Association of Smoking in the Home With Lung Cancer Worry, Perceived Risk, and Synergistic Risk
Purpose/Objectives: To examine the association of smoking in the home with lung cancer worry, perceived risk, and synergistic risk, controlling for sociodemographics, family history of lung cancer, and health-related self-concept. The hypothesis is that participants with smoking in the home would have higher scores for lung cancer worry, perceived risk, and synergistic risk.
Design: Cross-sectional baseline survey.
Setting: Participants recruited from an outpatient clinic and pharmacy at University of Kentucky HealthCare, an academic medical center.
Sample: 515 homeowners from a larger randomized, controlled trial aimed at reducing exposure to radon and secondhand smoke (SHS).
Methods: Homeowners were selected via quota sampling so that about half would have a smoker or smokers in the home.
Main Research Variables: Lung cancer worry and perceived risk; perception of synergistic risk of radon and SHS exposure; demographics.
Findings: Participants with smoking in the home had higher rates of lung cancer worry and perceived risk. In addition, those with less education and a family history of lung cancer and who were current smokers had higher lung cancer worry and perceived lung cancer risk scores. Predictors of perception of synergistic risk were marital status and health-related self-concept.
Conclusions: Homeowners with smoking in the home, less education, and a family history of lung cancer had greater lung cancer worry and perceived lung cancer risk. Lung cancer risk reduction interventions with vulnerable populations are needed.
Implications for Nursing: Nurses are in a unique position to target high-risk populations and identify opportunities to create teachable moments to reduce environmental risks of radon and tobacco smoke exposure.
Jump to a section
Lung cancer remains the leading cause of cancer death in the United States (Henley et al., 2014), although it is largely preventable by eliminating smoking, as well as exposure to radon and secondhand smoke (SHS) (Centers for Disease Control and Prevention [CDC], 2016c). Many people have heard that exposure to tobacco smoke is a cause of lung cancer because this information is widely available in the popular press. However, an estimated 25% of lung cancer cases globally occur in nonsmokers, resulting in about 300,000 deaths every year (Sun, Schiller, & Gazdar, 2007). The second leading cause of lung cancer among smokers and the leading cause among nonsmokers is radon exposure (Neri, Stewart, & Angell, 2013), causing about 15,000–22,000 lung cancer deaths annually in the United States (National Cancer Institute, 2011). More radon-related lung cancers occur in those with a history of smoking than in those without a history of smoking. Exposure to both radon and tobacco smoke produces a synergistic risk, increasing the likelihood of developing lung cancer (National Research Council, 1999) by nearly tenfold (U.S. Environmental Protection Agency, 2012), although radon exposure is a risk for smokers and nonsmokers. Among never smokers, exposure to radon may be more harmful to those who have also been exposed to SHS (Lagarde et al., 2001).
The home is the major source of SHS and radon exposure. Households with less educated parents or headed by a single parent are more likely to report smoking indoors (Klepeis et al., 2013; Zhang, Martinez-Donate, Kuo, Jones, & Palmersheim, 2012). Radon, an odorless, colorless radioactive gas, can enter a home by diffusion from the soil through concrete floors and walls, foundation cracks, floor drains, sump pumps, construction joints, and cracks or pores in hollow block walls (Kennedy, Probart, & Dorman, 1991; Radon Testing Corporation of America, n.d.).
People may perceive the presence of SHS and/or radon in the home as a threat, potentially prompting them to worry about lung cancer, which creates a teachable moment (TM) and stimulates action (McBride et al., 2008). The TM model defines TMs as health events that occur naturally and are believed to motivate individuals to make positive changes to reduce risk. A health event can serve as a cue to perceive a health threat, which can motivate an individual to reduce the threat (e.g., adoption of a smoke-free home) (McBride, Emmons, & Lipkus, 2003). TMs are characterized by three major psychosocial factors: perceived risk, emotions such as worry, and health-related self-concept. Because more radon-related lung cancers occur among those with a history of smoking (National Research Council, 1999; Sun et al., 2007), a fourth psychosocial factor was added, perceived synergistic risk, to better understand TMs for lung cancer risk reduction. Because health events, such as cancer diagnoses, are typically not predicted or randomly assigned, research on the TM has been challenging. A long-term goal of this research is to create TMs that can reduce lung cancer risk (e.g., radon testing).
