A Decision Aid for Patients With Minimally Suspicious Screening Mammograms: A Pilot Study
Objectives: To determine the feasibility and acceptability of using a decision aid (DA) in a breast surgery clinic.
Sample & Setting: 42 patients with minimally suspicious mammograms and two physicians participated in this study at an outpatient breast specialty clinic in Virginia.
Methods & Variables: A quasiexperimental single group pilot study was conducted to determine the feasibility of DecisionKEYS, a theory-based, interactive DA intervention. Patients with minimally suspicious mammogram results chose between breast biopsy or close imaging follow-up. The Decisional Conflict Scale was used to measure decisional conflict. The Decision-Making Quality Scale was used to evaluate the overall decision process. Postintervention physician and patient feedback evaluated feasibility and acceptability.
Results: Participants and physicians rated the DA as helpful. Decisional Conflict Scale scores were low before and after the intervention. Physicians reported the DA was feasible for workflow, and the majority reported using the DA in making final recommendations. Management recommendation (breast biopsy, close imaging follow-up) changed in 26 of 42 cases from pre– to postintervention. The majority of participants underwent breast biopsy.
Implications for Nursing: The feasibility and acceptability of the DA were beneficial to patients and clinic workflow.
Jump to a section
Screening mammography is a key tool in the secondary prevention of breast cancer. Sixty-six percent of adult U.S. women aged 40 years and older have undergone screening mammography in the previous two years (Centers for Disease Control and Prevention, 2018; Nattinger & Mitchell, 2016). In 2020, women underwent more than 38 million mammograms in the United States (U.S. Food and Drug Administration, 2021). Although screening mammography has proven beneficial, concerns remain regarding balancing the benefits of screening with the costs of overdiagnosis, frequency of callbacks for additional imaging (8%–11%), and false-positive biopsy results (5%–7%) (Monticciolo et al., 2017; Nattinger & Mitchell, 2016; Siu, 2016).
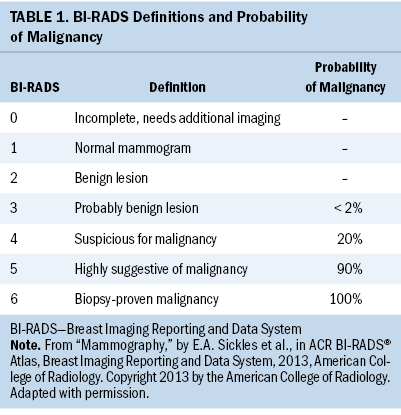
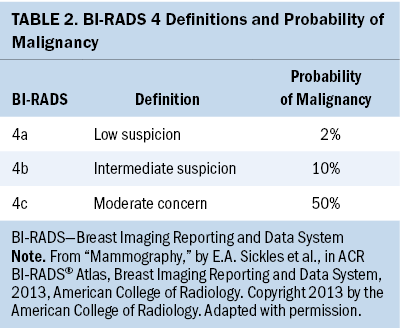
Minimizing patients’ screening-related costs has the potential to improve the patient screening experience. One opportunity for improvement is by strengthening the quality of patient decision-making in scenarios of clinical equipoise in breast imaging. Specifically, patients presenting with minimally suspicious breast imaging, frequently categorized as a Breast Imaging Reporting and Data System (BI-RADS) 4a by mammographers, may be reasonably managed with either close imaging and clinical follow-up or with needle biopsy. In addition, although the standard of care for patients found to have BI-RADS 3 lesions is short-term surveillance, a subset seeks breast biopsy. As shown in Tables 1 and 2, the estimated malignancy rate for BI-RADS 3 lesions is less than 2%, and for BI-RADS 4a lesions, the estimated malignancy rate is 2% (Sickles et al., 2013). However, patients significantly overestimate this malignancy risk (Grimm et al., 2018). In addition, 66% of women report that if there were any chance of cancer, they would choose breast biopsy over short-term follow-up (Grimm et al., 2018).
