Perceptions of Clinical Trial Participation in African American Cancer Survivors and Caregivers
Purpose: To explore the perceptions of African American survivors and caregivers about participation in clinical trials.
Participants & Setting: 13 participants were enrolled and participated in one of the focus groups, and 11 participated in two focus groups.
Methodologic Approach: A qualitative descriptive study using a community-based participatory research approach. Focus groups guided by Freire’s dialogic model explored the perceptions of African American patients with cancer about participation in clinical trials. Focus groups were audio recorded and transcribed.
Findings: The analysis identified three main themes: (a) facilitators for participation, (b) barriers to participation, and (c) facilitators and barriers playing dual roles.
Implications for Nursing: The under- representation of African American patients in clinical trials contributes to racial health disparity by negatively affecting health outcomes and quality of care delivered to this population. Oncology nurses are at the forefront of cancer care and in the best position to advocate for individuals with cancer, particularly those who face health inequalities. Findings from this study guided the recommendations.
Jump to a section
African American people, the second largest minority group in the United States, experience significant health inequities when compared to their White counterparts (Office of Minority Health, 2021). These disparities reflect social, cultural, and economic inequalities more than biologic differences (American Cancer Society, 2021; Colen et al., 2018; National Cancer Institute [NCI], 2020). Cancer remains the second leading cause of death in the United States despite novel therapies to treat and prevent various cancer types (Ahmad et al., 2021). However, cancer mortality is higher among African American people when compared to other ethnic groups, which adds to disparities in this population (NCI, 2020).
Clinical trials help develop new cancer treatments and reduce healthcare disparities; however, clinical trial cohorts often do not represent the affected population. The U.S. Food and Drug Administration (2020) reported that only 2% of female participants aged 65 years or older in oncology and hematology trials were Black. In addition, despite the widespread use of cancer immunotherapy, less than 4% of all patients enrolled across multiple trials of immune checkpoint inhibitors for lung cancer treatment were African American or Black (Nazha et al., 2019). To attain health equity among the African American population, studies investigating connections between social determinants of health and tumor biology must engage this population. To improve cancer delivery to this population, studies must elucidate more about the link between under-resourced neighborhoods and poor cancer care outcomes in African American patients (Ashing et al., 2021). Minimal representation of minority groups in clinical trials reduces the ability to generalize findings or create hypotheses about differences in results between subgroups. In addition, it minimizes opportunities for underrepresented groups to benefit from cutting-edge medical treatments (Ailawadhi et al., 2018).
Accrual of African American patients to clinical trials is challenging; challenges include organizational barriers, healthcare system fears and concerns, and negative attitudes and beliefs about clinical trials and the health system (Pariera et al., 2017; Webb Hooper et al., 2019). Many African American patients report feelings of fatalism that extend beyond individual opinions but are linked to wider historical and cultural issues (Somayaji & Cloyes, 2015). Historically, racism in the United States has tainted research; notable examples are the Tuskegee Study and the harvesting of Henrietta Lacks’ cancer cells without consent (Centers for Disease Control and Prevention, 2021; Skipper, 2020). In addition, studies exploring African American patients’ perceptions regarding clinical trial participation have identified mistrust in healthcare systems and research (Strekalova, 2018).
Despite research to identify and address barriers, clinical trial recruitment of African American patients remains low. Community-based participatory research (CBPR), a qualitative research method wherein researchers collaborate with community stakeholders to create and implement studies (Israel et al., 2019), has been used to investigate education interventions about clinical trials in the African American community. However, in those studies, interventions were created by the academic partners and not by the community stakeholders (Blakeney et al., 2015; Green et al., 2015; Michaels et al., 2015). To eliminate that gap, this study used a combination of CBPR and Freire’s (2000) dialogic model to embrace the social and cultural contexts that exist within the African American community. Freire’s model was developed for educating individuals considered oppressed or disadvantaged. African American patients fit this description through continuous racism and discrimination that have led to health disparities. This study design focuses on building trust, authenticity, and accountability among researchers and the community they aim to serve. This study investigated the perceptions of African American patients regarding clinical trials and life experiences contributing to underrepresentation in clinical trials.
