The Evolving Context of Driver Mutations: ROS1 Rearrangement in Metastatic Non-Small Cell Lung Cancer
The discovery of driver mutations in non-small cell lung cancer (NSCLC) has led to the advent of targeted therapy and changed the clinical landscape. ROS1 is a rare driver mutation found in 1%–2% of patients diagnosed with NSCLC. This case highlights a young woman of Asian descent with no history of smoking diagnosed with NSCLC and ROS1 rearrangement and discusses the implications for oncology nurses in clinical practice.
Jump to a section
A previously healthy, 30-year-old Filipino woman presented to an emergency department with complaints of shortness of breath and mild cough. She denied constitutional symptoms, such as night sweats, fevers, loss of appetite, or weight loss. Additional investigation revealed bilateral pleural and pericardial effusions with no obvious lung lesions or masses. The pericardial fluid was drained and preliminary cytology revealed atypical carcinoma cells. Her past medical history included an embryonic pregnancy and a benign breast cyst that was biopsied in the Philippines. She had immigrated to Canada two years earlier, was working full-time, and was living with her sister. She was planning on returning to the Philippines to wed and had a strong support system in Canada. She had never smoked cigarettes or consumed alcohol and had no family history of cancer. The patient was exposed to secondhand smoke as a child.
The patient was seen in an outpatient oncology clinic shortly after hospital discharge to review her final test results. Her Eastern Cooperative Oncology Group status was 0–1. Her pericardial fluid was positive for adenocarcinoma and immunohistochemistry (IHC) confirmed epidermal growth factor receptor (EGFR) negativity with no ALK rearrangement and negative KRAS. She was diagnosed with stage IV non-small cell lung cancer (NSCLC); however, the thoracic tumor board recommended a thoracoscopy and tissue biopsy to ensure that negative EGFR status was not a false-negative result—because the patient fit the clinical profile for EGFR positivity—and the limited sample may have been inaccurate.
Further tissue testing by polymerase chain rearrangement (PCR) confirmed EGFR and ALK negativity, and the decision was made to treat with the standard four cycles of cisplatin (Platinol®) and gemcitabine (Gemzar®) chemotherapy followed by pemetrexed (Alimta®) maintenance therapy. She tolerated induction and maintenance treatment with stable disease for a year and a half, at which time she began complaining of headaches. An urgent computed tomography scan of the brain confirmed the presence of brain metastases. She received whole-brain radiation and, after a two-week wash out period, started third-line docetaxel (Taxotere®) treatment. After only two cycles, she was clinically deteriorating and was admitted to the hospital with fatigue and hypoxia. She was enrolled into the palliative care program after reimaging confirmed further progression. Despite her poor prognosis, she continued to verbalize her desire to live and get married. She was not ready to die and hoped for some other treatment option.
Her pleural fluid was sent for ROS1 mutational analysis using fluorescence in situ hybridization (FISH) (of note, this test is not routinely done and has not yet been established as a standard of care at the institution). In the interim, the patient was discharged on home oxygen and started on erlotinib (Tarceva®), a broad-spectrum multikinase inhibitor, despite progressive shortness of breath. One week later, ROS1 results came back positive and the patient was started on crizotinib (Xalkori®) with immediate response. Within a week, her shortness of breath had completely resolved and her oxygen requirements decreased. After two weeks, her energy improved and she was able to walk longer distances without any oxygen support. Nine months later, she was discharged from the palliative care program and had stable disease with good partial response. She returned to work part-time and traveled to the Philippines to fulfill her wish to wed.
Lung Cancer and Driver Mutations
Lung cancer is a major cause of morbidity and mortality in the United States, with the fastest growing demographic of newly diagnosed individuals being nonsmoking women aged 30–50 years (Centers for Disease Control and Prevention, 2015; Sechler et al., 2013). About 80% of all lung cancers are classified as NSCLC, a distinction required for proper staging, treatment, and prognosis. The median age at diagnosis is 70 years, with less than 5% of patients diagnosed before age 50 years (Sacher et al., 2016). Lung cancer is characterized by a high mutational burden—compared to other forms of cancer—thought to be related to the mutagenic effect of carcinogen exposure (e.g., tobacco) (Devarakonda, Morgensztern, & Govindan, 2015). The shift from treating patients with conventional chemotherapy toward screening for the presence of specific mutational alterations driving the underlying carcinogenesis has led to the advent of personalized medicine in NSCLC.
