An Exploratory Study of Cognitive Function and Central Adiposity in Men Receiving Androgen Deprivation Therapy for Prostate Cancer
Objectives: To prospectively assess cognitive function, anthropomorphic measures, and bone mineral density in men receiving androgen deprivation therapy (ADT) for prostate cancer; explore relationships between cognitive function and central adiposity; and gather preliminary data from a personalized education, exercise, and nutrition intervention.
Sample & Setting: 33 participants consented from a randomized controlled intervention trial.
Methods & Variables: Neurocognitive performance and self-report of cognitive function were assessed at baseline and 6 and 12 months. Dual-energy x-ray absorptiometry (DEXA) scans were obtained at baseline and 6 months.
Results: No between-group differences in cognitive function were demonstrated. Increased visceral adiposity was not associated with decrements in visuospatial abilities. Significant increases in fat mass without increases in body mass index or waist–hip ratio provided further evidence for DEXA as the preferred central adiposity measure.
Implications for Nursing: Well-powered prospective research is needed to fully characterize the effects of ADT on cognitive function and the potential benefits of exercise and nutrition-based interventions.
Jump to a section
Significant metabolic risks are inherent during androgen deprivation therapy (ADT) for prostate cancer (Melloni & Roe, 2020). Testosterone suppression causes decreases in bone mineral density (BMD), central weight gain (subcutaneous and visceral fat), and sexual dysfunction (Nguyen et al., 2018) and may impair cognitive function, negatively affecting quality of life (Shahinian et al., 2006).
Cognitive decline represents an area of major clinical concern, particularly considering the potential irreversibility of the loss. The trajectory of cognitive function decline for men with prostate cancer has not been well characterized. Testosterone metabolites (dihydrotestosterone for spatial ability and estradiol for verbal memory) are important for cognitive function (Hampson et al., 2015) (see Figure 1). Central adiposity and secretory products related to visceral fat, including inflammatory cytokines such as tumor necrosis factor and interleukin-6 (Montague & O’Rahilly, 2000), are postulated to impair cognitive function (Dahl et al., 2010). Because ADT is a known risk factor for increased central adiposity, it may affect cognition directly and indirectly. However, study results have been mixed (Treanor et al., 2017). 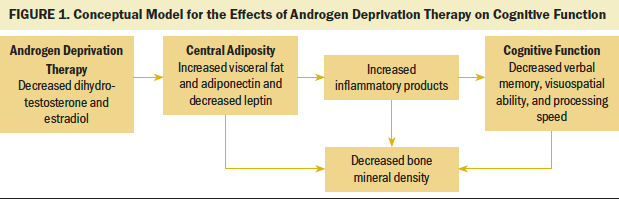
The relationship between increased body mass index (BMI), increased visceral fat, and BMD is somewhat contradictory. Although increased BMI typically is associated with increased BMD, there is an inverse relationship between visceral fat and BMD (Eckstein et al., 2016). This inverse relationship likely is because of a chronic inflammatory state and cytokine-related stimulation of bone resorption. The association of visceral fat with increased production of adiponectin and reduced leptin levels may contribute to bone mass loss. Decreased BMD may be predictive of cognitive decline in older adults (Kang et al., 2018). Common measures of obesity and central adiposity include BMI and waist–hip ratio, respectively. Dual-energy x-ray absorptiometry (DEXA) scanning, typically employed to measure BMD, is gaining favor as a clinically accessible and more precise estimate of fat distribution and lean muscle mass (Bi et al., 2015).
The purpose of this exploratory substudy was to (a) prospectively assess cognitive function, anthropomorphic measures, and BMD in men with prostate cancer receiving ADT; (b) explore relationships between cognitive function and central adiposity; and (c) gather preliminary data on the effects of a personalized education, exercise, and nutrition intervention.
Methods
This is a secondary analysis from a randomized controlled interventional study, “Staying Strong and Healthy During ADT for Men” (R01NRO14518), which is being conducted to minimize ADT-associated metabolic risks. Parent study participants were randomized into intervention or attention control groups. The intervention included six months of personalized education, prescribed aerobic and resistance exercise, and nutritional coaching (three months of weekly calls followed by three months of monthly calls) (Manson et al., 2019). The attention control group received general education about prostate cancer and related treatments, as well as provision of resources for available support groups (six monthly calls). Following institutional review board approval of the substudy, all subsequent men consented to the parent study were invited to participate in this substudy. Men who were within 90 days of ADT initiation were eligible for participation in the substudy. Informed consent for the substudy was obtained prior to data collection.
