Synergistic Interactions Among Fatigue, Sleep Disturbance, and Depression in Women With Breast Cancer: A Cross-Sectional Study
Objectives: To assess the symptom cluster of fatigue, sleep disturbance, and depression among female patients with breast cancer receiving chemotherapy, and to evaluate its impact on quality of life (QOL) and symptom severity.
Sample & Setting: 372 patients receiving adjuvant chemotherapy recruited from two tertiary hospitals in China.
Methods & Variables: Symptom severity and QOL were evaluated using the Brief Fatigue Inventory, Pittsburgh Sleep Quality Index, Patient Health Questionnaire–9, and Functional Assessment of Cancer Therapy–Breast on the eighth day after receiving chemotherapy.
Results: All symptoms positively correlated with each other. Although the symptom cluster was significantly associated with decreased QOL, individualized symptom severity worsened as the number of symptoms increased.
Implications for Nursing: The prevalence of and interactions among fatigue, sleep disturbance, and depression negatively affect symptom severity and patients’ QOL. Conducting early assessment followed by effective cluster-based interventions is needed to manage this symptom cluster.
Jump to a section
Breast cancer is the most prevalent cancer type in women worldwide and in China, with a report showing that there were 416,371 new female breast cancer cases in China in 2020 (Globocan, 2021). Cancer itself and its curative treatment modalities cause various side effects during the disease trajectory. As the most commonly used adjuvant systemic treatment, chemotherapy usually induces a series of unpleasant physical and psychological symptoms (e.g., gastrointestinal symptoms, fatigue, sleep disturbance, depressed mood) that seldom occur in isolation (Chui, 2019). One study suggested that chemotherapy-related symptoms were often more distressing than symptoms caused by other treatments (Sibeoni et al., 2018). Of note, an average of about 17 symptoms were reported by patients with breast cancer who received chemotherapy (Begum et al., 2016).
Background
When two or more concurrent symptoms share some interrelationships, they are considered to have formed a symptom cluster (Kim et al., 2005). Previous studies have used various symptom inventories, such as the MD Anderson Symptom Inventory and Symptom Experience Scale, and analytical techniques (e.g., factor analysis, latent analysis) to identify symptom clusters in patients who received chemotherapy for breast cancer (Albusoul et al., 2017; Hsu et al., 2017). This de novo approach, which identifies symptom clusters from all possible symptoms (Miaskowski, 2016), is known for its ability to consider a comprehensive range of symptoms. Although inconsistent findings were drawn from these studies, the three following common symptoms were found to frequently cluster together: fatigue, sleep disturbance, and depression (Albusoul et al., 2017; Hsu et al., 2017).
In contrast with the de novo approach, other studies have focused on commonly observed symptom clusters. These studies, which usually adopt a symptom-specific instrument to evaluate each symptom, are often referred to as prespecified approaches (Miaskowski, 2016). Using prespecified approaches, fatigue, sleep disturbance, and depression attracted attention from oncology researchers because of their impact on patients with cancer (Jim et al., 2011; Yennurajalingam et al., 2016), particularly those who suffer from breast cancer (So et al., 2021). Because the symptoms often coexist and influence each other, they are associated with lower quality of life (QOL) among patients. One review suggested that fatigue, depression, and insomnia formed a common symptom cluster that appeared across various cancer diagnoses (Donovan & Jacobsen, 2007). Another study examined the fatigue, sleep disturbance, and depression cluster in patients with breast cancer (Fiorentino et al., 2011). The results showed that the prevalence rate of each symptom ranged from 30% to 60% for fatigue, 20% to 70% for sleep disturbance, and 31% to 37% for depression. These rates varied across different diseases and treatment conditions (Fiorentino et al., 2011). Although previous studies claimed that fatigue, sleep disturbance, and depression formed a symptom cluster, the occurrence rates of these concurrent symptoms were reported individually rather than in combination. Therefore, the interactions between symptoms in this cluster remained unclear. In addition, symptom severity fluctuated along the disease trajectory. In one study (Bower et al., 2011), patients with breast cancer who had received chemotherapy experienced significantly higher levels of fatigue, sleep disturbance, and depression than those who did not receive chemotherapy. Of note, some studies found that symptom occurrence and severity often peaked within a few days after the start of chemotherapy during a 21-day treatment cycle, whereas the lowest scores were often obtained prior to the next treatment cycle (Hsu et al., 2017; Sullivan et al., 2018). Future research on this symptom cluster should consider the time frame of symptom measurement that best represents patients’ symptom burden (Miaskowski, 2016).
Despite the significant burden of symptoms on these patients, studies focused on the unique relationships among fatigue, sleep disturbance, and depression immediately after the start of chemotherapy are limited (Kim et al., 2005; Miaskowski et al., 2017). Statistical techniques used in these studies (e.g., exploratory factor analysis, principal component analysis) can only determine the existence of intercorrelations among symptoms but cannot explain the direction and nature of the relationships (Barsevick et al., 2006). Although evidence suggests that these relationships would exacerbate the negative impact associated with individual symptoms (Kim et al., 2005; Miaskowski et al., 2017), additional research is warranted to examine the synergistic effect of different levels of the fatigue, sleep disturbance, and depression cluster on QOL. Additional findings, including the occurrence rate of the cluster among patients with breast cancer receiving chemotherapy, the relationships among the number of symptoms within the cluster and QOL, as well as the symptom severity, will advance the knowledge of this symptom cluster. Appropriate interventions may be developed to modulate these relationships and improve symptom management (Berger et al., 2013).
The theory of symptom management was adopted as the theoretical framework in this study (Humphreys et al., 2014). In this theory, dynamic relationships among symptom experience, outcomes, and symptom management strategies exist under the influence of individual, environmental, and health and illness factors. The current study examined how the fatigue, sleep disturbance, and depression symptom cluster affects QOL and symptom severity, but the concepts of symptom experience and outcomes were used. In addition, the researchers investigated the contribution of various influencing factors, including sociodemographic and clinical characteristics of patients with breast cancer, to the relationships between symptom experience and outcomes.