Methods
A qualitative descriptive design was used to explore African American patients’ perceptions of clinical trial participation. A CBPR framework was used to build a partnership between community and academic partners whose research interests aligned. The community partner, the leader of Sisters R Us Circle of Survivors (SRUCOS), had worked with the academic partners in a previous study.
SRUCOS, a nonprofit organization, engages the community at large and provides ongoing support to women diagnosed with breast cancer; any-gender survivors of other cancers; and their caregivers, families, and friends. The academic partner included nursing faculty of the Thomas Jefferson University College of Nursing. Early in the study, partners agreed on their roles and contributions to the study, being equitable and based on expertise. All partners undertook numerous interactions and decisions throughout each stage of the research process.
Theoretical Framework
This study used Freire’s (2000) dialogic model, a construct for liberation of oppressed populations through critical consciousness. Exploring participants’ history and personal narratives, Freire’s model helped address prevailing issues in the African American community, such as racism and discrimination, at all levels. Further, it examined the participants’ clinical trial perceptions and searched for resiliency and protective factors that potentially decrease their perceptions of prejudice and unfairness regarding clinical trial participation.
Participant Selection and Setting
The target population was African American individuals diagnosed with cancer and/or caregivers of people with cancer. Inclusion criteria consisted of (a) self-identifying as African American/Black, (b) speaking English, (c) being aged 18 years or older, and (d) being able to commit to four focus group sessions.
The Thomas Jefferson University Institutional Review Board approved this study. Participants were recruited by SRUCOS in Philadephia, Pennsylvania. Recruitment was conducted by purposive and snowball sampling and advertised through social media; email blasts; community health meetings; and flyers at community centers, barber shops, and churches. Participation was voluntary, and participants provided written consent. They self-screened for eligibility per inclusion criteria and selected one of four dates based on scheduling availability. Participants received gift cards for participation ($40 for two-hour sessions; $30 for one-hour sessions).
Data Collection
Freire’s (2000) dialogic model guided the semistructured script for the focus group interviews, including a four-step process (see Figure 1). Open-ended focus group questions appear in Figure 2. Participants also completed a researcher-developed demographic survey. 
Each focus group of five to six participants was led by two researchers, an academic nurse who facilitated the semistructured interviews and the SRUCOS leader who monitored the process and audio recorded the two-hour interviews that were conducted in a private room in a college building. Field notes were collected during the interviews; researchers coded data for triangulation. Data collection for each focus group was completed in two sessions. 
Data Analysis
Data collection and analysis occurred concurrently using NVivo software, version 12. Data saturation was achieved by the third focus group, wherein keywords were repeated by participants. Data were analyzed via qualitative descriptive approach and content analysis to verify saturation and gather rich descriptions of participants’ experience of clinical trials through (a) familiarizing through audio-recorded and written notes, (b) indexing data into keywords, (c) charting data into meaning units, (d) identifying content categories, and (e) reducing data into themes. Lincoln and Guba’s (1985) evaluative criteria of trustworthiness guided the study, including credibility, dependability, transferability, and confirmability; these were applied through audio recording the focus groups, transcribing the audio recordings verbatim, validating the data by the participants, and verifying consistency in the raw data among the researchers (four coders). The community partner was involved in data analysis. Member checking ensured credibility; to synthesize and analyze data, participants were given the opportunity to comment on the identified themes and add data where needed (Harvey, 2015). Descriptive statistics were used to summarize demographic data.