Substudy Assessments
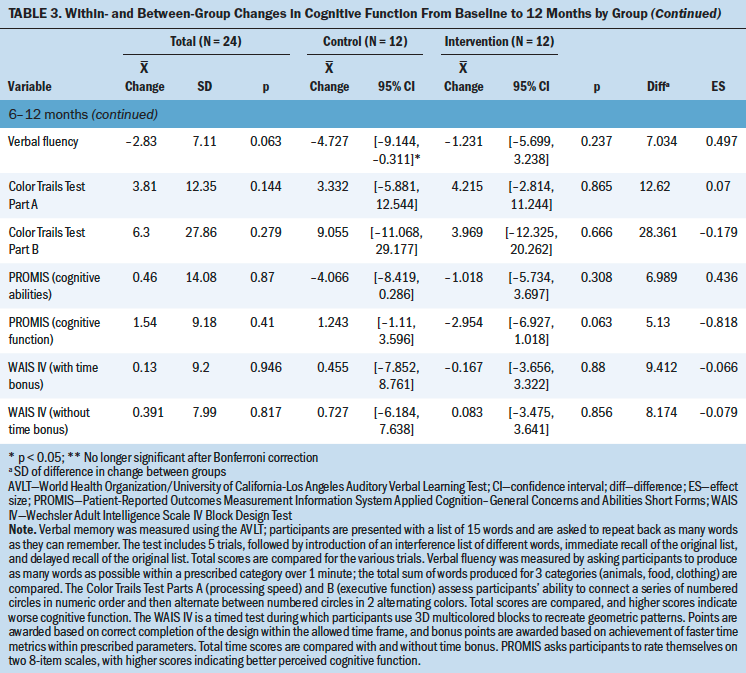
In-person neurocognitive tests and paper self-report cognitive function instruments were administered to 33 participants at baseline and 26 participants at 6 and 12 months. Neurocognitive tests were selected to align with the International Cancer and Cognition Task Force recommendations for the assessment of pertinent cognitive domains (verbal memory, verbal fluency, processing speed, and executive function) (Wefel et al., 2011). Visuospatial ability also was assessed. Neurocognitive testing was administered by two members of the study team, both of whom had doctoral-level neuropsychological training and experience. The eight-item Patient-Reported Outcomes Measurement Information System Applied Cognition–General Concerns and Abilities Short Forms have been well validated with cancer survivors and add minimal participant burden. The World Health Organization/University of California-Los Angeles Auditory Verbal Learning Test and Color Trails Test Parts A and B are preferred for those with limited English-language skills or low literacy.
Substudy funding supported DEXA scanning (GE Lunar iDXA software, version 13.5) sequentially for the first 24 consented substudy participants at baseline (n = 12 in the intervention group and n = 12 in the control group) and six months (n = 11 in the intervention group and n = 9 in the control group). In addition to BMD, DEXA measures of central adiposity included total fat mass (primary), estimated visceral tissue fat mass, and trunk fat mass. BMI and waist–hip ratio were clinically assessed.
Statistical Analyses
Standard descriptive statistics were computed for all variables based on level of measurement and observed distribution. Group baseline equivalence was compared using t test or Fisher exact test for continuous or categorical covariates, respectively. Between-group changes in DEXA scans and neurocognitive assessments (baseline to six months) were explored using student t test, with significance set at p < 0.05. Correlations were computed between cognitive assessments and DEXA scans to explore relationships between central adiposity and cognitive function. Linear regression modeling examined whether the intervention modified correlation between changes in fat mass and neurocognitive measures. SAS®, version 9.4, was used for statistical analyses. Because this study was a feasibility pilot, the sample size was not powered to detect statistically significant differences but will suggest direction and effect size for future studies.
Results
Participants were primarily older (aged older than 65 years), in a cohabiting relationship (n = 22), retired (n = 13), and White (n = 21). Most participants had some college education (n = 21) and metastatic disease (n = 11). ADT regimens varied from single agents to combined androgen blockade with or without radiation therapy and/or chemotherapy (see Table 1). 