Although breast biopsy is generally well tolerated, about one-third of patients report pain within the first two weeks after the procedure (Le et al., 2016). Complications of core needle biopsy occur in less than 2% of cases and include hematoma, bleeding, vasovagal reactions, and infection (Dahabreh et al., 2014). For stereotactic (mammogram-guided) breast biopsy, the complication rate has been reported as high as 20% (Al-Harethee et al., 2013).
In addition to clinical complications that may arise from the procedure itself, there is concern about patient anxiety resulting from abnormal breast imaging. In a prior study by the authors’ research group, 12 out of 20 patients reported anxiety at the time of abnormal breast imaging, which is consistent with data from other published studies (Dengel & Chu, 2018; Health Quality Ontario, 2016). In addition, women report anxiety about the effects of a breast biopsy on other medical conditions and future breast cancer risk (Schonberg et al., 2014).
Objectives
A patient decision aid assists patients and providers in selecting treatment or care options. A patient decision aid is designed to provide specific decision context and content from clinical experts and facilitate shared, informed decision-making between patients and their providers. A Cochrane Review on the utility of decision aids (DAs) for making health decisions highlights that DAs can enhance communication between patients and clinicians and help patients become active participants in the decision-making process (Stacey et al., 2017). In addition, DAs are useful in situations where there is not a clear best choice and the treatment options are preference-sensitive (Stacey et al., 2017). The use of DAs across a range of conditions has been associated with decreased patient anxiety at the time of medical decision-making (Stacey et al., 2017). When individuals are faced with decisions that involve risk or uncertain outcomes, decisional conflict can arise (Janis & Mann, 1977; O’Connor, 1993). To the authors’ knowledge, no DA is currently available for patients who present with minimally suspicious breast imaging and must choose between undergoing breast biopsy or close imaging follow-up. The authors’ institution has developed a series of oncology DAs, called DecisionKEYS, which have been highly acceptable to patients, feasible to apply in the clinical setting, and overall rated as valuable by patients and clinicians (Hollen et al., 2013; Jones et al., 2013). The authors developed a new DA based on the DecisionKEYS model. This DA included mammography-specific content to improve decision quality for patients presenting to a breast specialist with minimally suspicious imaging. The primary aim of this study was to assess the feasibility and acceptability of using the DA in a breast surgery clinic. Secondary aims were assessment of reported decision-making quality and of decisional conflict pre– and postintervention.
Methods
Theoretical Framework
Janis and Mann’s (1977) conflict theory of decision-making is the basis for the intervention that was assessed in this pilot study (Janis, 1981). According to the theory, preconditions such as risk, hope, and time predict the degree of stress a person feels when making a decision, which influences the quality and style of decision-making. Based on the decision-making style and quality, an individual can feel either regret or satisfaction about their decision (Janis, 1981; Janis & Mann, 1977). This theory has been used globally as the basis of decision support for research for patients with breast cancer (Hollen, 1994; Hollen et al., 2013).
Design and Setting
This pilot study evaluated the feasibility and acceptability of a theory-based interactive DA for 42 patients presenting to a breast specialist to discuss management of minimally suspicious breast imaging. Data on participant impact, physician feedback, and biopsy rate were collected. Two breast surgeons participated in the study. The study was conducted in the outpatient setting at a private practice breast specialty clinic located on the East Coast of the United States.
Intervention
This study evaluated the addition of a theory-based, interactive DA to standard care for patients with minimally suspicious breast imaging results. The intervention was designed for use in cases where the physician felt that breast biopsy and close imaging follow-up were equally valid. Review of the literature and of publicly available databases found no previously designed DA available to assist in this specific medical decision. The authors developed a novel DA based on their institution’s prior DecisionKEYS interventions in oncology care, which used the Janis and Mann (1977) conflict theory of decision-making as a framework (Hollen et al., 2013; Janis, 1981).
Before study enrollment, the DA was reviewed for face validity by a panel of four experts, including two independent breast specialists and two independent decision-making experts. The DA provides patients with a comprehensive cognitive-behavioral skills program, giving information on management choices while teaching patients how to improve the quality of their decision-making.