Results
Sample Characteristics
The first session had 13 participants, and the second had 11 participants. Demographic characteristics are listed in Table 1. Sample attrition was attributed to inability to attend future sessions because of scheduling conflicts and was not associated with any particular age group. 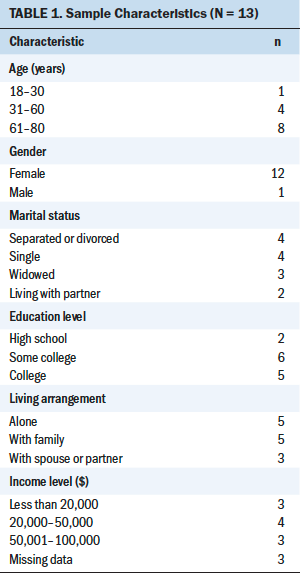
Themes
Data analysis revealed three major themes related to clinical trial participation: (a) facilitators of participation, (b) barriers to participation, and (c) facilitators and barriers playing dual roles.
Facilitators of Participation
Three facilitators for clinical trial participation were identified: support from others, religion/faith, and self-advocacy.
Support from others: Receiving support from family, friends, and support groups positively influenced the participants’ perceptions of and willingness to participate in clinical trials:
If they [family] didn’t approve of it, I don’t know if it would stop me or not, but it would be great to have their approval. . . . If I had a good, strong feeling about the trial, that it would help me, I think I would get the last decision. . . . Family sometimes doesn’t always understand what it is that you’re actually trying to do. As long as . . . it’s not going to hurt me, if I’m already terminal, I’m going for every study there is . . . but I also got to watch out for myself.
Support groups play a big part in understanding what your journey might be like . . . or just your concerns about your well-being . . . with a person who had some of those experiences that you didn’t.
A lot of us . . . don’t have that structure in our families and our friends. . . . We don’t have the people that we need to help us through certain situations.
Religion/faith: Most participants discussed how faith and religion affected their decision to participate in clinical trials. They spoke passionately about faith and explained they would pray about their decision because “God has the ultimate say.”
One participant said, “A relationship with God, it trumps everything. . . . If you don’t take that time to pause and talk to your higher power, your God, whoever you choose—it’s a mess.” Another participant said the following: “I take everything to the Lord. . . . He is very much important in my life. . . . I’m a child of God. . . . He has the final say. . . . Prayer changes things.”
The church community had mixed effects on the participants’ decisions. Some participants acknowledged having strong faith but not needing support from the church community.
Self-advocacy: Despite the myriad positive and negative influences in their lives, many participants communicated that they were the ultimate decision-maker in their care. This concept outweighed support from family, friends, and healthcare providers.
Personal advocacy . . . is something that I feel is positive. If you just feel that you can do something to help yourself, I just think that would be the way in which a person would even get into the ideology that they would do a clinical trial. We need to know how it’s going to help us.
Self-advocacy was mentioned during discussion of decision-making and emerged as a theme elsewhere, including its importance in patient–provider communication.
Barriers to Participation
Participants shared several barriers to clinical trial participation, including racism, media, and social determinants of health.
Racism: Participants discussed the impact of current and historical racism on their perceptions of clinical trial participation. Historical events, as previously mentioned, and current institutional racism have led to ongoing fear of clinical trial participation.
Those men in Tuskegee . . . had the medicine that could help them, [but] it was withheld to see just how bad it would go, and they let them die. . . . They could have given them [the treatment]. . . . Racism is not dead.
The history that we know most of all is the Tuskegee experiment. . . . It was not a good outcome for . . . African Americans. . . . I think that does make people leery, and the stigma of racism, how . . . a person would think about . . . a trial.
Regarding Henrietta Lacks’ cells, a participant shared the following: “[It] was really deep because, with the information . . . not being shared, they didn’t necessarily tell her that it was helping. [They] didn’t give her the credit for the outcome of the research.”
Ethnic concordance between patients and practitioners also affected the participants’ decisions about clinical trial participation. Participants felt that sharing the same ethnicity with practitioners and the research team who introduced them to clinical trials would improve participation rates based on increased levels of trust. One participant said, “We’ve gotten used to dealing with Caucasians in certain professional positions to trust them. . . . I would probably still have that stigma because we have a history.” Another said, “It depends on who the person is [and] . . . how they’re coming at you [to suggest clinical trials].”