The current study used a prespecified approach to explore the occurrence rate of the fatigue, sleep disturbance, and depression symptom cluster among postsurgery patients with breast cancer who received different cycles of adjuvant chemotherapy, and to investigate how this symptom cluster influences patients’ QOL and symptom severity. The five research questions that guided this study were as follows:
• What is the individual prevalence of fatigue, sleep disturbance, and depression in patients with breast cancer who received adjuvant chemotherapy?
• Are these three symptoms associated with each other in forming a symptom cluster?
• What are the proportions of participants who experienced one, two, three, or none of the symptoms in the cluster?
• What is the association between the number of symptoms experienced and QOL and symptom severity scores?
• What is the association between the existence of the symptom cluster and patients’ QOL scores after controlling for potential confounding factors?
Methods
Design and Participants
This study used a descriptive, cross-sectional design. Eligible participants were recruited using convenience sampling from the inpatient wards of the Departments of Breast Surgery at two tertiary public hospitals in Xi’an, China, from September to December 2018. Eligible participants had to meet the following criteria: (a) Chinese women aged 18 years or older, (b) newly diagnosed with stage I–III breast cancer who had undergone breast surgery, (c) currently receiving adjuvant chemotherapy, and (d) able to communicate verbally in Chinese. Patients who had recurrent or metastatic breast cancer, were concurrently diagnosed with other types of cancer, or were diagnosed with mental disorders (e.g., schizophrenia) as identified in medical records were excluded.
An online sample size calculator was used to estimate the proportion of patients with the fatigue, sleep disturbance, and depression symptom cluster in the target population. Based on a previous report that assumed one-third of these patients would be affected by the symptom cluster (Fiorentino et al., 2011), this study required a sample size of 340 participants to meet the expected sample size, with a 5% margin of error at a 5% level of significance.
Measures
The Chinese version of the nine-item Brief Fatigue Inventory (BFI) was used to evaluate participants’ level of fatigue. The original scale was developed for the rapid assessment of global fatigue in English-speaking cancer populations (Mendoza et al., 1999). Three items measure fatigue severity, and the other six items evaluate the impact of fatigue on patients’ daily activities. Each item is rated on a scale ranging from 0 (no fatigue or interference) to 10 (worst fatigue and interference). A global fatigue severity score is calculated by averaging all items on the BFI, with higher scores indicating higher levels of fatigue. The scale was translated into Chinese and validated among 249 Chinese patients with cancer with multiple diagnoses (Wang et al., 2004). The internal consistency was excellent for both dimensions (Cronbach’s alpha = 0.92–0.9). Good convergent validity was shown to have moderate to strong correlation (r = –0.44 to –0.71) with the fatigue-related constructs of the SF-36®. No (0), mild (1–3), moderate (4–6), and severe (7–10) fatigue are defined based on the global fatigue score (Mendoza et al., 1999). The internal consistency of the BFI in the current study was very good (Cronbach’s alpha = 0.92).
The Chinese version of the Pittsburgh Sleep Quality Index (PSQI) was adopted for its specific design in assessing sleep quality in clinical populations (Buysse et al., 1989). Nineteen questions are used to calculate seven component scores that represent subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction aspects. Each component score ranges from 0 (no difficulty) to 3 (severe difficulty). A global score reflecting overall sleep quality is calculated by summing all component scores, with higher global scores indicating poorer sleep quality. Miladinia et al. (2018) suggested that global scores of 0–5 indicate no sleep disturbance, scores of 6–10 indicate some sleep disturbance, and scores of 11–21 indicate severe sleep disturbance, respectively. The Chinese version of the PSQI showed good internal consistency (Cronbach’s alpha = 0.85) and convergent validity with the Insomnia Severity Index (r = 0.84) (Lu et al., 2014). In the current study, the PSQI demonstrated good internal consistency (Cronbach’s alpha = 0.81).
Depression was evaluated using the Chinese version of the Patient Health Questionnaire–9 (PHQ-9), which was developed to measure the severity of depressive symptoms based on nine questions (Kroenke et al., 2001). A four-point Likert-type scale is used to rate individual items, with total scores ranging from 0 (no depression) to 27 (severe depression). A total score of 0–4 represents the absence of depressive disorder. Mild (score of 5–9), moderate (score of 10–14), moderately severe (score of 15–19), and severe (score of 20–27) depression are defined based on the total depression score (Kroenke et al., 2001). The psychometric test of the Chinese version of PHQ-9 was conducted in a Chinese community setting (Wang et al., 2014), with good internal consistency (Cronbach’s alpha = 0.86) and acceptable concurrent validity with the Self-Rating Depression Scale (r = 0.29). The PHQ-9 showed good internal consistency in the current study (Cronbach’s alpha = 0.78).
The Chinese version of the Functional Assessment of Cancer Therapy–Breast (FACT-B) was used to assess participants’ overall QOL. The FACT-B was developed based on the general FACT questionnaire, which consists of four subscales (physical well-being, social and family well-being, emotional well-being, and functional well-being), and a breast cancer subscale to specifically evaluate QOL in patients with breast cancer (Brady et al., 1997). Each of the 37 items are rated using a five-point Likert-type scale ranging from 0 to 4, with higher total scores indicating better QOL. The Chinese version of the FACT-B demonstrated good reliability in five domains (Cronbach’s alpha = 0.59–0.82) and criteria-related validity with the Quality of Life Instrument–Breast Cancer Patient Version (r = 0.73) in Chinese patients with breast cancer (Wan et al., 2007). A good internal consistency of the FACT-B was identified in the current study (Cronbach’s alpha = 0.86).
