Temporary Stoppages and Burden of Treatment in Patients With Cancer
Objectives: To examine the effect of burden of treatment and multimorbidity on the relationship between baseline characteristics and oral oncolytic agent (OOA) temporary stoppages.
Sample & Setting: 272 patients newly prescribed OOAs at six National Cancer Institute–designated comprehensive cancer centers.
Methods & Variables: Patients were randomly assigned to an adherence and symptom management group or a usual care/control group. Temporary OOA stoppages, symptom interference, OOA regimen complexity, and multimorbidities were explored. Data were collected at four-week intervals for 12 weeks.
Results: Burden of treatment variables and multimorbidity had no significant effect on OOA temporary stoppages. Women and those prescribed kinase inhibitors were significantly more likely to experience a temporary stoppage.
Implications for Nursing: Oncology nurses are in a crucial position to educate patients on self-management of OOAs and symptoms. Nurses should be aware of patients who may be more susceptible to severe symptoms, including those with multimorbidities. Future research is needed to better understand OOA stoppages and factors associated with preventing stoppages.
Jump to a section
Individual characteristics may play a role in the treatment regimens of patients with cancer. Age, sex, body mass index (BMI), race, and other characteristics have been extensively researched in patients with cancer to facilitate providers’ decision-making ability regarding treatment plans (Tawfik et al., 2016). However, the presence of multimorbid conditions in patients with cancer brings about unique challenges for providers and patients (Sarfati, Koczwara, & Jackson, 2016). As life expectancy increases, the issue of cancer and multimorbidity will become a growing consideration for researchers and clinicians. For patients, the issue of cancer and multimorbidity brings about an increased number of tasks needed to manage their diseases, as well as their perspective of their cancer and multimorbidities and the associated workload. The workload for patients with cancer who are prescribed oral oncolytic agents (OOA) presents unique challenges because they experience greater responsibility for self-management than those treated with IV chemotherapy (Zerillo et al., 2017). Unlike IV chemotherapy, patients prescribed OOAs must manage their medications and side effects in the home (Salgado et al., 2017).
Burden of treatment (BOT) is defined as the combination of a patient’s workload and his or her perspective of the condition and workload (Eton et al., 2015; Sav et al., 2013; Tran, Barnes, Montori, Falissard, & Ravaud, 2015). The need to examine this concept is related to the shift to shorter inpatient stays and a greater emphasis on patient self-management in the home and, therefore, a greater workload or burden (Pefoyo et al., 2015; Williams et al., 2016). Tran et al. (2015), Sav et al. (2013), and Eton et al. (2015) are the three primary contributors to BOT conceptual literature. Their work began with the conceptualization of BOT, and since then, they have started to empirically measure the concept within certain chronic disease populations.
The existing BOT literature has focused on several chronic conditions, including HIV/AIDS (Gao, Nau, Rosenbluth, Scott, & Woodward, 2000; Lépine & Briley, 2011; Mrazek, Hornberger, Altar, & Degtiar, 2014), mental illness (Lépine & Briley, 2011; Mrazek et al., 2014), diabetes (Brown, Nichols, & Perry, 2004), cardiovascular diseases (Stange, Kriston, von-Wolff, Baehr, & Dartsch, 2013), cystic fibrosis (Sawicki et al., 2013), celiac disease (Shah et al., 2014), hyperlipidemia (Ridgeway et al., 2014), asthma (Eton et al., 2013; Ridgeway et al., 2014), and stroke (Ridgeway et al., 2014; Tran et al., 2012). In addition, BOT has been examined among individuals with multimorbidity or the coexistence of more than one chronic condition (Pefoyo et al., 2015). These chronic condition studies have examined BOT through varied designs and variables of interest. Qualitative studies identified themes, such as tasks required of patients to manage their conditions, difficulties with access to care, the impact of tasks on self-management, identification of problem-solving techniques and coping strategies, the importance of social support, and positive aspects of the healthcare system (Ridgeway et al., 2014; Tran et al., 2015). Quantitative studies have used indicator variables to examine BOT as an outcome variable or predictor of patient and clinical consequences. Indicator variables were used in place of the actual variable or concept because it may be more feasible to collect the indicator variable rather than a BOT-specific measure. Examples of indicator variables include treatment complexity (Ridgeway et al., 2014; Sawicki et al., 2013), number of medications (Lhuilier, Brugeilles, & Rolland, 2015; Mrazek et al., 2014), number of interactions with healthcare providers (Cheng & Levy, 2017; Henry et al., 2008; Presley et al., 2017), and difficulty managing treatment (Lhuilier et al., 2015; Ridgeway et al., 2014; Shah et al., 2014).