The prevention of lung cancer is of importance to oncology nurses because it is the second most common cancer diagnosed in men and women, causing one of every four cancer deaths in the United States (American Cancer Society, 2016). Equally important is the identification of evidence-based strategies to educate the public and create behavior change to reduce environmental risks. The purpose of this study was to examine the association of smoking in the home with lung cancer worry, perceived risk, and synergistic risk (SHS plus radon), controlling for sociodemographics, family history of lung cancer, and health-related self-concept. The current authors hypothesized that participants with smoking in the home would have higher scores for lung cancer worry, perceived risk, and synergistic risk, as well as that these outcomes would be associated with demographic characteristics and health-related self-concept.
Methods
Design and Sample
The study was a descriptive correlational design. The data were collected during the baseline (i.e., preintervention) assessment of a larger randomized, controlled trial to test the effects of a dual home screening intervention for reducing home exposure to radon and SHS. A quota sample of homeowners were recruited from clinics and a pharmacy at an academic medical center, as well as from community locations (e.g., health fairs, homeowners’ association meetings), in central Kentucky and enrolled on site. Smoking in the home was assessed, and homeowners within each of the equally sized “smoking in the home” strata (i.e., those with and without smoking in the home) were randomly assigned to the intervention or control group for the larger trial. The study was approved by the University of Kentucky Medical Institutional Review Board.
Measures
Smoking in the home was assessed by asking the following question, answered with a “yes” or “no” response: “Do you or any other members of your household smoke cigarettes, cigars, or pipes?” Lung cancer worry was measured using a series of four items shown to be associated with taking health-promoting actions and adapted from the three-item validated Cancer Worry Scale (Lerman, Trock, Rimer, Boyce, et al., 1991; Lerman, Trock, Rimer, Jepson, et al., 1991). The first item—“How much do you currently worry about getting lung cancer someday?”—was measured using a five-point Likert-type scale ranging from 1 (not at all) to 5 (all the time). The other three items—“How much do worries about lung cancer impact your mood?”; “How much do worries about lung cancer impact your daily activities?”; and “When you worry about lung cancer, how difficult is it to control these worries?”—were measured using a four-point Likert-type scale ranging from 1 (not at all) to 4 (all the time). To ensure that all four items received equal weight for the final lung cancer worry score, the first item was multiplied by a factor of 4/5, resulting in a value of 4 as the maximum contribution from each item. A summary score was created, with higher scores representing greater lung cancer worry. The Cronbach alpha for this sample was 0.82.
Lung cancer risk was measured using a single ordinal item: “How would you rate your risk of developing lung cancer in your lifetime on a scale of 0–10?” Higher scores indicated elevated risk (Hahn, Rayens, Hopenhayn, & Christian, 2006). Similarly, synergistic risk was measured using a single item asking participants to rate the risk of developing lung cancer from being exposed to radon and smoking a pack of cigarettes per day, compared to the risk of only smoking a pack of cigarettes per day with no radon exposure. Participants rated risk on a five-point Likert-type scale ranging from 1 (much less risky) to 5 (much more risky).