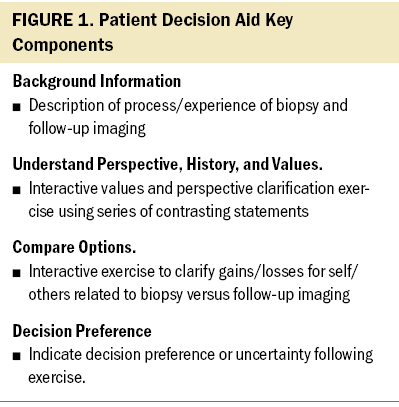
The DA is composed of three sections. Section one provides the participant with background information, outlining the respective risks and benefits of follow-up imaging and breast biopsy. The physician introduces section one and presents treatment options to consider. Sections two and three are interactive and are meant to elicit feedback from the participant and enhance communication between participants and healthcare professionals. Section two clarifies patient perspectives, history, and values. Patients use a two-column table to assess 11 aspects of their values or personal history that may affect their decision-making process or management recommendations. Section three, which is a comparison of options, uses a decision balance sheet to facilitate participant engagement in weighing gains and losses. For example, undergoing biopsy offers the gain of “having a definitive explanation of abnormal imaging in two to four days,” and choosing observation offers the potential gain of “avoiding a procedure.” A detailed summary of the content and components of the DA is shown in Figure 1. Sections two and three involve an interactive process with the consulting physician or a nurse and are designed to assist the patient in balancing choices, clarifying beliefs and values, noting concerns and conflicts, and discussing what decision they are leaning toward.
Participants
Forty-two patients and two breast surgeons participated in this study. Patient inclusion criteria were (a) able to understand English, (b) aged 18 years or older, and (c) diagnosed with minimally suspicious breast imaging for which the breast specialist had clinical equipoise regarding whether the patient should undergo a breast biopsy or close imaging follow-up alone. Typically, patients with imaging findings are classed as BI-RADS 3 or 4. Patients were eligible regardless of whether their lesions were palpable. Ineligible patients included those with known BRCA1/BRCA2 or other breast-related genetic mutations, atypia, lobular carcinoma in situ, those with a diagnosis of breast cancer within three years of the diagnostic breast imaging, and those with severe psychiatric comorbidity that would preclude full study participation. Patients who declined enrollment reported feeling confident in their first choice.
Instruments
Study measures consisted of two instruments to evaluate decision-making, two participant information forms, a participant evaluation form, and a physician evaluation form.
Demographic and clinical characteristics: The participant information forms included a form developed by the study team to collect participant sociodemographic data, including age, race, marital status, education level, income, health insurance status, and primary language. A medical record review form developed by the study team was used to record age, BI-RADS category, pathology results, additional imaging, and biopsy type.
Decisional Conflict Scale (DCS): Decisional conflict was evaluated by a 10-item low literacy version of the DCS. This instrument was selected because it is a 10-item, three-response category that participants could easily read and give their response. The conceptual framework of this scale is Janis and Mann’s conflict theory of decision-making (O’Connor, 1993). This scale has been tested in 63 women who were using a decision aid to assist in considering breast cancer options (α = 0.86) (O’Connor, 1993). Items are scored cumulatively. A score of 0 indicates no decisional conflict, and a score of 100 indicates very high decisional conflict (O’Connor, 1993).
Decision-Making Quality Scale (DMQS): The seven-item Likert-type DMQS was used to measure quality of decision-making. DMQS criteria were derived from empirical research and include considerations such as the incorporation of values and preferred outcomes, exploration of potential decision options, seeking out additional information as needed, and plan development. The DMQS was tested in five studies, three of which were among patients with cancer. In these studies, coefficient alphas ranged from 0.71 to 0.9 for internal consistency. The DMQS was also reviewed by three experts in decision theory for content validity. All study samples indicated that the instrument was feasible and acceptable (N = 766) (Hollen, 1994). Each item is scored on a four-point scale, with 0 indicating not true at all and 3 indicating very true. The possible range of total scores is 0-21. A score of 15 or higher is defined as high-quality decision-making (Hollen, 1994).