Although studies have examined BOT across several chronic conditions, only three studies were found that examined BOT within individuals with cancer. Two of these studies analyzed secondary data from large data sets: SEER–Medicare (Presley et al., 2017) and an institutional cancer database (Cheng & Levy, 2017). The third study recruited patients from a national survey database and had direct contact with participants through online and telephone surveys (Henry et al., 2008). These studies operationalized BOT as the number of days interacting with the healthcare system, type of interaction (i.e., receiving cancer treatment, clinic visit, or emergency department/acute stay), number of physicians patients interacted with, number of medications, and symptom burden (Cheng & Levy, 2017; Henry et al., 2008; Presley et al., 2017). Cheng and Levy (2017) and Henry et al. (2008) found that later-stage cancer and increased side effects from treatment were associated with increased BOT. However, these studies did not examine the impact of multimorbidities on BOT.
Looking more specifically at cancer and multimorbidity within these studies, Presley et al. (2017) collected data on patients’ multimorbid conditions and examined the impact on patients’ BOT, using the number of days interacting with the healthcare system as an indicator for BOT. When comparing patients who received the same cancer treatment, they found that patients with three or more multimorbidities experienced a significantly higher level of burden than those with less than three multimorbidities. Although this is the only study that examined BOT within patients with cancer and multimorbidities, other research has illustrated the impact of multimorbidities on a number of patient and healthcare outcomes. These patient outcomes include symptoms and side effects, adverse events (e.g., hospitalizations), quality of life, functional status, increased costs, disease exacerbation, and mortality (Sarfati et al., 2016; Spoelstra et al., 2015; Sun et al., 2016; Verbrugghe et al., 2016; Williams et al., 2016; Winn, Keating, & Dusetzina, 2016).
Patients with cancer prescribed OOAs experience unique challenges when compared to patients receiving IV chemotherapy. Although trained healthcare professionals administer IV chemotherapy in a controlled environment, OOAs lack such firsthand monitoring, thereby shifting responsibility to patients and their caregivers (Arthurs et al., 2015; Bassan et al., 2014; Hall et al., 2016; Mathes, Antoine, Pieper, & Eikermann, 2014; Zerillo et al., 2017). OOA regimens provide a sense of convenience for patients via home administration. However, OOA regimens can be complex, comprising multiple OOAs with varying dosages, cycles (days on and days off), and specific instructions (Accordino & Hershman, 2013; Hall et al., 2016). These regimens increase patients’ BOT and lead to difficulties in medication management and subsequent regimen modifications (Trivedi et al., 2014). Such modifications may include dose changes, temporary stoppages, permanent stoppages, and the adding or switching of a medication (McNamara et al., 2016; Salgado et al., 2017; Verbrugghe et al., 2016). Regimen modifications can be brought on by side effects and toxicities, interactions, patient and provider decisions, or disease progression (Salgado et al., 2017). OOA regimen changes can alter the effectiveness of the medication if patients are not receiving an adequate dose or require increased rest periods.
Individuals with cancer prescribed OOAs require more self-management than those receiving IV chemotherapy. Adding multimorbidities to complex OOA regimens increases the workload for patients to manage their conditions. Limited research has been conducted on BOT in individuals with cancer and multimorbidities who are prescribed OOAs. Because most cancer and multimorbidity management takes place in the home, it is imperative that patients and providers have open communication and effective education regarding side effects that can lead to treatment modifications. Effective communication is needed for providers to educate patients to properly manage their OOAs and symptoms and side effects, as well as multimorbidities.
The purpose of this study was to evaluate the effect of BOT indicator variables (OOA regimen complexity and symptom interference) on the relationship between baseline characteristics (age, sex, employment status, marital status, multimorbidity, OOA drug class, insurance type, and OOA co-pay) and OOA temporary stoppages. The objective was to determine what variables might have an impact on the OOA regimen modifications, as well as whether or not variables associated with the BOT might affect the direct relationship between baseline characteristics and OOA temporary stoppages. This study provides new evidence for clinicians to encourage patients to proactively seek care for early management of symptoms and insight for future research in patients with cancer and multimorbidities.
Methods
This study is a secondary data analysis of a two-arm, multisite randomized controlled trial testing adherence and symptom management interventions in patients with cancer who are newly prescribed OOAs (Sikorskii et al., 2018). Permission for this secondary analysis was granted by the principal investigators. The experimental arm received a weekly symptom assessment with referral to a symptom management toolkit for symptoms rated 4 or greater on a severity subscale and an adherence intervention comprised of daily reminders using an automated interactive voice response system. Participants in the control group received standard care without toolkit referrals or reminder calls.