Participants’ sociodemographic and personal characteristics were assessed; they are listed in Table 1. Because few participants indicated minority racial and ethnic group membership, categories were combined to form a single measure of race or ethnicity (i.e., “White and non-Hispanic” versus “other”). Family history of lung cancer was determined by asking the following question, answered with a “yes” or “no” response: “Has anyone in your family ever been told they have lung cancer?” Personal smoking was determined by asking the following question: “When was the last time you smoked a cigarette?” Current smokers indicated having smoked in the past 30 days, whereas former or never smokers had either not smoked in the past month or in their lifetime. 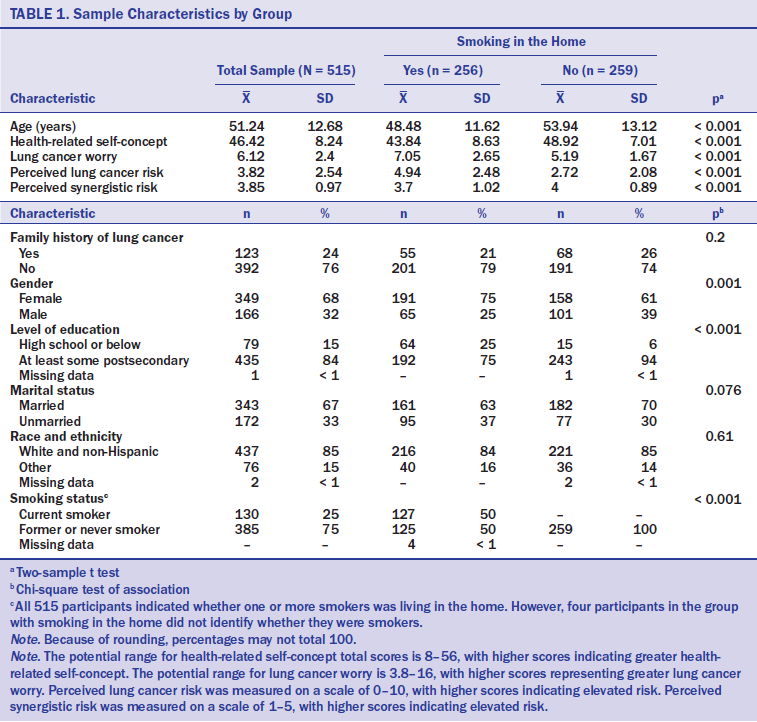
Health-related self-concept was measured using the eight-item validated health-protective motivation subscale of the Generalized Health-Related Self-Concept–76 (Wiesmann, Niehörster, Hannich, & Hartmann, 2008). The subscale measures beliefs and attitudes toward health-enhancing behaviors (one item is, “I look after my health consciously”) and behavioral intentions (one item is, “In general, practicing healthy behaviors is good for me”). Participants indicated their level of agreement with the subscale statements using a seven-point Likert-type scale ranging from 1 (disagree entirely) to 7 (agree entirely). All items except one were positively worded so that higher scores would reflect more health-enhancing beliefs; the one negatively worded item was reverse-coded to create the summary score. The summary score was calculated by summing the eight items; the potential range is 8–56, with higher scores indicating greater health-related self-concept. The Cronbach alpha for this sample was 0.91.
Analytic Strategy
Study variables were summarized using descriptive statistics, including means and standard deviations or frequency distributions. Bivariate analysis, including the two-sample t test and chi-square test of association, was used to compare study variables between those with and without smoking in the home. Multiple linear regression was used to test for associations of demographic and personal variables, smoking-related indicators, and health-related self-concept with lung cancer worry, perceived lung cancer risk, and synergistic risk. Variance inflation factors were used to determine if multicollinearity was present in the regressions. All data analysis was conducted using SAS®, version 9.4, with an alpha level of 0.05 throughout.
Results
Compared to participants with smoking in the home, participants without smoking in the home were older (48.5 years versus 53.9 years, respectively; p < 0.001). Participants from households with smoking were more likely to be female (75%) compared to those from nonsmoking households (61% female; p < 0.001). The association of home smoking status with race and ethnicity was not significant; in the total sample, 85% were White and non-Hispanic. Participants with smoking in the home were less likely to report having completed postsecondary education (75%) compared with those without smoking in the home (94%) (p < 0.001). The percentage of married participants did not differ significantly between the two home smoking groups (i.e., those with and without smokers in the home); 67% of all participants were married. The association between family history of lung cancer and home smoking group was not significant; 24% of all participants had a family history of lung cancer.
Consistent with the design, personal smoking status was correlated with home smoking group. Of those who lived in a home with one or more smokers, 50% were current smokers, whereas none of those living in a home without smokers personally smoked (p < 0.001). Among those living in a home with smokers, 52% of female participants were nonsmokers, compared to 40% of male participants who did not currently smoke (chi-squared = 3, p = 0.085). Health-related self-concept was significantly lower among those living in a home with smoking, and lung cancer worry and perceived lung cancer risk were higher, compared with those living in a home without smoking (p < 0.001 for each of these three group comparisons). Perceived synergistic risk was lower among those living in a home with smoking, compared to participants living in a home without smoking (p < 0.001).