Generational differences in opinion on clinical trials emerged. Older participants held a bias against them, which heightened their wariness in participating. They worried that history would be repeated and felt they might be treated as “guinea pigs.” However, younger participants trusted clinical trials more. One participant suggested generational differences reflected that the “younger generation was more educated and did further research on the historical events,” whereas the older generation was “more upset on past events.” Overall, participants were familiar with the history of racism in research and were not confident that having additional information about clinical trials would improve participation rates. As one participant said, “The obvious [reason] is . . . we have that history of racism and discrimination. . . . [It] is not going to go anywhere. It’s still going to be there.”
Media: Media influence added to negative perceptions about clinical trials, specifically advertisements that presented a “long list of side effects from medications” and entertainment that recounted historical depictions of the impact of research on African American patients. One participant said, “When you see the books and the movies over and over again . . . racism . . . still happens today. . . . I think it just magnifies it, and it just makes things worse.”
Social determinants of health: Social determinants of health, including transportation, child care, health insurance, and health literacy, affected participants’ decisions to participate in clinical trials. Several participants discussed lack of reliable transportation, specifically a ride home because of the clinical trial being held in an inconvenient location. Childcare obligations added to restrictions. As one participant said, “Additional support, lack of support from the caregiver or myself, I think that needs to be added in.”
Health insurance was another participation barrier. One participant said, “What kind of carrier you have and the opportunities to be in clinical trials. . . . that plays a big part in care and treatment. That’s a barrier for some people with getting what they need.” Another said, “If you don’t have the right insurance, you might not be offered that clinical trial. On that end, there’s access, but for our end, there’s no access.”
Health literacy was a multifaceted barrier. Participants mentioned having difficulty accessing information about clinical trials and agreed more accessible clinical trial education was necessary to improve participation:
If there was more knowledge, if there was more education, then people will be open. You don’t want to do something that you don’t understand. . . . Since there is no understanding, there’s no participation. So when you educate, then when you know better, you do better. For me to go onto a website and try to navigate that at stage III breast cancer, absolutely, that wouldn’t happen.
Don’t just give me the paper and tell me to take it home and read about it. Just try to explain it because a lot of times, you can’t comprehend it, going through what you’re going through. This isn’t sinking in.
In addition to improving content, participants discussed communication strategies to facilitate participation. One participant said, “Language can subconsciously . . . facilitate the conversation and can help in the recruitment.”
Participants indicated that the amount of information presented was another participation barrier. As one participant said, “It’s sometimes so much information. I was getting so bombarded with information about what I should do for treatment . . . I couldn’t even understand the treatment, let alone the clinical trials.”
Facilitators and Barriers Playing Dual Roles
Patient–provider communication and trust/mistrust emerged as subthemes that could play dual roles as clinical trial participation facilitators or barriers.
Patient–provider communication: Patient–provider communication was a powerful facilitator or barrier for clinical trial participation. Satisfactory interactions with their healthcare providers, where participants felt respected and listened to, motivated them to participate in clinical trials. However, if they felt mistreated by their providers, they did not consider participating in clinical trials.
Participants who had satisfactory interactions said the following:
Yes, I have a good primary doctor. He’s second to none. My therapist is second to none. These things are important when you’re going through this life anyway.
I would definitely probably tell my social worker first because if there’s any information out there, she would search and she would get back to me and tell me what was available. I would definitely talk to my doctor, but I think I would definitely get more information from my social worker.
Participants who had negative interactions said the following:
I had a horrible situation the first time with breast cancer, and it was a woman. [The physician] said, “Oh my God, your breasts are so big. We’re going to have difficulty.”
I was there with the doctor, and I was asking him about the side effects of the medication. He tells me, “Well, I can’t put you through medical school in 20 minutes.” That was his answer to me.