Intervention evaluation: To assess participants’ and clinicians’ perceptions of the utility and impact of the DA on each consultation, an evaluation form developed by the study team was completed by the study participants and the breast specialists administering the intervention.
Procedures
The institutional review board at the study site reviewed and approved the study. Participants were enrolled and informed consent was obtained by the study nurse, after which participants received and reviewed the DA and study forms during their consultation appointment with the breast specialist. In this study, the physician administered the measures and provided support after the intervention.
Before reviewing the DA, participants completed the DMQS and DCS scales. Each participant completed the intervention DA at the time of their initial consultation and before deciding to select biopsy or close observation. After completing the DA, during the same appointment time, the consulting surgeon reviewed each participant’s DA responses and carried out an interactive discussion with the participant to further clarify the participant’s feedback. Staff conducted other aspects of the consultation as usual. At the end of the visit, the consulting breast specialist completed an evaluation of the DA. Patients who did not elect to participate in the study received standard care and education on clinical options.
Data Analysis
All data were encoded by participants’ study IDs. Statistical analysis was performed using SAS, version 9.4. Primary descriptive results were based on all eligible participants that entered the study, regardless of compliance with study procedures. Descriptive statistics were calculated from physician evaluation forms to describe clinician perceptions of the feasibility and acceptability of the DA. Descriptive statistics were also calculated for participant sociodemographic data, biopsy rates, and pathology results.
Results
Sociodemographic and Health Status
A total of 42 individuals with minimally suspicious mammogram results participated in the study. Of the 42 participants, 2 presented with BI-RADS 3 and 40 presented with BI-RADS 4, 29 of whom were categorized as BI-RADS 4a. Nineteen additional patients were eligible but declined to participate. Of the 19 who chose not to participate, 12 opted for immediate biopsy and 7 opted for observation. All participants had health insurance. Most were white, had partners, and had completed at least some postsecondary education.
Decision-Making Measures
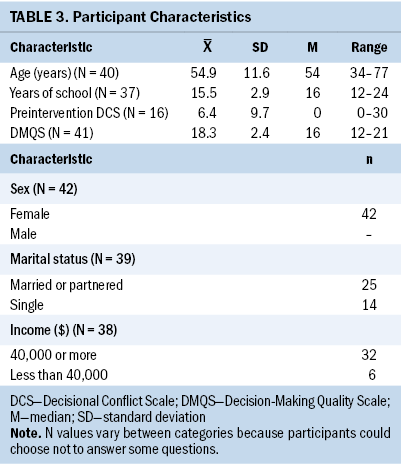
DMQS scores were high among participants, indicating high-quality decision-making. Of 41 participants in the DMQS, 38 had DMQS scores of 15 or greater. Not all participants completed the preintervention and postintervention DCS scale (see Table 3). DCS scores were low before (range = 0–30, median = 0) and after (range = 0–20, median = 0) the intervention, indicating overall low levels of decisional conflict.
Participant and Physician Evaluation
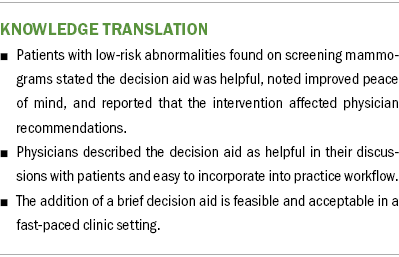
Table 4 presents data on patient and physician evaluation of the DA. Participant perception of the intervention was assessed through the participant feedback form. Of 41 participants, most agreed or strongly agreed that the DA was helpful in their decision-making (n = 38) and gave greater peace of mind about their decision (n = 39). Participants reported that the physician incorporated the results of the DA into their consultation (n = 38).