Social support was evaluated using the Chinese version of the 12-item Multidimensional Scale of Perceived Social Support (MSPSS), which measures perceived social support from family, friends, and significant others (Zimet et al., 1988). Higher mean scores indicate better social support. Although the Chinese version of the MSPSS had good reliability (Cronbach’s alpha = 0.92) and item subscale correlation (all > 0.4) (Zhou et al., 2015), a Cronbach’s alpha coefficient of 0.92 also indicated a very good internal consistency of the MSPSS in the current study.
A questionnaire designed by the investigators was used to collect data on sociodemographic (age, transportation time from home to hospital, education level, marital status, personal monthly income, and insurance status) and clinical characteristics (clinical stage, type of surgery received, chemotherapy regimens, menstrual status, and comorbidity status). Permission for scale use was obtained from the original authors prior to data collection.
Procedures
Because a symptom cluster is defined as the intercorrelation among coexisting symptoms (Kim et al., 2005), measurement of various symptoms should be conducted in a consistent time frame. Because symptoms usually aggravate and peak at one week after receiving chemotherapy (Sullivan et al., 2018), data collection was conducted on day 8 of any 21-day chemotherapy cycle to capture the experience of symptoms during the past seven days.
To ensure reliability, three research assistants (RAs) were trained for data collection. The training focused on the purpose of the study, the procedure of obtaining the written informed consent from participants, the process of conducting interviews, communication skills, and the management of completed questionnaires. The RAs were expected to refrain from judgement to avoid influencing participant responses. Return demonstration and role-play were used to evaluate the performance of the RAs.
Eligible participants were identified through a review of hospital medical records. Patients were approached during their hospital stay for postsurgery chemotherapy. Some may have received one or more cycles of adjuvant chemotherapy previously. The trained RAs would introduce the details of the study to eligible participants, and interested participants were required to provide written informed consent. Questionnaires were completed by the participants or the RAs in-person, or via telephone if the patients were discharged, on day 8 of any 21-day chemotherapy cycle. The RAs then extracted sociodemographic and clinical information from the medical records.
This study was approved by the Survey and Behavioural Research Ethics Committee of the Chinese University of Hong Kong (Ref. No.: SBRE-18-033) and the Biomedical Research Ethics Committee of Xi’an Jiaotong University (Ref. No.: 2018-517).
Data Analysis
Descriptive statistics (frequencies and means) were computed for sociodemographic and clinical characteristics, the level of perceived social support of participants, the prevalence and severity of individual symptoms, and the level of QOL of participants. To define the presence of fatigue, sleep disturbance, and depression among participants in this study, scores of 1 or greater (Mendoza et al., 1999), 6 or greater (Miladinia et al., 2018), or 5 or greater (Kroenke et al., 2001) that corresponded to each questionnaire were adopted as cutoff points. A symptom cluster was operationalized as the presence of at least two of these symptoms (Kim et al., 2005).
Spearman correlation coefficients were used to examine the correlations among fatigue, sleep disturbance, and depression. One-way analyses of variance were performed to compare QOL and symptom severity scores among various groups of participants who experienced different numbers of symptoms. The analyses tested whether there was a significant linear trend for QOL and symptom severity across these groups with an increasing number of symptoms. A two-sample independent t test was used to compare the QOL score and symptom severity score among the following: (a) participants who had no symptoms or one symptom and those who had two to three symptoms and (b) participants who had two symptoms and those who had three symptoms. In addition, hierarchical multiple regression analysis was used to examine the associations between the occurrence of the symptom cluster and QOL scores. Potential confounding factors, such as sociodemographic and clinical characteristics and social support, were adjusted in the final model (Al-Naggar et al., 2016; Ambroggi et al., 2015; Sharma & Purkayastha, 2017).