Sample and Setting
Participants in the parent study were recruited from six National Cancer Institute–designated cancer centers across the Midwest and eastern United States (University of Michigan, Indiana University, Ohio State University, University of Pittsburgh, Northwestern University, and Yale University). The institutional review boards at Michigan State University and all recruitment sites approved the parent study. Patients agreed to enroll by signing a consent form and could withdraw from the study at any point. Inclusion criteria for the parent study were being aged 21 years or older, being able to speak English, being newly prescribed one of the designated OOAs (U.S. Food and Drug Administration-approved), having an Eastern Cooperative Oncology Group performance score of 0–2 or a Karnofsky score of 50 or greater (Karnofsky & Burchenal, 1949; Oken et al., 1982), and actively receiving cancer treatment from one of the participating cancer centers. Patients were excluded if they had difficulty hearing on the telephone, had limited or no access to a touch-tone phone, had cognitive deficits as determined by recruiters, or were receiving hospice care. The parent study sample size was calculated with a power of 0.8, with a total of 272 patients consenting to participate in the parent trial. All 272 participants who completed the baseline interview were included in this secondary data analysis with no missing data for included variables.
Data Collection
Data were collected via telephone by trained interviewers at baseline and at 4, 8, and 12 weeks. Data collected included OOA prescription information and pill counts, demographics, insurance coverage, social support, symptoms, multimorbidities, and interactions with healthcare providers. Data were also collected during the experimental group’s daily reminder calls and participants’ automated weekly symptom calls. After participants completed the study, trained abstractors at the respective cancer centers completed medical record audits. Quality assurance checks were completed on each medical record audit as well as during randomly selected interviews.
Measures
Participant variables included age, sex, marital status, and employment status. Healthcare system variables included the type of insurance (government versus private) and presence of an OOA out-of-pocket cost at baseline. Disease and treatment characteristics included OOA drug class and the number of multimorbidities requiring medication management. Multimorbidities were based on preexisting (before the study period) prescriptions for medications primarily used to treat the specific conditions, which were found in the medical record. Two nurses on the research team reviewed the multimorbid conditions and medications. Trial group (experimental versus control) was also included as a variable.
BOT indicator variables were used for both participant workload and perspective. Participant workload was captured through OOA regimen complexity scores, which were measured using an adapted version of the Medication Regimen Complexity Index (MRCI) (George, Phun, Bailey, Kong, & Stewart, 2004). The MRCI uses variables that are weighted based on how each variable contributes to the overall complexity of a patient’s medication regimen. The MRCI was tested by an expert panel for validity and inter-rater reliability (> 0.9) in patients with chronic obstructive pulmonary disease. A higher score indicates a more complex medication regimen. The adapted MRCI included all of the original MRCI variables plus a variable that added 1 point if participants were receiving concurrent IV chemotherapy. The indicator BOT patient perspective variable was baseline symptom interference. The Cancer Symptom Experience Inventory (CSEI) measured symptom interference, which contains reports on 18 symptoms commonly associated with OOAs (Given et al., 2008). The CSEI has been tested in individuals with cancer for validity and internal consistency reliability, with a Cronbach alpha of 0.8. The 0–9 interference scores for each of the 18 symptoms were summed (range = 0–162), with higher score indicating greater interference with daily activities. Symptom interference was included instead of symptom severity because it better captures patient perspectives of how symptoms influence daily living.
The outcome variable is whether or not participants experienced a temporary stoppage of their OOA regimen during the parent trial (12 weeks). Temporary stoppages were defined as any interruption in the OOA regimen with which they later continued back on the regimen. Stoppage data were collected at each recruitment site through detailed prescribing information and modifications of patients’ cancer treatment regimens through the electronic health record.
Data Analysis
Descriptive statistics were used to measure baseline characteristics and the frequency of OOA temporary stoppages. Effect coding was used for the categorical predictor variables within the models. To standardize continuous variables, the group means for regimen complexity, symptom interference, and number of multimorbid conditions were subtracted from each patient’s value. 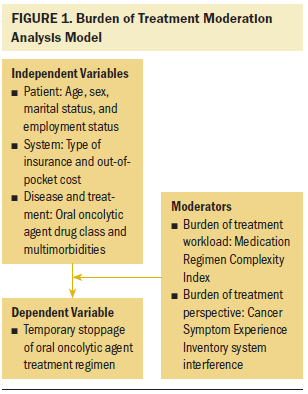
For the primary research question, a moderation analysis was implemented using two multiple logistic regression models, based on Baron and Kenny (1986). Figure 1 provides an illustration of the moderation analysis model with all included variables. The outcome for both models was whether an individual experienced a temporary stoppage during the 12-week parent trial. Because the primary focus for this work is the burden of patients with cancer and multimorbid conditions, participants’ multimorbidities were the baseline characteristic variable included in the interaction term with the BOT indicator variables (regimen complexity and symptom interference). Chi-square tests and simple logistic regressions were completed for categorical and continuous variables, respectively, to test the main effect of each predictor on the outcome of temporary stoppages. Significant main effects and the moderation variables were included in the final regression models. If the moderation interaction terms were found to be nonsignificant, then a main effects regression model including the significant predictors and three moderation variables (multimorbidity, regimen complexity, and symptom interference) would be analyzed. Included data met assumptions of statistical tests. Data were analyzed using STATA/IC, version 14.0.