DEXA Comparisons and Neurocognitive Scores
Total fat mass significantly increased for the DEXA sample (N = 20) from baseline to six months, and changes in BMI were correlated with changes in total fat mass (r = 0.58476, p = 0.0068). No significant changes were noted for BMI, waist–hip ratio, or BMD. A between-group reduction in estimated visceral fat mass was seen for the intervention group (p = 0.0173) (see Table 2). 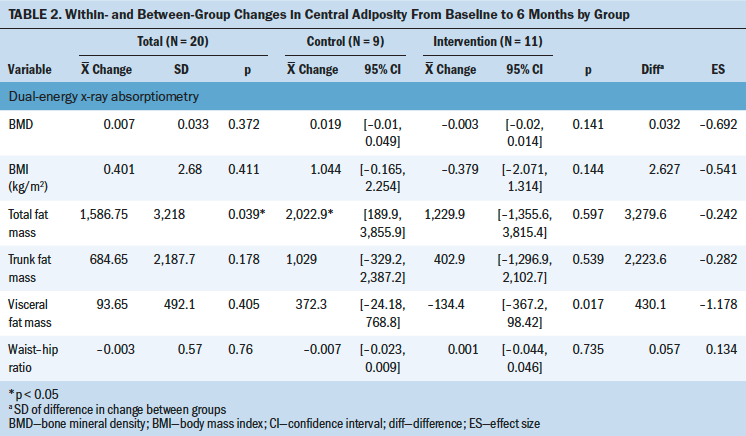
Neurocognitive test scores were commensurate with age-related published norms. Significant improvements were noted from baseline to six months prior to Bonferroni correlation (see Table 3). No change in self-report of cognitive function was noted. No between-group differences were observed for any of the neurocognitive variables. The control group demonstrated a significant within-group decrease in verbal fluency at two months. 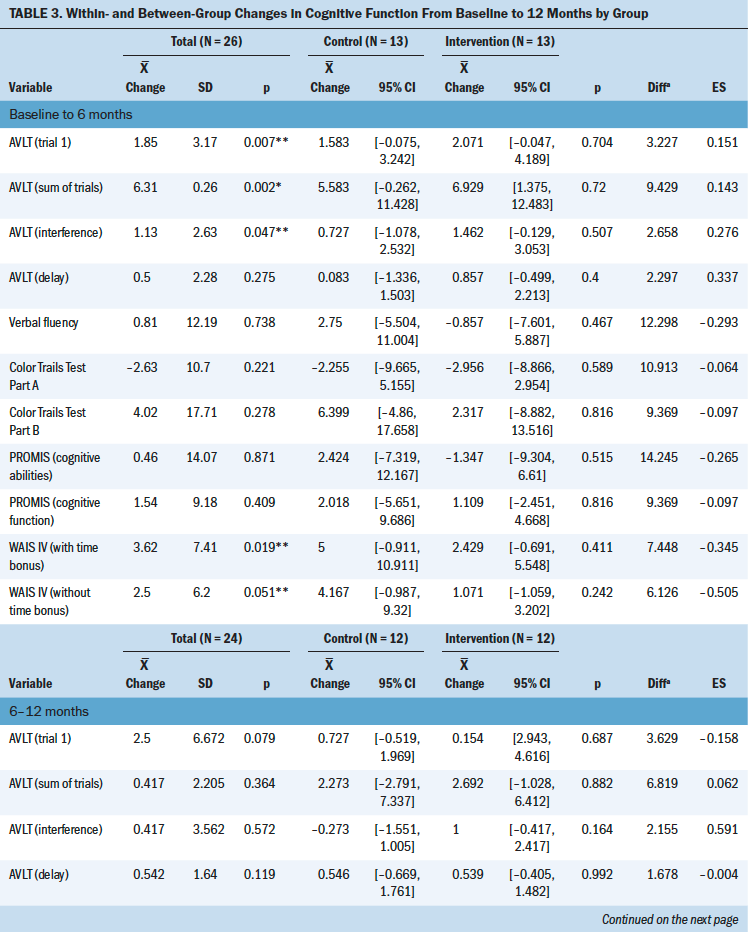
Correlations and Linear Regression
Significant correlation was seen between changes in total fat mass and BMI (r = 0.58476, p = 0.0068), but not between total fat mass and waist–hip ratio (r = 0.13646, p = 0.5662). A correlation of borderline significance was noted between total fat mass and scores on the Color Trails Test Part A (increased time indicates poorer processing speed) (r = 0.42416, p = 0.0623).