Data analyses were performed using IBM SPSS Statistics, version 25.0. All statistical tests were two-sided, and statistical significance was set at p < 0.05.
Results
Participant Characteristics
A total of 372 participants completed the study questionnaires. The average age of participants was 50.05 years (range = 20–74 years). Most participants completed secondary education or higher (n = 292, 79%) and were married (n = 360, 97%). About two-thirds (n = 248, 67%) of participants were employed, whereas less than one-third (n = 120, 32%) of participants had a medium to high monthly income. Eighty-three percent (n = 310) of participants had their medical expenses covered by insurance. In terms of diagnoses, 77% (n = 285) of participants were diagnosed with stage I–II breast cancer. More than half (n = 214, 58%) of participants were postmenopausal at diagnosis. All participants underwent either mastectomy or lumpectomy before receiving chemotherapy, and 14% (n = 52) reported at least one comorbidity, with hypertension and diabetes being the most prevalent (see Table 1). 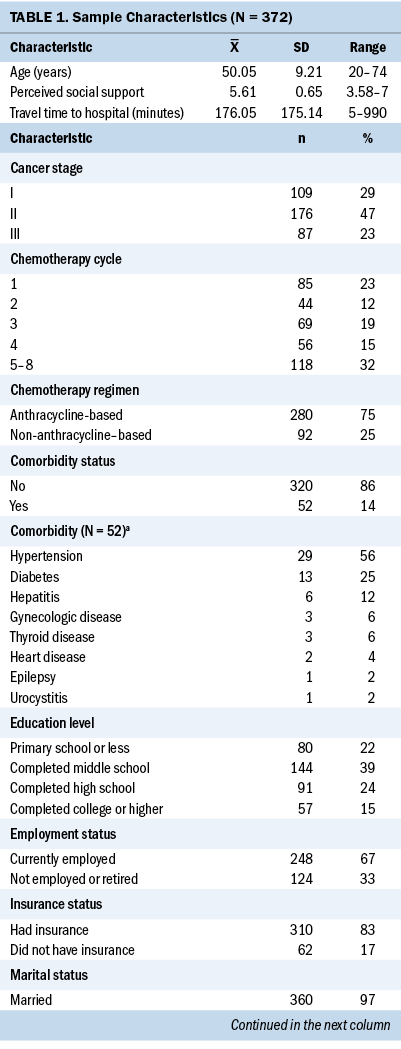
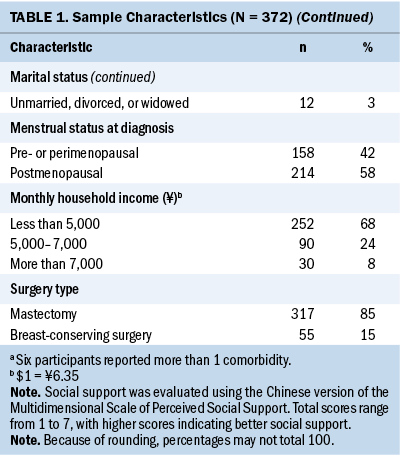
Prevalence of and Correlations Among Individual Symptoms
Almost all participants reported mild to severe fatigue (n = 369, 99%), 87% (n = 325) reported sleep disturbance, and 94% (n = 347) reported depression. Regarding mean severity scores for each symptom, moderate levels of fatigue (mean = 5.53, SD = 1.76, range = 0.11–9.22) and depression (mean = 10.4, SD = 3.94, range = 0–23) were reported, whereas mean sleep disturbance severity scores just reached the severe level (mean = 10.18, SD = 3.66, range = 1–19). Spearman correlation analyses showed that fatigue, sleep disturbance, and depression were positively correlated with each other, with correlation coefficients ranging from 0.45 to 0.57 (p < 0.001 for all variables) (see Table 2). 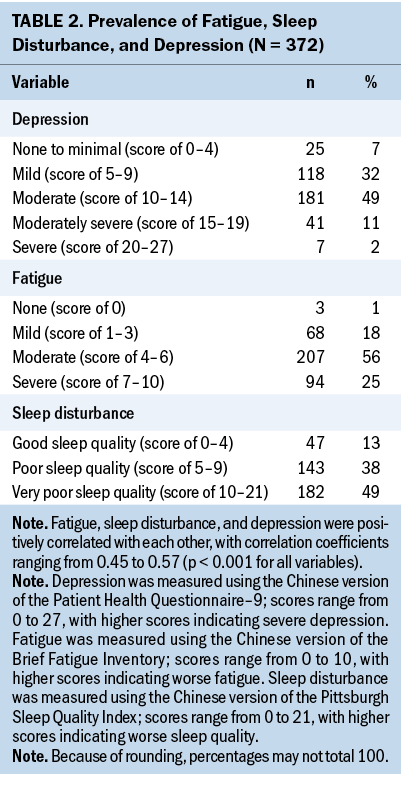
Prevalence and Effects of Multiple Symptoms
Only 4% (n = 14) of participants reported one symptom or no symptoms. Individuals who reported no symptoms (n = 3, 1%) or one symptom (n = 11, 3%) were combined into a single category because of the small numbers for each category. The proportion of participants who experienced any two symptoms was 12% (n = 44) (n = 11 for fatigue and sleep disturbance and n = 33 for fatigue and depression, respectively). As many as 84% (n = 314) of participants experienced all three symptoms (see Table 3). 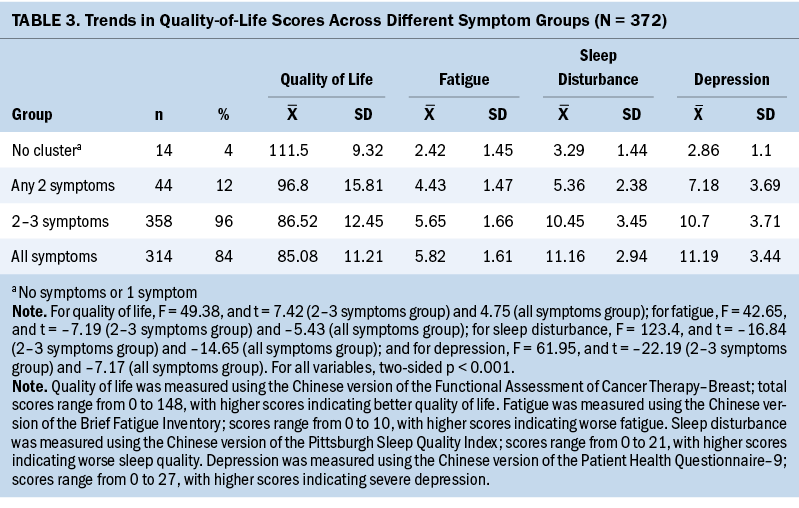
As the number of symptoms increased, mean QOL scores decreased from 111.5 (SD = 9.32) to 85.08 (SD = 11.21), and mean scores for individual symptoms increased from 2.42 (SD = 1.45) to 5.82 (SD = 1.61) for fatigue, from 3.29 (SD = 1.44) to 11.16 (SD = 2.94) for sleep disturbance, and from 2.86 (SD = 1.1) to 11.19 (SD = 3.44) for depression. The results of the one-way analyses of variance demonstrated that QOL scores and symptom intensity significantly differed across these three groups and had significant linear trends (p < 0.001 for all trends).
Compared with participants who reported no symptoms or one symptom, those who experienced two to three symptoms in the cluster reported significantly lower QOL scores and higher symptom severity scores (p < 0.001 for all symptoms). In addition, participants who experienced all three symptoms reported significantly lower QOL scores and higher symptom severity scores when compared with those who experienced any two symptoms (p < 0.001 for all symptoms).