Trust/mistrust: Trust in the medical system was a clinical trial participation facilitator, and mistrust was a barrier. Participants discussed instances of trust and mistrust in healthcare providers, but responses largely described mistrust in providers and pharmaceutical companies.
If the point is to get us more involved in clinical trials, the synthesis of everything that has been said is communication. It is trust, it is respect, and it absolutely is us being at the table. We have to know that our presence is valued at the front end, at the back end, and all the way in the middle throughout the entire process.
So, now it comes down to the health professionals that are conducting these studies. Have they been manipulated to not lure but encourage these patients to participate to measure the effectiveness of the drug? Or are they being manipulated because there’ll be some kind of financial gain in there for them? Now, we have to put our trust in the health professionals that are conducting this, and I would like to believe that most of them would do this for the good of the patient or for medicine, not just for financial gains because that would be awful.
Discussion
Themes
Racial disparities persist in cancer clinical trial participation despite research to understand and efforts to improve them (Ailawadhi et al., 2018). Participants recognized that lack of knowledge about clinical trials was an obstacle for their participation in research. Disproportionately low health literacy among African American patients persists (Muvuka et al., 2020), and studies have associated limited health literacy and lack of information about clinical trials, or research literacy, with poor clinical trial participation (Evans et al., 2012; Ford et al., 2013). Increasing knowledge about clinical trials could increase willingness to participate in them (Echeverri et al., 2018; Simon et al., 2019, 2021), which was evident with this study’s participants. In addition, their physicians’ processes for providing information about clinical trials did not inspire confidence in them. Consistent with Clark et al. (2019), participants in this study indicated that physicians offered insufficient time to communicate appropriate information or answer their questions about clinical trials. Of note, participants acknowledged feeling more comfortable communicating with other healthcare members, including social workers, nurses, and nurse practitioners.
Trust/mistrust in the healthcare system is a powerful predictor for clinical trial participation. Provider trust is built from high-quality care, honest communication, and respect between clinicians and patients; however, fear of exploitation and conspiracy between medical and research teams continues to be reported in studies exploring African American patients’ perceptions about clinical trials (Haynes-Maslow et al., 2014; Martinez et al., 2017; Somayaji & Cloyes, 2015). In this study, participants with good patient–provider relationships expressed trust in them and were more open to participating in clinical trials. Conversely, if their physicians treated them in a condescending manner, participants mistrusted them and were less willing to participate in clinical trials.
Participants cited clinical trial participation barriers, including the legacy of racism in the United States, effects from media coverage, and social determinants of health, that contribute to health inequities that prolong social, environmental, and economic disparities among African American patients. The ongoing structural and institutional racism that currently persists in society has continued the cycle of unconscious bias experienced by African American patients (Swartz & Titanji, 2020) and contributes to hesitancy in enrolling in clinical trials. This study’s participants mentioned that the U.S. history of racism and research atrocities committed against African American people affected their perceptions of clinical trials and made them fearful of large institutions. These factors contribute to negative perceptions about clinical trials. Interestingly, knowledge of the facts from those studies was not clear among this study’s participants (Centers for Disease Control and Prevention, 2021; Skipper, 2020).
Research has shown that overcoming systemic, individual, and interpersonal barriers to participation requires multilevel interventions. Knowledge about improved safety regulations and protection of the rights and well-being of study participants through institutional review boards contributes to younger African American patients’ increased willingness to participate in clinical trials (Clark et al., 2019; Henderson et al., 2020).
Media affected participants’ decisions to participate in clinical trials. A Pew survey found that African American people tend to trust their local news more than national news, because they feel more connected to local representatives (Atske et al., 2019); however, participants in this study broadly distrusted the media. Their perceptions of the media were similar to survey results from the Opportunity Agenda (2021), which found that media typically offered inaccurate representations of African American people, leading to conscious and unconscious biases.