Associations of Worry and Perceived Risk Outcome Variables
The variance inflation factors for the set of possible independent variables (i.e., age, gender, race and ethnicity, marital status, education, family history of lung cancer, personal smoking status, smoking in the home, and health-related self-concept) were all less than 1.7, suggesting that multicollinearity was not a factor. Smoking in the home was predictive of lung cancer worry and perceived risk of lung cancer. Other significant predictors of lung cancer worry included postsecondary education, family history of lung cancer, and current smoking status (see Table 2). The model was significant overall (p < 0.001), with an R2 of 0.24. Those with postsecondary education scored an average of 1.3 points lower on the lung cancer worry scale (p < 0.001), whereas those with a family history of lung cancer scored an average of 0.6 points higher on the worry scale (p = 0.004) (points refer to how much average lung cancer risk score changes as a result of changing the value of the significant predictors in the model). Current smokers scored 1.1 points higher on lung cancer worry than former or never smokers (p < 0.001), and participants with smoking in the home rated their lung cancer worry one point higher than those in homes without smoking (p < 0.001). The other variables in the model were not significantly associated with lung cancer worry. The model with the outcome of perceived risk of lung cancer was significant overall (R2 = 0.31, p < 0.001). Participants with postsecondary education rated their risk as nearly one point lower (0.9) than those with, at most, a high school degree (p = 0.001). Those with a family history of lung cancer rated their perceived risk of developing the disease as 1.3 points higher, on average, compared to those without a family history of lung cancer (p < 0.001). Current smokers rated their perceived risk as 1.5 points higher than nonsmokers (p < 0.001), and participants living in a home with one or more smokers rated their lung cancer risk as 1.2 points higher, on average, than those in homes without smokers (p < 0.001). Similar to the lung cancer worry model, the other variables did not predict perceived risk of lung cancer.
[[{"type":"media","view_mode":"media_original","fid":"30211","attributes":{"alt":"","class":"media-image","height":"472","typeof":"foaf:Image","width":"947"}}]]
Smoking in the home was not associated with synergistic risk perception. The significant predictors of perception of synergistic risk included marital status and health-related self-concept. The model was significant overall (p < 0.001), but the R2 was relatively modest, at 0.09. Compared to unmarried participants, those who were married perceived the synergistic risk of smoking one pack of cigarettes per day and radon exposure as being about 0.2 points higher, on average (p = 0.02). For each 10-point increase in health-related self-concept, the perception of synergistic risk increased by an average of 0.2 points (p = 0.004). Synergistic risk perception was not related to family history of lung cancer, personal smoking, or other demographic or personal variables.
Discussion
This study examined the association of smoking in the home with lung cancer worry, perceived risk, and synergistic risk, controlling for demographic and personal variables. As hypothesized, those with smoking in the home had greater lung cancer worry and perceived lung cancer risk than those without smoking in the home. Study participants who were current smokers also reported greater lung cancer worry and risk of developing lung cancer. Similarly, Finney Rutten, Blake, Hesse, Augustson, and Evans (2011) observed that current smokers perceive their lung cancer risk to be very high and report worry about lung cancer more frequently than former and never smokers. Those who are at high risk for developing cancer report more worry (McCaul, Branstetter, O’Donnell, Jacobson, & Quinlan, 1998). However, smokers may underestimate their risk of developing lung cancer (McCoy et al., 1992; Weinstein, Marcus, & Moser, 2005). The fact that those with tobacco smoke exposure in this study reported more lung cancer worry and greater perceived risk than nonsmokers and those without smoking in the home provides support for creating and testing targeted TM interventions to reduce SHS and increase access to tobacco treatment (McBride, Emmons, & Lipkus, 2003). Homeowners with less education reported greater lung cancer worry and perceived lung cancer risk. What may partially explain this finding is that individuals with less education are more likely to be smokers (CDC, 2016a) and that smokers in this study reported greater lung cancer worry and perceived risk. Individuals with less education tend to fall into a lower socioeconomic status (SES) group, which bears a disproportionate burden of tobacco-related illness and death. This is, in part, attributable to disparities in access to health care and smoking cessation programs (American Legacy Foundation, 2009), as well as the tobacco industry’s targeted promotion to low SES populations (Apollonio & Malone, 2005). A systematic review of behavior change interventions with low-income groups revealed small positive effects on healthy behaviors, including smoking cessation (Bull, Dombrowski, McCleary, & Johnston, 2014); more research is needed on effective lung cancer risk reduction approaches concerning radon and tobacco smoke for low-income groups. Consideration of health literacy is important in this population because it may affect individuals’ ability to engage in self-care. Key components of health literacy are using plain language people can understand the first time they hear it, relaying the most important points first, breaking complex information into simpler pieces, defining technical terms, and using active voice (U.S. Department of Health and Human Services, n.d.). In addition to behavior change interventions, policy approaches affecting low income populations are critical, including promoting smoke-free public housing, improving access to tobacco treatment, counteracting tobacco industry messages, and funding culturally and linguistically appropriate educational interventions. Hewett, Sandell, Anderson, and Niebuhr (2007) found that 48% of renters in multiunit housing facilities had experienced SHS exposure at some point, and 54% expressed interest in living in smoke-free housing. Policy interventions are particularly important for those living in public housing who are more affected by SHS (Winickoff, Gottlieb, & Mello, 2010) and may not feel empowered to request smoke-free housing (Hennrikus, Pentel, & Sandell, 2003). Findings paired with existing evidence provide support for more research on radon and SHS exposure, lung cancer worry, and perceived risk in low SES populations.