Association Between the Symptom Cluster and Quality-of-Life Scores
A hierarchical multiple regression model was conducted to investigate the association between the presence of the symptom cluster and QOL scores. Before implementing the regression analysis, four variables (education level, monthly income, chemotherapy cycle, and clinical stage) were translated into dummy variables. The results and the dummy variables are presented in Tables 4 and 5. When the fatigue, sleep disturbance, and depression cluster variable (0–1 symptom group versus 2–3 symptoms group) was included in the regression model, it was found to be a significant influencing factor (beta = –0.36, p < 0.001), which explained the variance of 13% of QOL. When other potential influencing factors were gradually added for the sociodemographic, clinical, and social support aspects as three additional blocks in the regression model, all factors accounted for the variance of 28% of QOL in the final model. Among all the entered variables, only the symptom cluster (beta = –0.314, p < 0.001), age (beta = 0.24, p < 0.001), and perceived social support (beta = 0.244, p < 0.001) significantly influenced QOL scores. No multiple collinearity issue was observed during each regression analysis (variance inflation factor less than 5). 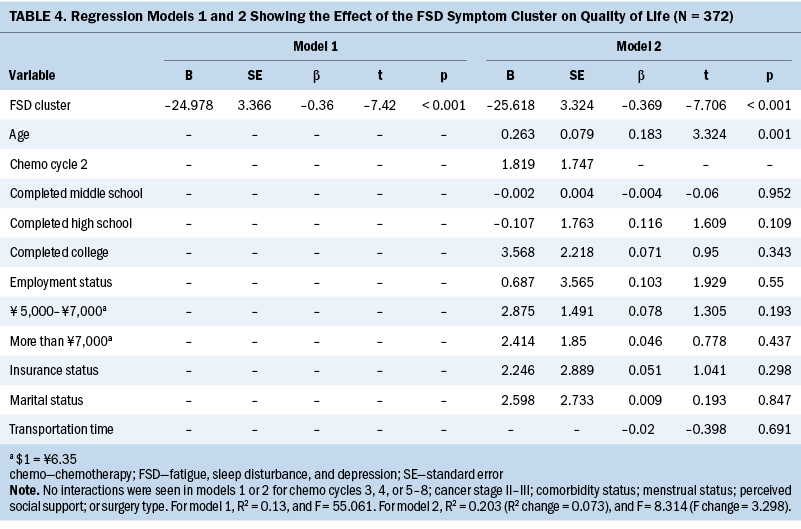
Discussion
This study examined the occurrence of the symptom cluster of fatigue, sleep disturbance, and depression in women with breast cancer treated with surgery followed by adjuvant chemotherapy. The co-occurrence of and intercorrelations among fatigue, sleep disturbance, and depression suggested that these three symptoms comprised a symptom cluster affecting most of these women. This cluster and the number of symptoms experienced were associated with symptom severity and QOL. These results suggest the potential synergistic effect of these symptoms on patient outcomes. 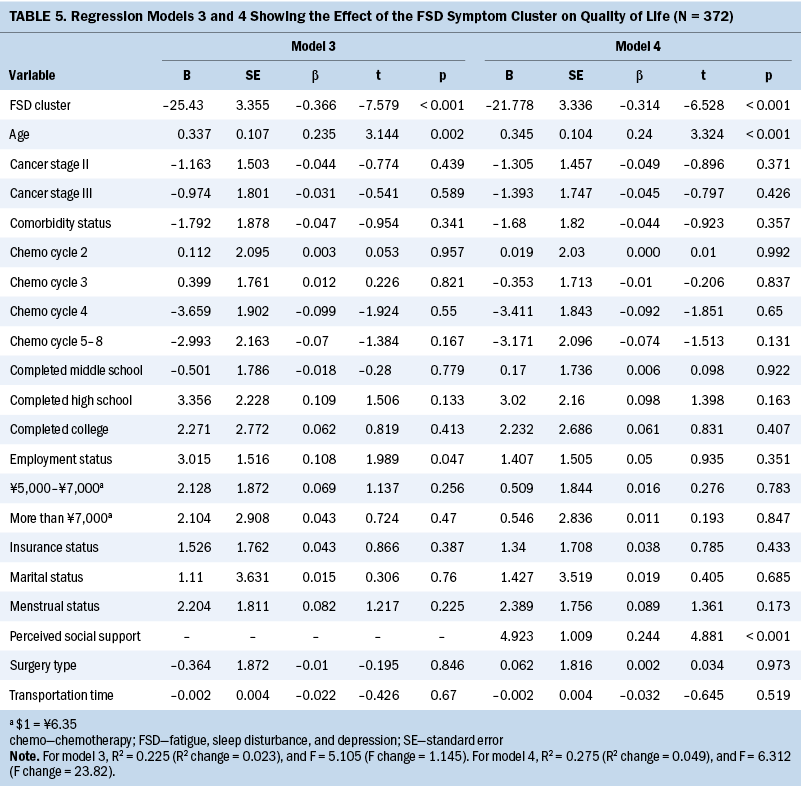
High Prevalence of the Symptom Cluster
In contrast with previous studies of patients with breast (Liu et al., 2009) and other cancer-related conditions (Lin et al., 2013), very high prevalence rates of fatigue (99%), sleep disturbance (87%), and depression (94%) were reported in the current study. The results also suggested that more than 80% (n = 314) of participants concurrently experienced all three symptoms. Although mean scores for fatigue, sleep disturbance, and depression reached moderate levels, the high occurrence rate and moderate intensity of this symptom cluster may be because of the standardized chemotherapy regimens adopted at the study sites. Female patients who suffer from cancer, particularly breast cancer, are more likely to have psychosocial issues than male patients with cancer (Koyama et al., 2016). In addition, the course of chemotherapy was reported to be more distressing when compared to other cancer treatments, such as surgery and radiation therapy (Chui, 2019; Sibeoni et al., 2018).
