the analysis. All authors contributed to the manuscript preparation. Chan can be reached at chanyn@live.unc.edu, with copy to ONFEditor@ons.org. (Submitted March 2021. Accepted May 24, 2021.)