Results
The parent trial sample of 272 was split evenly between men and women and had a mean age of 61 years (see Table 1). Thirty-two percent of the sample (n = 88) was employed either part- or full-time, and 61% (n = 167) were married at the time of their baseline interview. Fifty-seven percent (n = 154) of participants’ primary insurance was government coverage, which included Medicare, Medicaid, and Veterans Affairs (VA). For their initial OOA prescription, 53% (n = 143) had an out-of-pocket cost, with a mean cost of $258. Of the 28 different primary OOAs prescribed to patients, 82% were either in the cytotoxic agent (n = 95) or kinase inhibitor (n = 127) drug classes. In addition to being prescribed an OOA, 27% of the sample (n = 73) was also receiving IV chemotherapy at baseline. 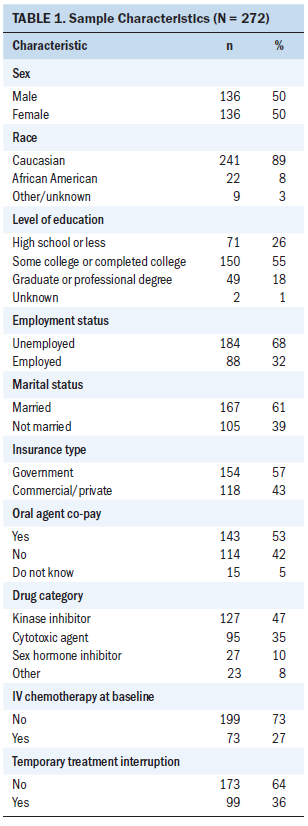
In addition to their cancer, participants had an average of 3.39 chronic conditions requiring medication management (see Table 2), with an average of 12 non-cancer treatment medications per participant. The most common multimorbidities were cardiovascular disease, peptic ulcer disease, depression, diabetes, hyperlipidemia, and renal disease. Participants’ mean treatment regimen complexity score was 6.81 (SD = 2.67, range = 1–16). Participants had a mean baseline symptom interference score of 17.84 (SD = 20.08, range = 0–119). During the course of the 12-week parent trial, 36% (n = 99) experienced at least one temporary stoppage of their cancer treatment regimen. Table 3 shows the frequency of temporary interruptions by characteristics. 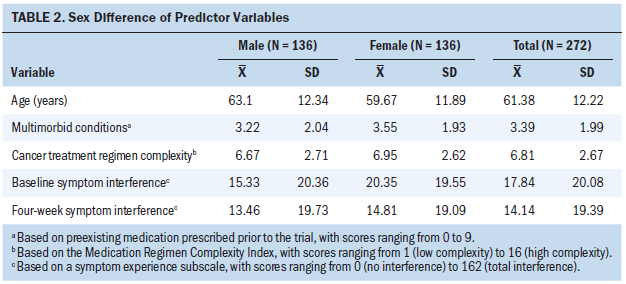
For the final moderation regression models, chi-square tests showed that sex and OOA drug class had significant group differences in terms of temporary stoppages. Women were more likely to experience a temporary stoppage during the course of the trial (p < 0.001). After testing OOA drug class using logistic regression with effect coding, those prescribed cytotoxic agents (p = 0.049) and kinase inhibitors (p = 0.004) experienced a greater number of temporary stoppages than those taking sex hormone inhibitors and other OOAs. Age, marital status, employment status, type of insurance, out-of-pocket cost, and experimental/control group showed no significant differences of temporary stoppages and, therefore, were not included in the final moderation regression models. 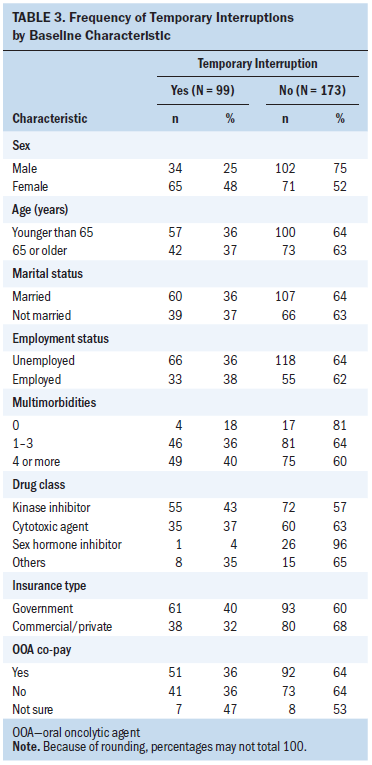
Both of the interaction terms between symptom interference and multimorbidity and OOA regimen complexity and multimorbidity were nonsignificant within their respective models. Therefore, a regression model was conducted that included sex, OOA drug class, symptom interference, regimen complexity, and multimorbidity (see Table 4). Much like the interaction terms, the main effects for the BOT variables were nonsignificant. However, like the moderation models, sex and OOA drug class were significant. Women (p = 0.018) and participants prescribed kinase inhibitors (p = 0.004) were more likely to experience a temporary stoppage during the parent trial. However, cytotoxic agents were no longer statistically significant. 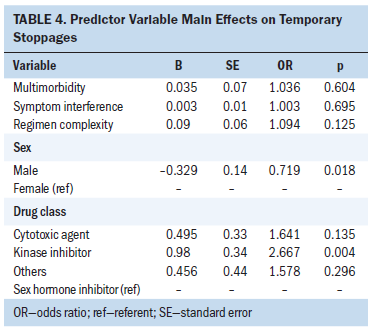
Discussion