This study found that a large proportion of patients with breast cancer experienced fatigue, sleep disturbance, and depression simultaneously, and these symptoms were significantly correlated with each other. Because a symptom cluster is defined as a group of coexisting and interrelating symptoms, which may have a common mechanism (Kim et al., 2005), the results of the current study suggest that a mechanism underlying this symptom cluster may exist. One study suggested potential mechanisms underlying this prespecified symptom cluster, including various pro- and anti-inflammatory cytokines and markers (e.g., tumour necrosis factor-alpha, interleukin-1, C-reaction protein) (Bower et al., 2011). Previous studies have also found that there could be other mechanisms related to this symptom cluster, such as chemotherapy (Bower et al., 2011), immune responses (Jaremka et al., 2012), and impaired circadian rhythms (Fiorentino et al., 2011). Additional research addressing these underlying mechanisms is needed, which could guide the evolution of effective symptom cluster interventions.
Negative Effect of the Symptom Cluster on Symptom Intensity
Because the presence of a symptom was defined by the cutoff symptom severity score, it was not surprising that the no symptom cluster group (0–1 symptom) represented the lowest symptom scores. However, as participants experienced an additional symptom, the severity of all three symptoms would aggravate simultaneously. A similar trend was represented in a study of patients with lung cancer after surgery (Lin et al., 2013), which found that pain, fatigue, disturbed sleep, and distress ranked as the top four symptoms in terms of prevalence and severity. Patients who reported coexistence of these symptoms had higher symptom severity scores as compared to those who had fewer symptoms. However, no clear reason could explain this trend. This finding suggested the synergistic effect of the symptom cluster, and future research can adopt advanced statistical techniques to examine the temporal interactions among symptoms within a cluster. These results also indicate that early intervention may be helpful to prevent symptom clusters and escalation of symptom severity.
Negative Effect of the Symptom Cluster on Quality of Life
QOL scores decreased when participants experienced more symptoms within the cluster. Of note, differences in mean QOL scores between groups were greater than 8, which indicates a clinically meaningful difference (Cella et al., 2002). The decrease in QOL mean scores was larger between the no symptoms to one symptom and two to three symptoms groups than the score between any groups of two or all three symptoms. Such differences suggested a stronger effect of the symptom cluster on QOL than any individual symptoms. In addition, the hierarchical multiple regression analysis indicated that the symptom cluster was negatively associated with patients’ QOL after controlling for potential influencing factors in patients with breast cancer (Al-Naggar et al., 2016; Sharma et al., 2017). Among the various sociodemographic and clinical characteristics evaluated, only age and perceived social support were associated with QOL. Other characteristics, including transportation time, education level, marital status, employment status, monthly income, chemotherapy cycle, surgery type, menstrual status, and comorbidity status, were not significant. Although reasons for these nonsignificant results are unclear, participants in this study reported a very high prevalence of the symptom cluster. The occurrence of the symptom cluster alone explained some degree of variances. Because QOL is an important clinical outcome in patients with cancer, effective symptom management for relieving fatigue, sleep disturbance, and depression should be developed and provided to patients who receive chemotherapy for breast cancer.
Limitations
One limitation of this study was the use of convenience sampling in a northwestern city in mainland China, which may limit the generalizability of the results in other races and regions. There may also be unobserved confounding factors that were not considered or controlled. To address this limitation, consideration of additional factors, such as coping style and genetic composition, is warranted. Future studies may examine the relationships among these factors and the symptom cluster of fatigue, sleep disturbance, and depression to determine the mechanisms underlying these symptoms.
Implications for Nursing
According to the theory of symptom management (Humphreys et al., 2014), timing is an essential component of every symptom management strategy. The results of this study suggested that the symptom cluster of fatigue, sleep disturbance, and depression occurred shortly after administration of chemotherapy and negatively affected symptom severity and QOL. Although early intervention would achieve better symptom control and QOL outcomes in the long-term (Zimmermann et al., 2014), assessment and management of these distressing symptoms should be initiated as soon as chemotherapy is administered. In fact, cluster-based interventions could be a more cost-effective option in oncology settings. Based on the theory of symptom management (Humphreys et al., 2014), these interventions may modulate the physiologic factors underlying the fatigue, sleep disturbance, and depression symptom cluster. Because more than one biologic pathway may contribute to the symptom cluster of fatigue, sleep disturbance, and depression, interventions that can influence multiple biologic pathways simultaneously warrant consideration in managing this symptom cluster and promoting patients’ QOL (Mustian et al., 2016). For example, exercise is considered beneficial in bringing about positive effects on body’s multiple functions and systems, such as the inflammatory response, immunity (Koelwyn et al., 2015; Sprod et al., 2010), circulation, metabolism (Tang et al., 2010), and neuroendocrine (Sprod et al., 2010). 
Conclusion
The symptom cluster of fatigue, sleep disturbance, and depression is highly prevalent in patients with breast cancer who receive adjuvant chemotherapy. These symptoms interact and have negative synergistic effects on symptom severity and patients’ QOL. The results of this study suggest the need for exploring effective interventions to improve management of this symptom cluster among these patients.
The authors gratefully acknowledge the Departments of Breast Surgery at the First Affiliated Hospital of Xi’an Jiaotong University and the Shaanxi Province Oncology Hospital for their cooperation with this study.
About the Author(s)
Xiaole He, PhD, is a research secretary in the Nursing Department at Huizhou Central People’s Hospital in Guangdong, and was, at the time of the writing, a PhD candidate in the Nethersole School of Nursing in the Faculty of Medicine at the Chinese University of Hong Kong; Marques Shek Nam Ng, RN, PhD, is a research assistant professor and Kai Chow Choi, PhD, is a senior research fellow, both in the Nethersole School of Nursing in the Faculty of Medicine at the Chinese University of Hong Kong; Lulu Li, MPhil, is a head nurse in the Department of Integrative Medicine at the Cancer Center of the Fifth Affiliated Hospital of Sun Yat-Sen University in Guangdong; and Wenqian Zhao, MPhil, and Mengyue Zhang, MPhil, are PhD candidates, and Winnie Kwok Wei So, RN, PhD, FAAN, is a professor, all in the Nethersole School of Nursing in the Faculty of Medicine at the Chinese University of Hong Kong, all in China. No financial relationships to disclose. He and So contributed to the conceptualization and design. He, Li, Zhao, and Zhang completed the data collection. He and Choi provided statistical support. He, Ng, and So provided the analysis and contributed to the manuscript preparation. Ng can be reached at marquesng@cuhk.edu.hk, with copy to ONFEditor@ons.org. (Submitted June 2021. Accepted October 22, 2021.)