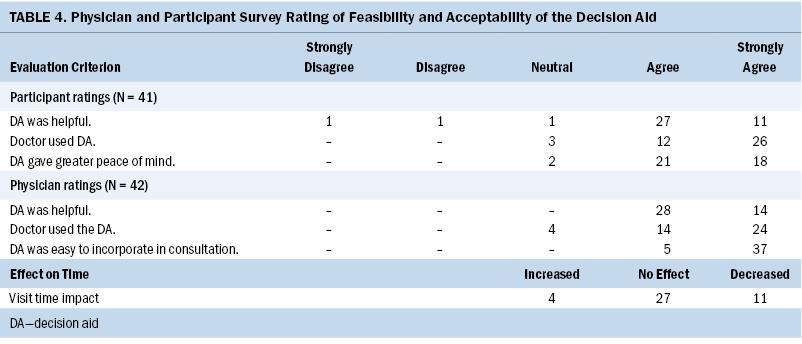
The two physician evaluators indicated that the intervention was helpful with decision-making in 42 out of 42 cases. Most reported that they were able to incorporate participants’ responses to the DA into the decision-making process (n = 38). Physician evaluators reported that DA implementation reduced perceived visit time in 11 visits, did not change perceived time in 27 visits, and only increased the perceived time requirement in 4 of the 42 cases. Physician evaluators agreed or strongly agreed that the intervention was easily incorporated into their workflow for all visits.
Impact on Decision Outcome
Before the intervention, 4 out of 15 participants preferred biopsy, 3 out of 15 preferred observation, and 8 out of 15 were uncertain about their preference. Following the intervention and during the same clinic visit, 16 out of 39 participants favored biopsy, 19 out of 39 preferred observation, and 4 out of 39 remained uncertain about their preference. In addition, physicians identified any slight preference on their own part regarding management before and after the intervention. Before the DA review with patients, the two physicians preferred biopsy in 7 of the 42 cases. After the intervention, physicians preferred biopsy in 21 of the 42 cases (see Table 5).
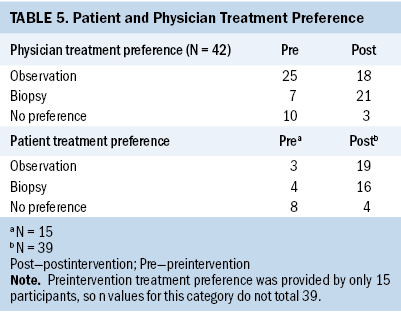
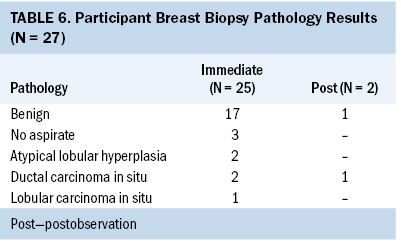
Of the 42 cases, most participants (n = 27) underwent breast biopsy. Of these, almost all biopsies (n = 25) were performed immediately following their initial consultation, and very few (n = 2) were performed after their observation period. Of the 27 patients who underwent biopsy, 21 had benign pathology or nothing to evaluate, and 3 had ductal carcinoma in situ (DCIS) (see Table 6). No invasive cancers were identified.
Discussion
This study demonstrates the feasibility of adding a brief, clinic-based DA to usual care for patients with minimally suspicious breast imaging presenting to a breast specialist. Participants and physicians indicated that the DA helped facilitate decision-making during the consultation. Physician participants felt the intervention was easily incorporated into clinical workflow and did not increase the visit time. Interestingly, in about one-fourth of the cases, using the DA resulted in physicians’ perceived decrease in visit length, perhaps reflecting the efficacy of the DA in focusing the discussion and allowing participants time to process the material while the treating physician completed a separate task. Patients and clinicians often cite time constraints as a barrier to engaging in shared decision-making (Joseph-Williams et al., 2014; Légaré et al., 2008). Thus, the potential for an intervention to be time neutral and even time-saving is valuable and bears further examination.