Patients with cancer are continuing to live longer because cancer treatments have become more effective. Consequently, this longer survival time increases the chances that a patient with cancer will have other chronic conditions that require medical management (Sarfati et al., 2016). With the population of patients with cancer and multimorbid conditions rising, it is important to understand the BOT these individuals face while managing their cancer and multimorbid conditions (Sarfati et al., 2016; Williams et al., 2016). This study is the first to examine the BOT of patients prescribed OOAs who must also medically manage other multimorbid conditions. Similar to previous cancer-related BOT studies (Cheng & Levy, 2017; Henry et al., 2008; Presley et al., 2017), this current analysis used indicator variables to measure BOT components. However, unlike previous studies, this analysis included an indicator variable for patient perspective (patient-reported symptom interference) as well as an indicator variable for patient workload (cancer treatment regimen complexity).
OOAs are a last line of treatment for most patients with cancer, and patients could theoretically be taking them until the end of life (Kawakami et al., 2015; Winn et al., 2016). Symptom burden is a primary concern for oncology providers in the management of OOAs—increased symptom burden is a primary reason for patients needing temporary stoppages or dose changes with their OOA regimens (Hall et al., 2016). Symptoms were the most common reason for temporary stoppages in the parent trial. Of the 99 participants who experienced a temporary stoppage, the most common symptoms and toxicities associated with temporary stoppages were fatigue, anemia, abnormal blood counts, cold or flu-like symptoms, nausea and vomiting, skin rash, and hand-foot syndrome. In addition, about 25% of the sample experienced a permanent stoppage of their treatment regimen (Sikorskii et al., 2017). Permanent stoppages are primarily driven by disease progression and, therefore, patients do not have as much say in the decision as they do with temporary stoppages (Lo et al., 2015).
The results of the initial group difference analysis indicate that women and those prescribed kinase inhibitors and cytotoxic agents were more likely to experience a temporary stoppage of their cancer treatment regimen. The two moderation regression models showed similar results. However, when factoring in sex, multimorbidity, treatment regimen complexity, and baseline symptom interference, cytotoxic agents no longer had a significant difference in terms of temporary stoppages, whereas kinase inhibitors remained significant. Given the different symptom profiles associated with certain OOAs, differences in temporary stoppages among OOA drug classes were expected (Cornelison, Jabbour, & Welch, 2012; Dasanu, 2012). The most commonly prescribed kinase inhibitors in the parent trial were palbociclib, pazopanib, sorafenib, and regorafenib.
To explore the significant influence of sex on temporary interruption, additional analyses were completed examining the differences between men and women. Although women had a significantly higher mean symptom interference score at baseline (p = 0.038), there was no difference in symptom interference between men and women after taking their OOAs for four weeks. With symptoms being the primary driver behind temporary stoppages, it is unclear why women experienced a significantly higher number of temporary interruptions than men in the parent trial. This could possibly be related to women taking OOAs that had higher proportions of temporary stoppages. Future research will be needed to examine sex differences related to OOA regimen modifications.