A linear regression with changes in Wechsler Adult Intelligence Scale IV Block Design Test scores as the dependent variable and changes in total fat mass, the intervention, and their interaction as independent variables produced a significant interaction (p = 0.0423). The regression coefficient of total fat mass for the control group was negative and marginally significant (r = –0.0022, p = 0.0768), suggesting that, on average, control group participants who gained more fat tended to improve less in performance on the Block Design Test. Comparatively, the regression coefficient of total fat mass for the intervention group was small and nonsignificant (r = 0.00073, p = 0.2717), suggesting that changes in total fat mass were not associated with changes in scores on the Block Design Test. This finding may reflect that the intervention mitigated the negative effect of increased fat mass on visuospatial ability in this small substudy. Adding the difference in metabolic equivalent minutes per week, which was an assessment of physical activity intensity levels obtained from the International Physical Activity Questionnaire administered as a component of the parent study, did not significantly contribute to the regression model, and the significance of the interaction effect between changes in total fat mass and the intervention was maintained (p = 0.019).
Linear regression with changes in BMD as the dependent variable and changes in waist–hip ratio, the intervention, and their interaction as the independent variables yielded a significant interaction (p = 0.006). The regression coefficient of waist–hip ratio for the control group was significant (r = –0.86298, p = 0.001). Therefore, greater increases in waist–hip ratio were associated with less increases in BMD. In contrast, the regression coefficient of waist–hip ratio for the intervention group was not significantly different from 0 (r = 0.14273, p = 0.1645), indicating that the intervention may have significantly minimized the effect of increased waist–hip ratio on BMD reduction (see Table 4). 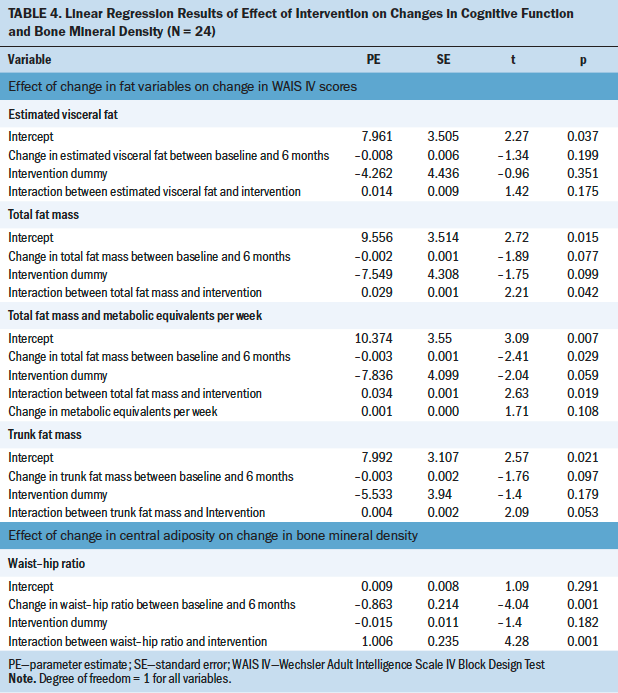
Discussion
As expected, the DEXA sample experienced a significant increase in central adiposity (total fat mass). The results did not show a significant increase in BMI or waist–hip ratio. This disparity lends further evidence to support DEXA for measurement of central adiposity. A significant between-group reduction in estimated visceral fat was noted, providing preliminary evidence that the intervention may be effective in reducing visceral weight gain.
The expected correlation between increases in central adiposity and decreases in neurocognitive test performance was only seen with borderline significance (p = 0.0623) for changes in total fat mass and processing speed (Color Trails Test Part A). However, interaction results were demonstrated between increases in total fat mass, Block Design Test performance, and the intervention, potentially indicating a reversal of the expected direction of the association between visceral fat gains and visuospatial ability test performance. The authors speculated that individual variance in activity level might be influencing this interaction. However, adding metabolic equivalent minutes to the regression model did not change the significance of the treatment interaction or the direction of the relationships. One potential explanation is that the intervention eliminated the mediating effect of inflammation between fat increases and decreased visuospatial ability.
Some studies have demonstrated visuospatial ability reductions in individuals receiving ADT, whereas others have shown no ADT-related cognitive effects (Joly et al., 2006; Marzouk et al., 2018). One systematic review indicated that ADT-related declines in visuospatial ability range from 24% to 69% (Treanor et al., 2017). An earlier review indicated that men receiving ADT performed worse on visuomotor tests (including the Wechsler Adult Intelligence Scale IV Block Design Test) without detriments for other cognitive domains (McGinty et al., 2014). Research also has demonstrated both ADT-associated decrements in visuospatial ability and improvements in verbal memory (Treanor et al., 2017). Researchers have questioned whether the lack of consistency regarding ADT-associated cognitive effects is because of a lack of sensitivity of available neurocognitive tests for the subtle cognitive changes reported by this population (Kluger et al., 2020). Qualitative research has shown an increase in self-report of cognitive problems in the absence of decrements on performance during neurocognitive tests (Wu et al., 2016). The results of the current study were not significant for self-reported changes in cognitive function, although a large effect size (d = –0.82) indicated worse cognitive function at 12 months for the intervention group. The current study’s results did not show a strong correlation between increased visceral fat and decreased BMD; however, regression analyses indicated that the intervention may have neutralized the effect of visceral fat on BMD.