Participants’ views in this study align with research demonstrating that lack of access to research sites, lack of health insurance, childcare concerns, and educational disparities were barriers to clinical trial participation and retention (Asare et al., 2017; Henderson et al., 2020). Such barriers affect African American peoples’ ability to access quality health care and fully engage with the healthcare team. Another barrier is not being recruited into clinical trials because of provider biases that African American people are “noncompliant or difficult to reach” (Asare et al., 2017, p. 21); therefore, they are not given the opportunity to participate. To address these concerns, key strategies recommended for cancer clinical trial recruitment include having diverse recruitment staff, having flexibility in study requirements, and having resources to address competing needs and priorities (Barrett et al., 2017; Regnante et al., 2019).
This study’s participants also discussed facilitators to participation. Participants cited multiple sources of support that may influence clinical trial participation, including family, friends, and support groups. Studies show that formal and informal peer support of patients with cancer improves coping skills, reduces isolation, allows for information sharing, enhances understanding of the cancer experience, increases confidence in talking with physicians, and improves quality of life (Hu et al., 2019; Lee et al., 2016). Similar to this study’s results, previous research demonstrates the importance of social support from family and friends in clinical trial decision-making processes for African American patients (Rivers et al., 2013).
Religious and spiritual beliefs can influence cancer treatment–related decision-making (Kelly et al., 2021). Participants discussed the importance of faith in decision-making, but some emphasized the church community rather than religion. Younger participants in this study emphasized spirituality and prayer over organized religion or a church community. However, consistent with the literature, older African American people felt the church was the centerpiece of religious, social, and political life, and was an effective source of clinical trial–related education (Frew et al., 2016).
Study participants described self-advocacy regarding treatment-related decision-making and communication with healthcare providers. Self-advocacy in people with cancer is a process of internalizing and activating resources into actions to overcome cancer- and treatment-related obstacles (Hagan & Donovan, 2013). Self-advocacy facilitates leveraging personal strengths, skills, and resources to achieve goals. Although no studies explore self-advocacy in African American patients with cancer as influencing clinical trial participation, research has shown self-advocacy as a positive factor in accessing specialty care for this population (Cowan et al., 2019).
Community-Based Participatory Research
The CBPR conceptual model that served as a framework to assess this study involves four domains: context, partnership process, research and intervention, and outcomes (Oetzel et al., 2018) (see Table 2). 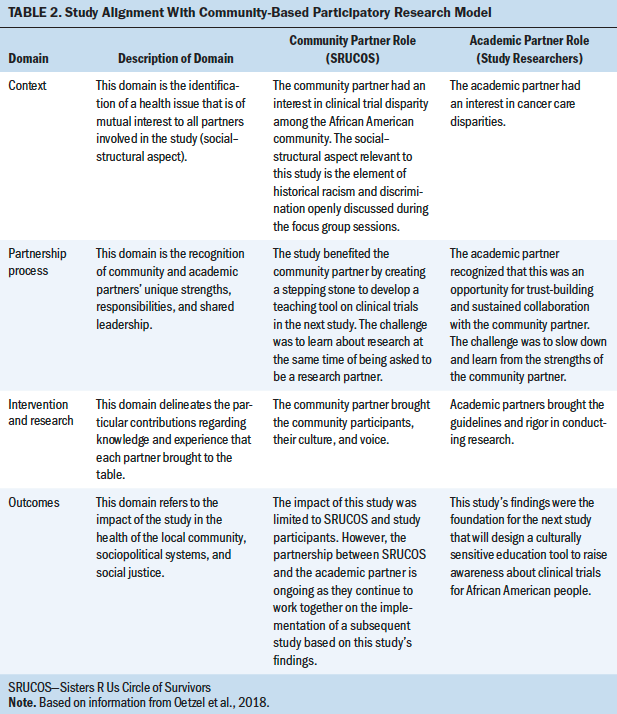
Limitations
Participants were recruited from one city, limiting the transferability of the findings to other regions. Although data saturation was achieved, the small sample size also limited the transferability. Another limitation was gender uniformity, because only one participant identified as male, and all other participants identified as female. This may have limited capturing unique experiences among genders.