The results of the moderation regression analyses showed no significant affect on temporary stoppages within the two models resulting from the main effects of cancer treatment regimen complexity (patient workload), baseline symptom interference (patient perspective), multimorbidity, and their interaction terms. This was not an expected finding because the literature has described the impact of multimorbidities on the management of cancer treatment and overall quality of life of patients with cancer (Sarfati et al., 2016; Sogaard, Thomsen, Bossen, Sorensen, & Norgaard, 2013; Tawfik et al., 2016; van Leersum et al., 2013; Williams et al., 2016). The reason for the lack of significant findings could be related to the measurement of multimorbidities, which was operationalized based on non-cancer prescription medications within patients’ medical record audit. The same could be said for the use of BOT indicator variables as opposed to a BOT-specific measure. Although not statistically significant, patients with multimorbid conditions had a higher frequency of temporary stoppages than patients with no multimorbidities. Given this, more research is needed to examine the impact of multimorbid conditions on the OOA regimen modifications and patient symptom burden.
Limitations
Using indicator variables for the BOT components was a limitation of this secondary analysis. Other measures for patient workload and perspective, such as a validated BOT scale, may have yielded different results within the study sample. Inclusion of ICD-10 diagnoses within the parent trial may have improved the measurement of multimorbidities. The sample lacked racial diversity and was highly educated, and, therefore, was not representative of the general U.S. population. Finally, the follow-up period of the parent trial was 12 weeks. A longer follow-up time may have shown different results as patients’ cancer treatments progressed.
Implications for Research and Practice
OOAs provide a sense of convenience for patients with cancer, unlike traditional IV chemotherapy. However, with that convenience comes an increased responsibility to manage their cancer treatment and side effects. Oncology nurses are at the forefront to address the challenges for patients being prescribed OOAs for the first time. This study showed that certain patient groups are more susceptible to experiencing temporary stoppages of their OOA regimens. These stoppages can be the result of a number of reasons. One of the most common reasons is related to severe symptoms and side effects (Sikorskii et al., 2017). Patients with cancer are required to do a great deal of the symptom management at home with less frequent interaction with their oncologist and nurses than those receiving IV chemotherapy. Oncology nurses need to emphasize the importance of patients proactively seeking out care to properly manage their OOAs and effectively manage symptoms. Increased intervention by oncology nurses through closer monitoring and individualized education plans may help patients better self-manage symptoms.
With the current rise in the prevalence of individuals with cancer and multimorbid conditions, the research is far behind the pace needed to meet the challenges facing this population. BOT is a vital concept to answering the question of why these individuals have difficulties in managing the challenges going on in their lives. This study is an initial step to start to answer that question. Future research will need to include BOT measurement development, pilot studies, and eventually RCTs to address and target the BOT experienced by this population. This secondary analysis discusses some of the difficulties experienced by patients in managing OOAs along with other diseases. Future work will need to further examine BOT and develop interventions that can be translated to practice for oncology teams to provide strategies to patients who are struggling to manage their cancer and other conditions. 
Conclusion
The current study found that 36% of patients experienced a temporary stoppage in their cancer treatment regimen during the course of 12 weeks. With a short observation period, this shows the volatility of cancer treatment for those prescribed OOAs. Women and patients prescribed kinase inhibitors were more likely to experience temporary stoppages than men and patients prescribed other OOA classes, respectively. These patients are in a difficult period of their cancer care trajectory—most have failed prior lines of treatment. If these patients have other chronic conditions as well, the complexity of management and level of burden is increased. As the population lives longer, patients with cancer and multimorbid conditions will become a norm. Continued research efforts within this population will be vital for practice guidelines and empowering patients to effectively self-manage their OOAs and multimorbid conditions in the home.
About the Author(s)
Eric Vachon, PhD, RN, was, at the time of this writing, a doctoral student, Barbara Given, PhD, RN, FAAN, is a university distinguished professor, and Charles Given, PhD, is a professor, all in the College of Nursing at Michigan State University in East Lansing; and Susan Dunn, PhD, RN, FAHA, is an associate professor in the College of Nursing at the University of Illinois at Chicago. This research was funded by a Doctoral Degree Scholarship in Cancer Nursing from the American Cancer Society (grant/award number: 130704-DSCN-17-085-01-SCN) and from the Michigan State University Graduate School through the Dissertation Completion Fellowship. B. Given has previously received a grant from the National Institutes of Health and has served as the chair of Sparrow Hospital Board in Lansing, MI. All authors contributed to the conceptualization and design. Vachon, B. Given, and C. Given completed the data collection. Vachon provided statistical support. Vachon and B. Given provided the analysis. Vachon, B. Given, and Dunn contributed to the manuscript preparation. Vachon can be reached at vachoner@msu.edu, with copy to ONFEditor@ons.org. (Submitted September 2018. Accepted March 8, 2019.)