Limitations
The study sample was quite small for the number of comparisons investigated. Eligibility criterion (within 90 days of initiating ADT) may have precluded obtaining true baseline neurocognitive assessments because of variability in the onset of side effects, potentially reducing the detectable amount of change in cognitive function. Treatment regimen variability also may have influenced study results. DEXA was not conducted at 12 months, which limited the authors’ ability to assess the effect of time on the relationships between neurocognitive function and central adiposity. 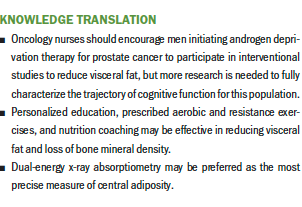
Conclusion
The results of this study were encouraging because gross deficits in neurocognitive performance and self-report of cognitive function were not seen during 12 months of ADT. No between-group differences in cognitive function were demonstrated despite a significant reduction in visceral fat for the intervention group. Increased visceral adiposity was not shown to be associated with decrements in visuospatial abilities. Well-powered prospective research is needed to fully characterize the potential impact of ADT on cognitive function for men with prostate cancer, as well as the potential effects of exercise and nutrition-based interventions in this population. Collection and analysis of serum markers of inflammation will be important to further explore the relationship between changes in visceral fat and visuospatial ability in men with prostate cancer. Men initiating ADT for prostate cancer should be encouraged to take part in exercise and nutrition-based interventional studies to reduce visceral fat and further advance the science in this area. Significant increases in fat mass without significant increases in BMI or waist–hip ratio provided further evidence that DEXA may be preferred for measuring central adiposity.
The authors gratefully acknowledge Jennifer Heins, PA, Jim Kovarik, PA, and Xinglei Shen, MD, for referring eligible patients for the study, and Nichole Cortez, RD, LD, Loran Griffith, MS, RDN, CSO, LD, William Hendry, BS, Carrie Michel, MS, RND, CSO, LD, CNSC, Hilary Robertson, MS, RDN, LD, Deana Wilhoite, MS, RN, and Jaime Ward, BS, for assistance with data collection.
About the Author(s)
Jamie S. Myers, PhD, RN, AOCNS©, FAAN, is a research associate professor and Alana Manson, PhD, is a postdoctoral fellow, both in the School of Nursing at the University of Kansas; Sandra A. Billinger, PT, PhD, FAHA, is a professor in the Department of Physical Therapy, Rehabilitation Science, and Athletic Training and the Department of Neurology at the University of Kansas Medical Center; William Parker, MD, is an assistant professor in the Department of Urology at the University of Kansas Medical Center and a urologist in the University of Kansas Health System; Jill Hamilton-Reeves, PhD, RD, CSO, is an associate professor in the Department of Urology and the Department of Dietetics and Nutrition at the University of Kansas Medical Center; Francisco J. Diaz, PhD, is an associate professor and Ron Krebill, MPH, is a senior research analyst, both in the Department of Biostatistics and Data Science at the University of Kansas Medical Center; and Sally Maliski, PhD, RN, FAAN, is the dean of and the Beverly Gaines Tipton Endowed Professor in Oncology Nursing in the School of Nursing at the University of Kansas, all in Kansas City. This research was supported by the University of Kansas Cancer Center 2016 Pilot Award, the National Institute of Nursing Research (R01NRO14518, NCT02969577), and the National Cancer Institute Cancer Center Support Grant (P30 CA168524), using the Nutrition Shared Resource. Myers, Manson, Billinger, Parker, Hamilton-Reeves, and Maliski contributed to the conceptualization and design. Myers, Manson, Billinger, Hamilton-Reeves, and Maliski completed the data collection. Myers, Billinger, Hamilton-Reeves, Diaz, Krebill, and Maliski provided the analysis. Diaz and Krebill provided statistical support. Myers, Manson, Billinger, Parker, Hamilton-Reeves, Diaz, and Maliski contributed to the manuscript preparation. Myers can be reached at jmyers@kumc.edu, with copy to ONFEditor@ons.org. (Submitted May 2021. Accepted August 14, 2021.)