Implications for Nursing
Oncology nurses are at the forefront of cancer care and in the best position to advocate for people with cancer, particularly those who face health inequities. Results from this study guide recommendations for oncology nurses and other healthcare professionals to reduce clinical trials disparities in African American patients with cancer.
First, the participants recommended providing appropriate, culturally sensitive education on clinical trials. In the clinical setting, nurses should inform African American patients with cancer of the clinical trials available to them as a first step toward increasing their participation in cancer research. In the community setting, enlisting African American individuals with cancer into train-the-trainer programs on clinical trials has shown to be an effective method to improve cancer research literacy (Green et al., 2015; Michaels et al., 2015). In addition, participants suggested that the process for providing education about clinical trials could improve if healthcare providers become more aware and sensitive to the African American culture, such as by recognizing how historical research atrocities experienced by African American people affect their feelings toward clinical trials (Warren et al., 2019).
Second, oncology nurses must understand they are not immune to racism (FitzGerald & Hurst, 2017). The seminal report Unequal Treatment: Confronting Racial/Ethnic Disparities in Healthcare by the Institute of Medicine states that “bias, stereotyping, prejudice, and clinical uncertainty on the part of healthcare providers may contribute to racial and ethnic disparities in healthcare” (Smedley et al., 2003, p. 11). Unfortunately, conscious and unconscious bias continue to shape the lives of African American people. Oncology nurses should promote self-awareness by educating themselves; understanding that unconscious bias is a normal aspect of human cognition can help nurses address their own biases in an open and healthy manner (Marcelin et al., 2019). Nurse managers should help implement organizational strategies to promote a culture of inclusion and humility among the nursing staff, such as training on diversity and inclusion (Marcelin et al., 2019).
Third, oncology nurses could encourage clinical trial participation in African American patients with cancer by improving their overall experience while navigating the healthcare system. This study and others have found that trust/mistrust in the healthcare system results from how African American patients with cancer live through multiple encounters with healthcare providers. A positive experience increases trust; a negative experience increases mistrust (Martinez et al., 2017). Oncology nurses can promote trust by improving patient–provider relationships, listening to patients’ concerns, and serving as patient advocates.
Fourth, given that participants expressed a strong sense of self-advocacy as a protective factor to coping with cancer, oncology nurses should enhance this strength through open dialogue, honest patient–nurse relationships, and promotion of cancer support groups (NCI, 2019). 
Conclusion
Nurses must encourage clinical trial participation to increase representation among underrepresented participants to obtain results from diverse groups. If nurses advocate for, recruit, and enroll patients from various ethnicities into clinical trials, their results will more accurately represent the U.S. population to improve future science and health outcomes for underrepresented groups. 
About the Author(s)
Clara Granda-Cameron, DNP, CRNP, AOCN®, is an assistant professor in the College of Nursing at Thomas Jefferson University in Philadelphia, PA; Yvonne McLean Florence, MDiv, is the founder and president of Sisters R Us Circle of Survivors in Philadelphia, PA; Lisa Whitfield-Harris, PhD, MBA, RN, is an associate professor and program director of Community Systems Administration, and Jeannette Kates, PhD, APRN, AGPCNP-BC, GNP-BC, is an associate professor and director of the Adult-Gerontology Primary Care Nurse Practitioner Program, both in the College of Nursing at Thomas Jefferson University; and Jessica Marie Lenzo, MPH, BSN, RN, is a clinical research nurse II at the Hospital of the University of Pennsylvania in Philadelphia. This study received funding from the Thomas Jefferson University Community Research Day funding mechanism. Granda-Cameron, Florence, Whitfield-Harris, and Kates contributed to the conceptualization and design. All authors completed the data collection, provided the analysis, and contributed to the manuscript preparation. Granda-Cameron can be reached at cxg075@jefferson.edu, with copy to ONFEditor@ons.org. (Submitted April 2021. Accepted August 27, 2021.)