Driver mutations are those key molecular alterations that occur within the genome of NSCLC cells that are critical for cell growth and survival and activate or drive cellular transformation (Sequist & Neal, 2016). Less essential mutations or alterations are known as passenger mutations. ROS1 is a receptor tyrosine kinase related to ALK on the insulin receptor family located on chromosome 6q22. Fusion with a portion of 1 of 12 different proteins leads to formation of ROS1 rearrangement, a driver mutation (Shaw et al., 2014; Takeuchi, Soda, & Togashi, 2012). ROS1 is not unique to NSCLC; it also is activated in a variety of other malignancies, such as cholangiocarcinoma, gastric and ovarian cancer, and glioblastoma (Shaw et al., 2014). ALK and ROS1 rearrangements rarely occur in the same tumor because most driver mutations are mutually exclusive (Wu et al., 2015).
To date, personalized therapy toward finding oncogene drivers for patients with NSCLC at diagnosis helps guide clinicians toward treatment with cytotoxic chemotherapy, or targeted therapies, based on molecular subgroups (Cao et al., 2016). About 1%–2% of NSCLC cases harbor ROS1 rearrangement, which was first identified in 2007 (Wu et al., 2015). With an estimated 200,000 new case of lung cancer annually in the United States, an estimated 4,000 cases of new ROS1 rearranged tumors will occur per year (Bergethon et al., 2012).
Clinical Characteristics of ROS1
In a review of 2,237 patients diagnosed with NSCLC at Dana-Farber Cancer Institute from 2002–2014, younger age was significantly associated with a targetable genotype (p < 0.01) (Sacher et al., 2016). Patient characteristics most commonly associated with ROS1 fusion in NSCLC are younger age, never smoked or light history of smoking, adenocarcinoma histology, and advanced stage or advanced grade disease (Bergethon et al., 2012; Mazieres et al., 2015). ROS1 also has been noted to be overrepresented in patients of Asian descent (Bergethon et al., 2012). Nonsmokers diagnosed with stage IV NSCLC are diagnosed at a more advanced stage compared to smokers. This may either be related to late presentation on the part of the patient or late diagnosis on the part of the clinicians because lung cancer in nonsmokers is comparatively less common (less than 10%) and not high on the diagnostic differential at presentation (Devarakonda et al., 2015).
Screening Modalities
Sacher et al. (2016) advocated that more aggressive efforts to routinely search for rare and even potentially novel targetable genetic alterations in patients younger than age 50 years are needed because that patient population has a 59% greater likelihood of harboring a targetable genotype (EGFR, ALK, ROS1, HER2, BRAF 6000E). This may be difficult for clinicians because no standard platform exists for testing, with break-apart FISH testing (see Figure 1) being the only assay approved by the U.S. Food and Drug Administration to detect ALK rearrangement in NSCLC (Cao et al., 2016; Sequist & Neal, 2016).
As the list of rare but potentially targetable genomic alterations increases, the potential cost and complexity of comprehensive genetic testing will continue to grow in parallel (Sacher et al., 2016). FISH, quantitative real-time PCR (qRT-PCR), and IHC all are effective methods for detection of ROS1 rearrangement, each with advantages and disadvantages (Cao et al., 2016) (see Table 1). Shan et al. (2015) compared screening efficacy for ROS1 rearrangement in 60 cases of lung adenocarcinoma. IHC identified 16 ROS1-positive cases compared with 13 cases by FISH and 20 by qRT-PCR. IHC is a reliable, rapid, and widely available screening tool for patients with NSCLC (Cao et al., 2016).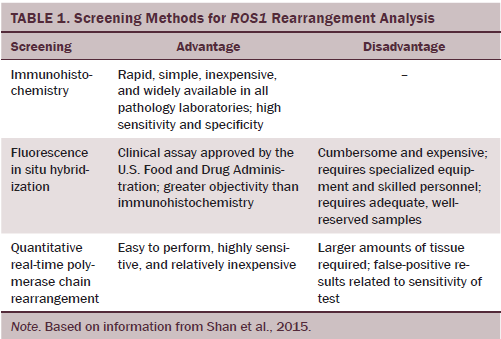
Financial, resource, and sampling drawbacks limit the application of FISH in all clinical institutions despite FISH testing being an effective diagnostic technology to detect chromosomal rearrangements in tumor tissue (Shan et al., 2015). Next-generation sequencing technology has been introduced to detect multiple alterations in lung cancer genes simultaneously.