Those with a family history of lung cancer reported greater lung cancer worry and perceived lung cancer risk. Consistent with recommendations from the CDC (2016b) and others (Ramsey, Yoon, Moonesinghe, & Khoury, 2006), assessment and consideration of family history of lung cancer needs to be integrated into cancer prevention and control programs and into all public health nursing settings. In contrast to the findings of this study, Weinstein et al. (2005) reported that having a family history of lung cancer was not associated with perceived risk among current and former smokers; a potential explanation for this finding is that smokers are likely to minimize their own health risks. However, because participants with a family history of lung cancer were more worried and viewed themselves more at risk in this study, those with a family history of lung cancer need targeted interventions to create TMs to reduce lung cancer risk. Nurses are in a unique position to intervene here. A diagnosis of lung cancer may motivate close friends and family members to quit smoking (Gritz et al., 2006; McBride, Emmons, & Lipkus, 2003; McBride, Pollak, et al., 2003). However, the majority of family members of patients with lung cancer continue to smoke after their family member’s diagnosis. In a study of families of patients with lung cancer, Butler, Rayens, Zhang, and Hahn (2011) found that 72% of family members planned to quit within the next six months. Motivation to quit smoking was positively correlated with perceived lung cancer risk. A diagnosis of lung cancer represents a TM for family members who may be amenable to tobacco treatment, as well as to radon and SHS risk reduction measures.
The model predicting synergistic risk for lung cancer was the weakest of the three models tested in this study. Those who were married perceived greater potential for synergistic risk than did unmarried participants. Synergistic risk for lung cancer was also rated higher among those with greater health-related self-concept scores. Perception of synergistic risk was not related to family history, smoking status, or smoking in the home. In contrast, family history of lung cancer and smoking in the home were related to lung cancer worry and perceived risk of lung cancer, which were measured in regard to how participants were personally affected. Synergistic risk was conceptualized in this study as the perception of risk of “someone” who smokes one pack of cigarettes per day and is exposed to radon. This difference in perspective (i.e., personal versus external to self) may be one reason why family history of lung cancer and smoking in the home did not predict synergistic risk for lung cancer. Measurement tools to assess personal synergistic risk are needed. Another reason that known risk factors for lung cancer may not have predicted synergistic risk may be a lack of public awareness that risk for lung cancer dramatically increases when an individual is exposed to radon and SHS (National Research Council, 1999). Alerting homeowners with smoking in the home to the combined risks of radon and SHS is a way to promote risk reduction behaviors. Developing and testing risk reduction interventions framed around the concept of synergistic risk and employed in the general population is warranted.
Participants with smoking in the home had poorer health-related self-concept and lower perception of synergistic risk based on the bivariate analysis. Smoking in the home may negatively affect perception of health status and lessen perceived quality of life. Perception of synergistic risk was lower among those with smoking in the home. Implications exist for educational and policy interventions, as well as for research related to the synergistic risk of tobacco smoke and radon exposure aimed at those with smoking in the home. Because smoking in the home was connected with all three of the major psychosocial factors associated with creating a TM (perceived risk, emotions such as worry, and health-related self-concept), testing for radon and SHS in the home may serve as a cue to perceive a health threat, which could then motivate homeowners to reduce the threat (McBride, Emmons, & Lipkus, 2003), such as radon mitigation and/or adoption of a smoke-free home (Hahn et al., 2014).