Participants in this study reported high-quality decision-making skills, as indicated by DMQS scores. These scores were unexpectedly high compared to findings from other studies (Hollen et al., 2013). High DMQS scores may reflect the unique qualities of this highly educated and insured study population (Ryan & Bauman, 2016). Alternatively, this could represent a ceiling effect because of cognitive bias relative to the decision faced by participants. In the original DecisionKEYS study, newly diagnosed women with breast cancer and their supporters making treatment decisions had higher baseline DMQS scores than patients making decisions about advanced lung cancer or advanced prostate cancer, which is consistent with the elevated baseline DMQS in this pilot study (Hollen et al., 2013). These findings in the area of breast disease management may be because breast cancer occurs more commonly than other cancers and abnormal mammogram results are relatively common. Patients have likely encountered similar decisions personally or with friends or family, and their experience may inform their management choices. The high DMQS score, representing the use of high-quality decision-making, is a limitation of the broader applicability of these findings and reflects the need to study the DA in a more heterogeneous group of patients.
The DCS scale measures overall decisional conflict. It has five subscales: (a) uncertainty, (b) informed, (c) values clarity, (d) support, and (e) effective decision (O’Connor, 1993). A low score on the uncertainty subscale reflects that the participant feels certain in their choice. Participant scores were generally low (mean = 35) on the DCS uncertainty subscale. This is lower than findings in previous studies of patients newly diagnosed with breast cancer (mean = 52.9), but consistent with findings for patients with advanced prostate cancer (mean = 36.4) and with advanced lung cancer (mean = 31.2) (Hollen et al., 2013). This could reflect a difference in patients’ confidence in their understanding of treatment options in the more straightforward clinical scenario as opposed to in more complex contexts.
The DCS was selected for this study because patients often indicate feelings of concern or uncertainty in navigating the decision-making process at the time of abnormal breast imaging (Schonberg et al., 2014; Solbjør et al., 2011). However, the low scores create a reverse ceiling effect, which suggests that another measure to capture other aspects of patients’ experiences may be useful in future studies. Participants did, however, report that the DA was valuable in their decision-making, suggesting that despite low reported levels of decisional conflict, the patient perception of decision-making quality may be improved with the implementation of the DA.
Interestingly, despite low levels of decisional conflict, the biopsy rate for this study was relatively low, at 64% of consented participants in contrast to the 80% of participants reported in prior descriptive work with a similar population (Dengel & Chu, 2018). This reduction may be explained by several factors seen in work on the value of DAs to participants, including greater confidence in the less-invasive option, more thorough understanding of risks and benefits, or increased trust in the relationship with and decision-making of their physician. The difference in physicians’ treatment preference may result from a clearer understanding of patient preference.
Prior work using similar DAs found that patients felt better able to engage in the decision-making process after learning about the costs and benefits of various treatment options (Jones et al., 2013). A similar process may be at work in this study, as participants gained a more complete understanding of the benefits and harms of biopsy versus observation. Future work exploring etiologies and examining whether this difference is persistent could be of use to patients and healthcare professionals. The finding of a decreased biopsy rate warrants investigation in a more extensive study to explore the possibility that using a DA may decrease the impact of false-positive imaging findings in the practice of screening mammography.
Three of the 27 participants who underwent breast biopsy were diagnosed with DCIS. Two participants underwent biopsy at the time of initial consultation, and one underwent biopsy after six-month follow-up imaging. All participants diagnosed with DCIS were managed according to standard clinical guidelines; there were no diagnoses of invasive cancer.
Limitations
The generalizability of the study findings is limited, as participants were enrolled from a single study site and were predominantly white, highly educated, insured, had high DMQS scores, and had a high household income. The potential to detect changes in DCS scores was limited by relatively low preintervention DCS scores, small sample size, and incomplete score collection. A further limitation is that not all study participants completed the DCS scale.
Despite these limitations, the current pilot study results support the feasibility and acceptability of a patient DA to facilitate optimal decision-making at the time of minimally suspicious breast imaging. Overall, physicians and patients reviewed the DA favorably. Given these findings, future research is warranted to further examine the impact of the DA on patient decision-making and decision quality.