References
Accordino, M.K., & Hershman, D.L. (2013). Disparities and challenges in adherence to oral antineoplastic agents. American Society of Clinical Oncology Educational Book, 271–276.
Arthurs, G., Simpson, J., Brown, A., Kyaw, O., Shyrier, S., & Concert, C.M. (2015). The effectiveness of therapeutic patient education on adherence to oral anti-cancer medicines in adult cancer patients in ambulatory care settings: A systematic review. JBI Database of Systematic Reviews and Implementation Reports, 13, 244–292. https://doi.org/10.11124/jbisrir-2015-2057
Baron, R.M., & Kenny, D.A. (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182.
Bassan, F., Peter, F., Houbre, B., Brennstuhl, M.J., Costantini, M., Speyer, E., & Tarquinio, C. (2014). Adherence to oral antineoplastic agents by cancer patients: Definition and literature review. European Journal of Cancer Care, 23, 22–35.
Brown, J.B., Nichols, G.A., & Perry, A. (2004). The burden of treatment failure in type 2 diabetes. Diabetes Care, 27, 1535–1540.
Cheng, A.C., & Levy, M.A. (2017). Data driven approach to burden of treatment measurement: A study of patients with breast cancer. AMIA Annual Symposium Proceedings, 1756–1763.
Cornelison, M., Jabbour, E.J., & Welch, M.A. (2012). Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: The role of the midlevel practitioner. Journal of Supportive Oncology, 10, 14–24. https://doi.org/10.1016/j.suponc.2011.08.001
Dasanu, C.A. (2012). Length of adjuvant imatinib therapy in GIST: Weighing benefits, side effects and costs. Journal of Oncology Pharmacy Practice, 18, 379–380.
Eton, D.T., Elraiyah, T.A., Yost, K.J., Ridgeway, J.L., Johnson, A., Egginton, J.S., . . . Montori, V.M. (2013). A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Related Outcome Measures, 4, 7–20. https://doi.org/10.2147/prom.s44694
Eton, D.T., Ridgeway, J.L., Egginton, J.S., Tiedje, K., Linzer, M., Boehm, D.H., . . . Anderson, R.T. (2015). Finalizing a measurement framework for the burden of treatment in complex patients with chronic conditions. Patient Related Outcome Measures, 6, 117–126. https://doi.org/10.2147/prom.s78955
Gao, X., Nau, D.P., Rosenbluth, S.A., Scott, V., & Woodward, C. (2000). The relationship of disease severity, health beliefs and medication adherence among HIV patients. AIDS Care, 12, 387–398. https://doi.org/10.1080/09540120050123783
George, J., Phun, Y.T., Bailey, M.J., Kong, D.C., & Stewart, K. (2004). Development and validation of the Medication Regimen Complexity Index. Annals of Pharmacotherapy, 38, 1369–1376. https://doi.org/10.1345/aph.1D479
Given, B., Given, C.W., Sikorskii, A., Jeon, S., McCorkle, R., Champion, V., & Decker, D. (2008). Establishing mild, moderate, and severe scores for cancer-related symptoms: How consistent and clinically meaningful are interference-based severity cut-points? Journal of Pain and Symptom Management, 35, 126–135. https://doi.org/10.1016/j.jpainsymman.2007.03.012
Hall, A.E., Paul, C., Bryant, J., Lynagh, M.C., Rowlings, P., Enjeti, A., & Small, H. (2016). To adhere or not to adhere: Rates and reasons of medication adherence in hematological cancer patients. Critical Reviews in Oncology/Hematology, 97, 247–262.
Henry, D.H., Viswanathan, H.N., Elkin, E.P., Traina, S., Wade, S., & Cella, D. (2008). Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Supportive Care in Cancer, 16, 791–801.
Karnofsky, D.A., & Burchenal, J.H. (1949). The clinical evaluation of chemotherapeutic agents in cancer. In C.M. MacLeod (Ed.), Evaluation of chemotherapeutic agents (pp. 191–205). New York, NY: Columbia University Press.
Kawakami, K., Nakamoto, E., Yokokawa, T., Sugita, K., Mae, Y., Hagino, A., . . . Hama, T. (2015). Patients’ self-reported adherence to capecitabine on XELOX treatment in metastatic colorectal cancer: Findings from a retrospective cohort analysis. Patient Preference and Adherence, 9, 561–567. h
Lépine, J.P., & Briley, M. (2011). The increasing burden of depression. Neuropsychiatric Disease and Treatment, 7(Suppl. 1), 3–7. https://doi.org/10.2147/ndt.s19617
Lhuilier, D., Brugeilles, F., & Rolland, D. (2015). Chronic disease and treatment burden for patients: The example of hepatitis C. Sante Publique, 27(1, Suppl.), S17–S21.