Those with smoking in the home tended to be younger, female, and less educated, as demonstrated in the bivariate analysis. Nationally, the highest smoking rates are among younger adults and those with lower education levels, although smoking rates tend to be higher among men than women (CDC, 2016a). In this study, more than half of the women living in homes with smokers were not smokers themselves, compared with only two-fifths of men in the same situation. Findings from this study are similar to those in a study reporting that, among women, those closer to the poverty level were less likely to have a smoke-free home (Shavers et al., 2006). Low parental education, unemployment, low household equivalent income, and single-parent family are independently associated with children’s SHS exposure in the home (Bolte & Fromme, 2008; Mills, White, Pierce, & Messer, 2011). Targeted interventions to promote smoke-free environments are needed with young, less educated female homeowners.
Limitations
A few limitations of this study exist. Because quota sampling was used to recruit participants (i.e., half in each home smoking stratum), the two groups differed somewhat demographically. This concern is lessened because of inclusion of demographics as controls in the multivariate analyses. In addition, the sample was largely White and non-Hispanic. Although this is consistent with the race and ethnicity distribution in the study’s geographic area, generalizability to other regions may be limited. Finally, the sample was relatively well educated compared to the general population, likely reflective of homeowner status as one of the inclusion criteria. This limits generalizability of these findings to more diverse populations, particularly those with lower socioeconomic status who may be more affected by tobacco use and lung cancer.
Implications for Nursing
Nurses, particularly oncology nurses, are well prepared for involvement in screening and prevention activities directed at lung cancer. Their expertise lends credibility and a perspective that is unique to their practice. Nurses are also in a key position to target high-risk populations and their families, as well as to identify opportunities for creating TMs to reduce risks of radon and tobacco smoke exposure. TMs are not necessarily random and can be created as a deliberate element of nurse–patient interactions. Education plays a large role in such interventions. For example, given a diagnosis of lung cancer, oncology nurses could talk about the risks of radon and ask the patient and his or her family to test their home.
Targeted campaigns have been shown to motivate people to consider smoking cessation and support smoke-free policy (Butler et al., 2014; Riker et al., 2015). In addition to information about the dangers of tobacco smoke and radon exposure, education about the synergistic effects of these two risk factors is essential. Nurses need to combine the message when providing evidence-based tobacco treatment and SHS reduction interventions by encouraging smoke-free homes and home radon testing. Home test kits for radon are commercially available at a low cost, but many may not know where or how to get radon test kits or even how to use or interpret them once purchased (Kennedy et al., 1991). Education-based interventions may serve as a cue, resulting in a perceived health threat, which can, in turn, motivate positive behavior change to eliminate tobacco smoke and radon exposure. Oncology nurses can also lead initiatives to promote smoke-free policy and mandatory radon testing, which have been shown to decrease smoking rates, as well as exposure to SHS and radon (Hahn, York, & Rayens, 2010; Lantz, Mendez, & Philbert, 2013).
Targeting those exposed to SHS in the home with a combined message to reduce radon and tobacco smoke is indicated and appropriate for nurses who work with this vulnerable population. Oncology nurses are also well positioned to be involved in research to develop and test lung cancer risk reduction interventions that create TMs with vulnerable populations. 
Conclusion
Results of this study reveal that homeowners with smoking in the home, less education, and a family history of lung cancer had greater lung cancer worry and perceived lung cancer risk. Those with smoking in the home were more likely to be younger, less educated, and female. Targeting patients and family members exposed to SHS in the home with a combined message to reduce radon and tobacco smoke is indicated. Findings establish the need to develop and test lung cancer risk reduction interventions that create TMs with vulnerable populations. Oncology nurses have special expertise that makes them ideal to encourage patients and their families, particularly those with smokers in the home, to reduce exposure to SHS and radon to prevent lung cancer.
About the Author(s)
Butler is an associate professor and assistant dean, Rayens is a professor, Wiggins is a statistician, Rademacher is a data manager, and Hahn is a professor and director, all in the College of Nursing at the University of Kentucky in Lexington. This project was funded by the National Institute of Environmental Health Sciences (NIEHS) and the National Institute of General Medical Sciences (NIGMS) (R01ES021502). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIGMS, or the National Institutes of Health. Hahn has received research funding and travel support from NIEHS. Butler, Rayens, and Hahn contributed to the conceptualization and design. Butler, Rademacher, and Hahn completed the data collection. Rayens and Rademacher provided statistical support. Butler, Rayens, and Rademacher provided the analysis. All of the authors contributed to the manuscript preparation. Butler can be reached at karen.butler@uky.edu, with copy to editor at ONFEditor@ons.org. Submitted May 2016. Accepted for publication July 21, 2016.