Implications for Nursing and Practice
In clinical settings, with a breast health nurse navigator, the nurse can also administer the DA to patients considering breast screening and detection choices. It is helpful for clinic nurses or breast health navigators to understand the potential uncertainty or anxiety that an abnormal screening mammogram may cause when patients are faced with the choice to biopsy or not. Further, nurse scientists can continue to explore the need for more decisional support in this setting.
Conclusion
Findings of this pilot study indicate possible benefits of further testing of the use of a DA intervention designed for the breast clinic setting. The use of a DA to improve the shared decision-making process can offer patients more peace of mind and also provide physicians the perception of better time management and decreased clinic time. DAs have the potential to streamline clinical conversations while capturing the goals of patients and the clinical team.

About the Authors
Crystal Chu, BSN, RN, and Jonathan Yoder, MSN, RN, are PhD students in the School of Nursing, Mark Smolkin, MS, is a biostatistician in the Department of Public Health Sciences in the School of Medicine, Patricia J. Hollen, PhD, RN, FAAN, is a professor emerita in the School of Nursing, and Lynn T. Dengel, MD, MSc, is an assistant professor at the Department of Public Health Sciences in the School of Medicine, all at the University of Virginia in Charlottesville, VA. Financial support for this study was provided entirely by a grant from the American Cancer Society (#81-001-30-IRG). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Preliminary results were reported at the Society for Medical Decision-Making 41st Annual Meeting, Portland, Oregon, October 23, 2019, and at the Virginia Surgical Society 2019 Annual Meeting, Richmond, Virginia, May 18, 2019. Chu, Yoder, Hollen, and Dengel contributed to the conceptualization and design. Chu, Yoder, and Dengel completed the data collection. Smolkin provided statistical support. Chu, Yoder, Smolkin, and Dengel provided the analysis. All authors contributed to the manuscript preparation. Chu can be reached at cdm9b@virginia.edu, with copy to ONFEditor@ons.org. (Submitted September 2021. Accepted February 11, 2022.)
References
Al-Harethee, W., Theodoropoulos, G., Filippakis, G.M., Papapanagiotou, I., Matiatou, M., Georgiou, G., . . . Zografos, G. (2013). Complications of percutaneous stereotactic vacuum assisted breast biopsy system utilizing radio frequency. European Journal of Radiology, 82(4), 623–626. https://bit.ly/3ApT7Ap
Centers for Disease Control and Prevention. (2018). Mammography. https://www.cdc.gov/nchs/fastats/mammography.htm
Dahabreh, I.J., Wieland, L.S., Adam, G.P., Halladay, C., Lau, J., & Trikalinos, T.A. (2014). Core needle and open surgical biopsy for diagnosis of breast lesions: An update to the 2009 report. Agency for Healthcare Research and Quality. www.effectivehealthcare.ahrq.gov/reports/final.cfm
Dengel, L., & Chu, C. (2018). Patient decision-making and quality of life following a mildly suspicious screening mammogram. [Unpublished poster presentation]. Sentara Martha Jefferson Hospital Research Day.
Grimm, L.J., Shelby, R.A., Knippa, E.E., Langman, E.L., Miller, L.S., Whiteside, B.E., & Soo, M.S.C. (2018). Patient perceptions of breast cancer risk in imaging-detected low-risk scenarios and thresholds for desired intervention: A multi-institution survey. Journal of the American College of Radiology, 15(6), 911–919. https://doi.org/10.1016/j.jacr.2018.02.010
Health Quality Ontario. (2016). Women’s experiences of inaccurate breast cancer screening results: A systematic review and qualitative meta-synthesis. Ontario Health Technology Assessment Series, 16(16), 1–22. http://www.hqontario.ca/Portals/0/documents/evidence/reports/review-scr…
Hollen, P.J. (1994). Psychometric properties of two instruments to measure quality decision making. Research in Nursing and Health, 17(2), 137–148. https://doi.org/10.1002/nur.4770170209
Hollen, P.J., Gralla, R.J., Jones, R.A., Thomas, C.Y., Brenin, D.R., Weiss, G.R., . . . Petroni, G.R. (2013). A theory-based decision aid for patients with cancer: Results of feasibility and acceptability testing of DecisionKEYS for cancer. Supportive Care in Cancer, 21(3), 889–899. https://doi.org/10.1007/s00520-012-1603-8
Janis, I.L. (1981). Counseling on personal decisions: Theory and research on short-term helping relationships. Yale University Press.