ROS1-Targeted Therapies and Survival Benefits
Crizotinib is a small molecule tyrosine kinase inhibitor of ALK, ROS1, and MET with potent antitumor activity (Shaw et al., 2014). In particular, ROS1 is highly sensitive to crizotinib in both previously treated and treatment-naive patients (Sequist & Neal, 2016).
Fifty patients with NSCLS and ROS1 rearrangement treated with crizotinib (80% previously treated with one or more prior therapies) resulted in 3 patients with complete response and 33 with partial responses (Shaw et al., 2014). The median duration of response was 17.6 months, and median progression-free survival (PFS) was 19.2 months. In a retrospective review of 32 eligible patients treated with crizotinib with subsequent discovery of ROS1 rearrangement, Mazieres et al. (2015) reported a median PFS of 9.1 months and 44% at 12 months. The median age was 50.5 years, with 65% female and 68% nonsmokers (overall response rate = 80%), in line with the patient profile for ROS1 rearrangement.
No prognostic difference in early-stage ROS1 rearranged NSCLC is apparent, but negative consequences do exist in patients with advanced-stage NSCLC relative to patients with an EGFR mutation (Chen et al., 2014). Regardless, the majority of patients treated with critzotinib will develop resistance in the first 12 months with two postulated mechanisms for resistance: (a) secondary mutations that hinder drug binding, and (b) activation of EGFR, which allows cancer cells to overcome crizotinib-mediated inhibition of ROS1 signalling (Camidge, Bang, & Kwak, 2012; Shaw et al., 2014). Second-generation inhibitors are in development to overcome crizotinib-resistant disease (Sequist & Neal, 2016).
Implications for Oncology Nurses
The treatment landscape in NSCLC is constantly evolving as new driver mutations and targeted therapies are developed and introduced into clinical practice. Oncology nurses will be challenged to stay current and informed about new discoveries and treatments, but also knowledgeable about different techniques for testing, advantages and limitations of each test, and local availability until standardization has occurred. Nurses also can advocate for personalized genotype screening and inquire about targeted therapies when individuals with high-risk characteristics present in practice (e.g., young, female, nonsmokers). Survival in younger individuals diagnosed with NSCLC is poor in comparison with other age groups, which may be a result of more aggressive disease biology than that found in older adult patients (Sacher et al., 2016). For this reason, clinicians should be particularly vigilant when lung cancer is suspected in younger individuals and advocate for early screening to detect genetic alterations prior to systemic intervention. Young, nonsmoking individuals and caregivers may require more education and counseling to overcome the uncertainty of their diagnosis, a role that oncology nurses are suited to fulfil.
Conclusion
In the case of a patient with terminal lung cancer, implementation of driver mutation screening and next-sequencing gene technology may challenge the known cancer trajectory and enable nurses to foster hope in patients where none previously existed. In the case study, in the absence of ROS1 driver oncogene testing, the patient would have died soon after being enrolled into palliative care because ROS1-positive tumors are not sensitive to erlotinib therapy (Davies et al., 2012). The patient’s younger age, desire to live, and maintenance of hope drove the decision to pursue additional molecular testing.