References
Albusoul, R.M., Berger, A.M., Gay, C.L., Janson, S.L., & Lee, K.A. (2017). Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. Journal of Pain and Symptom Management, 53(5), 880–886. https://doi.org/10.1016/j.jpainsymman.2016.12.332
Al-Naggar, R., Osman, M., & Al-Baghdadi, N. (2016). Study of quality of life and characteristic factors in women with breast cancer undergoing different types of therapy. Journal of Applied Pharmaceutical Science, 6(9), 147–152. https://doi.org/10.7324/japs.2016.60922
Ambroggi, M., Biasini, C., Del Giovane, C., Fornari, F., & Cavanna, L. (2015). Distance as a barrier to cancer diagnosis and treatment: Review of the literature. Oncologist, 20(12), 1378–1385. https://doi.org/10.1634/theoncologist.2015-0110
Barsevick, A.M., Whitmer, K., Nail, L.M., Beck, S.L., & Dudley, W.N. (2006). Symptom cluster research: Conceptual, design, measurement, and analysis issues. Journal of Pain and Symptom Management, 31(1), 85–95. https://doi.org/10.1016/j.jpainsymman.2005.05.015
Begum, M.S.N., Petpichetchian, W., & Kitrungrote, L. (2016). Symptom experience and quality of life of patients with breast cancer receiving chemotherapy in Bangladesh. Bangladesh Journal of Medical Science, 15(2), 201–206. https://doi.org/10.3329/bjms.v15i2.28747
Berger, A.M., Yennu, S., & Million, R. (2013). Update on interventions focused on symptom clusters: What has been tried and what have we learned? Current Opinion in Supportive and Palliative Care, 7(1), 60–66. https://doi.org/10.1097/spc.0b013e32835c7d88
Bower, J.E., Ganz, P.A., Irwin, M.R., Kwan, L., Breen, E.C., & Cole, S.W. (2011). Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29(26), 3517–3522. https://doi.org/10.1200/jco.2011.36.1154
Brady, M.J., Cella, D.F., Mo, F., Bonomi, A.E., Tulsky, D.S., Lloyd, S.R., . . . Shiomoto, G. (1997). Reliability and validity of the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument. Journal of Clinical Oncology, 15(3), 974–986. https://doi.org/10.1200/jco.1997.15.3.974
Buysse, D.J., Reynolds, C.F., Monk, T.H., Berman, S.R., & Kupfer, D.J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Cella, D., Hahn, E.A., & Dineen, K. (2002). Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Quality of Life Research, 11(3), 207–221. https://doi.org/10.1023/a:1015276414526
Chui, P.L. (2019). Cancer- and chemotherapy-related symptoms and the use of complementary and alternative medicine. Asia-Pacific Journal of Oncology Nursing, 6(1), 4–6. https://doi.org/10.4103/apjon.apjon_51_18
Donovan, K.A., & Jacobsen, P.B. (2007). Fatigue, depression, and insomnia: Evidence for a symptom cluster in cancer. Seminars in Oncology Nursing, 23(2), 127–135. https://doi.org/10.1016/j.soncn.2007.01.004
Fiorentino, L., Rissling, M., Liu, L., & Ancoli-Israel, S. (2011). The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: Severity of the problem and treatment options. Drug Discovery Today, 8(4), 167–173. https://doi.org/10.1016/j.ddmod.2011.05.001
Globocan. (2021). Population fact sheets. World Health Organization, International Agency for Research on Cancer. https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sh…
Hsu, H.T., Lin, K.C., Wu, L.M., Juan, C.H., Hou, M.F., Hwang, S.L., . . . Dodd, M.J. (2017). Symptom cluster trajectories during chemotherapy in breast cancer outpatients. Journal of Pain and Symptom Management, 53(6), 1017–1025. https://doi.org/10.1016/j.jpainsymman.2016.12.354
Humphreys, J., Janson, S., Donesky, D.A., Dracup, K., Lee, K.A., Puntillo, K., . . . Kennedy, C. (2014). Theory of symptom management. In M.J. Smith & P.R. Liehr (Eds.), Middle range theory for nursing (3rd ed., pp. 141–164). Springer. https://doi.org/10.1891/9780826195524.0007
Jaremka, L.M., Fagundes, C.P., Glaser, R., Bennett, J.M., Malarkey, W.B., & Kiecolt-Glaser, J.K. (2012). Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology, 38(8), 1310–1317. https://doi.org/10.1016/j.psyneuen.2012.11.016
Jim, H.S.L., Small, B., Faul, L.A., Franzen, J., Apte, S., & Jacobsen, P.B. (2011). Fatigue, depression, sleep, and activity during chemotherapy: Daily and intraday variation and relationships among symptom changes. Annals of Behavorial Medicine, 42(3), 321–333. https://doi.org/10.1007/s12160-011-9294-9
Kim, H.J., McGuire, D.B., Tulman, L., & Barsevick, A.M. (2005). Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing, 28(4), 270–282. https://doi.org/10.1097/00002820-200507000-00005
Koelwyn, G.J., Wennerberg, E., Demaria, S., & Jones, L.W. (2015). Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology, 29(12), 908–922.