Janis, I.L., & Mann, L. (1977). Decision making: A psychological analysis of conflict, choice, and commitment. Free Press.
Jones, R.A., Steeves, R., Ropka, M.E., & Hollen, P. (2013). Capturing treatment decision making among patients with solid tumors and their caregivers. Oncology Nursing Forum, 40(1), E24–E31. https://doi.org/10.1188/13.ONF.E24–E31
Joseph-Williams, N., Elwyn, G., & Edwards, A. (2014). Knowledge is not power for patients: A systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Education and Counseling, 94(3), 291–309. https://doi.org/10.1016/j.pec.2013.10.031
Le, M.T., Mothersill, C.E., Seymour, C.B., & McNeil, F.E. (2016). Is the false-positive rate in mammography in North America too high? The British Journal of Radiology, 89(1065), 20160045. https://doi.org/10.1259/bjr.20160045
Légaré, F., Ratté, S., Gravel, K., & Graham, I.D. (2008). Barriers and facilitators to implementing shared decision-making in clinical practice: Update of a systematic review of health professionals’ perceptions. Patient Education and Counseling, 73(3), 526–535. https://doi.org/10.1016/j.pec.2008.07.018
Monticciolo, D.L., Newell, M.S., Hendrick, R.E., Helvie, M.A., Moy, L., Monsees, B., . . . Sickles, E. (2017) Breast cancer screening for average-risk women: Recommendations from the ACR commission on breast imaging. Journal of the American College of Radiology, 14(9), 1137–1143. https://bit.ly/3pqRk7W
Nattinger, A.B., & Mitchell, J.L. (2016). Breast cancer screening and prevention. Annals of Internal Medicine, 164(11), ITC81–ITC96. https://doi.org/10.7326/AITC201606070
O’Connor, A.M. (1993). User manual: Decisional conflict scale. Ottawa Hospital Research Institute. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Con…
Ryan, C., & Bauman, K. (2016). Current Population Reports. United States Census Bureau. https://www.census.gov/library/publications/2016/demo/p20-578.html
Schonberg, M.A., Silliman, R.A., Ngo, L.H., Birdwell, R.L., Fein-Zachary, V., Donato, J., & Maracantonio, E.R. (2014). Older women’s experience with a benign breast biopsy—A mixed methods study. Journal of General Internal Medicine, 29(12), 1631–1640. https://doi.org/10.1007/s11606-014-2981-z
Sickles, E.A., D’Orsi, C.J., & Bassett, L.W. (2013). ACR BI-RADS® Mammography. In: ACR BI-RADS® atlas: Breast imaging reporting and data system. American College of Radiology. https://acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
Siu, A.L. (2016). Screening for breast cancer: U.S. preventive services task force recommendation statement. Annals of Internal Medicine, 164(4), 279–296. https://doi.org/10.7326/M15-2886
Solbjør, M., Forsmo, S., Skolbekken, J.A., & Sætnan, A.R. (2011). Experiences of recall after mammography screening: A qualitative study. Health Care Women International, 32(11), 1009–1027. https://doi.org/10.1080/07399332.2011.565530
Stacey, D., Légaré, F., Lewis, K., Barry, M.J., Bennett, C.L., Eden, K.B., . . . Trevena, L. (2017). Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 4(4), CD001431. https://doi.org/10.1002/14651858.CD001431.pub5
U.S. Food and Drug Administration. (2021). MQSA National Statistics. https://www.fda.gov/radiation-emitting-products/mqsa-insights/mqsa-nati…