References
Bergethon, K., Shaw, A.T., Ou, S., Katayama, R., Lovly, C.M., McDonald, N.T., . . . Iafrate, A.J. (2012). ROS1 rearrangements define a unique molecular class of lung cancers. Journal of Clinical Oncology, 30, 863–870. doi:10.1200/JCO.2011.35.6345
Camidge, D.R., Bang, Y.J., & Kwak, E. (2012). Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncology, 13, 1011–1019. doi:10.1016/S1470-2045(12)70344-3
Cao, B., Wei, P., Liu, Z., Bi, R., Lu, Y., Zhang, L., . . . Zhou, X. (2016). Detection of lung adenocarcinoma with ROS1 rearrangement by IHC, FISH, and RT-PCR and analysis of its clinocopathologic features. Journal of OncoTargets and Therapy, 9, 131–138.
Centers for Disease Control and Prevention. (2015). United States cancer statistics: Lung cancer. Retrieved from https://www.cdc.gov/cancer/lung/statistics
Chen, Y.F., Hsieh, M.S., Wu, S.G., Chang, Y.L., Shih, J.Y., Liu, Y.N., . . . Yang, P.C. (2014). Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. Journal of Thoracic Oncology, 9, 1171–1179. doi:10.1097/JTO.0000000000000232
Davies, K.D., Le, A.T., Theodoro, M.F., Skokan, M.C., Aisner, D.L., Berge, E.M., . . . Doebele, R.C. (2012). Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clinical Cancer Research, 18, 4570–4590. doi:10.1158/1078-0432.CCR-12-0550
Devarakonda, S., Morgensztern, D., & Govidan, R. (2015). Genomic alterations in lung adenocarcinoma. Lancet, 16, 342–351. doi:10.1016/S1470-2045(15)00077-7
Mazieres, J., Zalcman, G., Crino L., Biondani, P., Barlesi, F., Filleron, T., . . . Gautschi, O. (2015). Crizotnib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 cohort. Journal of Clinical Oncology, 33, 992–1001. doi:10.1200/JCO.2014.58.3302
Sacher, A.G., Dahlberg, S.E., Heng, J., Mach, S., Janne, P.A., & Oxnard, G.R. (2016). Lung cancer diagnosis in the young is associated with enrichment for targetable genomic alterations and poor prognosis. JAMA Oncology, 2, 313–320. doi:10.1001/jamaoncol.2015.4482
Sechler, M., Cizmic, A.D., Avasarala, S., Van Scoyk, M., Brzezinski, C., Kelley, N., . . . Winn, R.A. (2013). Non-small-cell lung cancer: Molecular targeted therapy ad personalized medicine-drug resistance, mechanisms, and strategies. Pharmacogenomics and Personalized Medicine, 6, 25–35.
Sequist L.V., & Neal, J.W. (2016) Personalized, genotype-directed therapy for advanced non-small cell lung cancer. Retrieved from http://bit.ly/2aJRAMm
Shan, L., Lian, F., Guo, L., Qiu, T., Ling, Y., Ying, J., & Lin, D. (2015). Detection of ROS1 gene rearrangement in lung adenocarcinoma: Comparison of IHC, FISH, and real-time RT PCR. PLOS One, 10, e0120422. doi:10.1371/journal.pone.0120422
Shaw, A.T., Ou, S.H., Bang, Y.J., Camidge, D.R., Solomon, B.J., Salgia, R., . . . Iafrate, A.J. (2014). Crizotinib in ROS1-rearranged non-small cell lung cancer. New England Journal of Medicine, 371, 1963–1971. doi:10.1056/NEJMoa1406766
Takeuchi, K., Soda, M., & Togashi, Y. (2012). RET, ROS1, and ALK fusions in lung cancer. Nature Medicine, 18, 378–381. doi:10.1038/nm.2658
Wu, S., Wang J., Zhou, L., Su, D., Liu, Y., Zhang, S., & Zeng, X. (2015). Clinicopathological characteristics and outcomes of ROS1-rearranged patients with lung adenocarcinoma without EGFR, KRAS mutations and ALK rearrangement. Thoracic Cancer, 6, 413–420. doi:10.1111/1759-7714.12191
About the Author(s)
DeCaire is a nursing unit manager and Streu is a clinical nurse specialist, both at CancerCare Manitoba in Winnipeg, Canada. No financial relationships to disclose. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. DeCaire can be reached at xdecaire@cancercare.mb.ca, with copy to editor at ONFEditor@ons.org.