Koyama, A., Matsuoka, H., Ohtake, Y., Makimura, C., Sakai, K., Sakamoto, R., & Murata, M. (2016). Gender differences in cancer-related distress in Japan: A retrospective observation study. BioPsychoSocial Medicine, 10(1), 10. https://doi.org/10.1186/s13030-016-0062-8
Kroenke, K., Spitzer, R.L., & Williams, J.B.W. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
Lin, S., Chen, Y., Yang, L., & Zhou, J. (2013). Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. Journal of Clinical Nursing, 22(9–10), 1281–1290. https://doi.org/10.1111/jocn.12228
Liu, L., Fiorentino, L., Natarajan, L., Parker, B.A., Mills, P.J., Sadler, G.R., . . . Ancoli-Israel, S. (2009). Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psycho-Oncology, 18(2), 187–194. https://doi.org/10.1002/pon.1412
Lu, T., Li, Y., Xia, P., Zhang, G., & Wu, D. (2014). Analysis on reliability and validity of the Pittsburgh Sleep Quality Index. Chongqing Medicine, 43(3), 260–263. https://doi.org/10.3969/j.issn.1671-8348.2014.03.002
Mendoza, T.R., Wang, X.S., Cleeland, C.S., Morrissey, M., Johnson, B.A., Wendt, J.K., & Huber, S.L. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85(5), 1186–1196.
Miaskowski, C. (2016). Future directions in symptom cluster research. Seminars in Oncology Nursing, 32(4), 405–415. https://doi.org/10.1016/j.soncn.2016.08.006
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P.A., Jacobsen, P., . . . Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute, 109(4), djw253. https://doi.org/10.1093/jnci/djw253
Miladinia, M., Baraz, S., Ramezani, M., & Malehi, A.S. (2018). The relationship between pain, fatigue, sleep disorders and quality of life in adult patients with acute leukaemia: During the first year after diagnosis. European Journal of Cancer Care, 27(1), e12762. https://doi.org/10.1111/ecc.12762
Mustian, K.M., Cole, C.L., Lin, P.J., Asare, M., Fung, C., Janelsins, M.C., . . . Magnuson, A. (2016). Exercise recommendations for the management of symptoms clusters resulting from cancer and cancer treatments. Seminars in Oncology Nursing, 32(4), 383–393. https://doi.org/10.1016/j.soncn.2016.09.002
Sharma, N., & Purkayastha, A. (2017). Factors affecting quality of life in breast cancer patients: A descriptive and cross-sectional study with review of literature. Journal of Mid-life Health, 8(2), 75–83. https://doi.org/10.4103/jmh.jmh_15_17
Sibeoni, J., Picard, C., Orri, M., Labey, M., Bousquet, G., Verneuil, L., & Revah-Levy, A. (2018). Patients’ quality of life during active cancer treatment: A qualitative study. BMC Cancer, 18, 951. https://doi.org/10.1186/s12885-018-4868-6
So, W.K.W., Law, B.M.H., Ng, M.S.N., He, X., Chan, D.N.S., Chan, C.W.H., & McCarthy, A.L. (2021). Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Medicine, 10(8), 2531–2565. https://doi.org/10.1002/cam4.3794
Sprod, L.K., Palesh, O.G., Janelsins, M.C., Peppone, L.J., Heckler, C.E., Adams, M.J., . . . Mustian, K.M. (2010). Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community Oncology, 7(10), 463–471. https://doi.org/10.1016/s1548-5315(11)70427-2
Sullivan, C.W., Leutwyler, H., Dunn, L.B., Cooper, B.A., Paul, S.M., Levine, J.D., . . . Miaskowski, C.A. (2018). Stability of symptom clusters in patients with breast cancer receiving chemotherapy. Journal of Pain and Symptom Management, 55(1), 39–55. https://doi.org/10.1016/j.jpainsymman.2017.08.008
Tang, M.F., Liou, T.M., & Lin, C.C. (2010). Improving sleep quality for cancer patients: Benefits of a home-based exercise intervention. Supportive Care in Cancer, 18(10), 1329–1339. https://doi.org/10.1007/s00520-009-0757-5
Wan, C., Zhang, D., Yang, Z., Tu, X., Tang, W., Feng, C., . . . Tang, X. (2007). Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Research and Treatment, 106(3), 413–418. https://doi.org/10.1007/s10549-007-9511-1
Wang, W., Bian, Q., Zhao, Y., Li, X., Wang, W., Du, J., . . . Zhao, M. (2014). Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. General Hospital Psychiatry, 36(5), 539–544. https://doi.org/10.1016/j.genhosppsych.2014.05.021
Wang, X.S., Hao, X.S., Wang, Y, Guo, H., Jiang, Y.Q., Mendoza, T.R., & Cleeland, C.S. (2004). Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C). Journal of Pain and Symptom Management, 27(4), 322–332. https://doi.org/10.1016/j.jpainsymman.2003.09.008
Yennurajalingam, S., Tayjasanant, S., Balachandran, D., Padhye, N.S., Williams, J.L., Liu, D.D., . . . Bruera, E. (2016). Association between daytime activity, fatigue, sleep, anxiety, depression, and symptom burden in advanced cancer patients: A preliminary report. Journal of Palliative Medicine, 19(8), 849–856. https://doi.org/10.1089/jpm.2015.0276
Zhou, K., Li, H., Wei, X., Yin, J., Liang, P., Zhang, H., . . . Zhuang, G. (2015). Reliability and validity of the multidimensional scale of perceived social support in Chinese mainland patients with methadone maintenance treatment. Comprehensive Psychiatry, 60, 182–188. https://doi.org/10.1016/j.comppsych.2015.03.007
Zimet, G.D., Dahlem, N.W., Zimet, S.G., & Farley, G.K. (1988). The multidimensional scale of perceived social support. Journal of Personality Assessment, 52(1), 30–41. https://doi.org/10.1207/s15327752jpa5201_2
Zimmermann, C., Swami, N., Krzyzanowska M., Hannon, B., Leighl, N., Oza, A., . . . Lo, C. (2014). Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet, 383(9930), 1721–1730. https://doi.org/10.1016/s0140-6736(13)62416-2