Lo, P.C., Dahlberg, S.E., Nishino, M., Johnson, B.E., Sequist, L.V., Jackman, D.M., . . . Oxnard, G.R. (2015). Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer, 121, 2570–2577. https://doi.org/10.1002/cncr.29397
Mathes, T., Antoine, S.L., Pieper, D., & Eikermann, M. (2014). Adherence enhancing interventions for oral anticancer agents: A systematic review. Cancer Treatment Reviews, 40, 102–108. https://doi.org/10.1016/j.ctrv.2013.07.004
McNamara, E., Redoutey, L., Mackler, E., Severson, J.A., Petersen, L., & Mahmood, T. (2016). Improving oral oncolytic patient self-management. Journal of Oncology Practice, 12, e864–e869.
Mrazek, D.A., Hornberger, J.C., Altar, C.A., & Degtiar, I. (2014). A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatric Services, 65, 977–987.
Oken, M.M., Creech, R.H., Tormey, D.C., Horton, J., Davis, T.E., McFadden, E.T., & Carbone, P.P. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 5, 649–655.
Pefoyo, A.J., Bronskill, S.E., Gruneir, A., Calzavara, A., Thavorn, K., Petrosyan, Y., . . . Wodchis, W.P. (2015). The increasing burden and complexity of multimorbidity. BMC Public Health, 15, 415. https://doi.org/10.1186/s12889-015-1733-2
Presley, C.J., Soulos, P.R., Tinetti, M., Montori, V.M., Yu, J.B., & Gross, C.P. (2017). Treatment burden of Medicare beneficiaries with stage I non–small-cell lung cancer. Journal of Oncology Practice, 13, e98-e107. https://doi.org/10.1200/JOP.2016.014100
Ridgeway, J.L., Egginton, J.S., Tiedje, K., Linzer, M., Boehm, D., Poplau, S., . . . Eton, D.T. (2014). Factors that lessen the burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Preference and Adherence, 8, 339–351. https://doi.org/10.2147/ppa.s58014
Salgado, T.M., Mackler, E., Severson, J.A., Lindsay, J., Batra, P., Petersen, L., & Farris, K.B. (2017). The relationship between patient activation, confidence to self-manage side effects, and adherence to oral oncolytics: A pilot study with Michigan oncology practices. Supportive Care in Cancer, 25, 1797–1807.
Sarfati, D., Koczwara, B., & Jackson, C. (2016). The impact of comorbidity on cancer and its treatment. CA: A Cancer Journal for Clinicians, 66, 337–350. https://doi.org/10.3322/caac.21342
Sav, A., King, M.A., Whitty, J.A., Kendall, E., McMillan, S.S., Kelly, F., . . . Wheeler, A.J. (2013). Burden of treatment for chronic illness: A concept analysis and review of the literature. Health Expectations, 18, 312–324. https://doi.org/10.1111/hex.12046
Sawicki, G.S., Ren, C.L., Konstan, M.W., Millar, S.J., Pasta, D.J., & Quittner, A.L. (2013). Treatment complexity in cystic fibrosis: Trends over time and associations with site-specific outcomes. Journal of Cystic Fibrosis, 12, 461–467.
Shah, S., Akbari, M., Vanga, R., Kelly, C.P., Hansen, J., Theethira, T., . . . Leffler, D.A. (2014). Patient perception of treatment burden is high in celiac disease compared with other common conditions. American Journal of Gastroenterology, 109, 1304–1311. https://doi.org/10.1038/ajg.2014.29
Sikorskii, A., Given, C.W., Given, B.A., Vachon, E., Krauss, J.C., Rosenzweig, M., . . . Majumder, A. (2018). An automated intervention did not improve adherence to oral oncolytic agents while managing symptoms: Results from a two-arm randomized controlled trial. Journal of Pain and Symptom Management, 56, 727–735. https://doi.org/10.1016/j.jpainsymman.2018.07.021
Sikorskii, A., Given, C.W., Given, B.A., Vachon, E., Marshall, V., Krauss, J.C., . . . Majumder, A. (2017). Do treatment patterns alter beliefs cancer patients hold regarding oral oncolytic agents? Psycho-Oncology, 27, 1005–1012. https://doi.org/10.1002/pon.4606
Søgaard, M., Thomsen, R.W., Bossen, K.S., Sørensen, H.T., & Nørgaard, M. (2013). The impact of comorbidity on cancer survival: A review. Clinical Epidemiology, 5(Suppl. 1), 3–29. https://doi.org/10.2147/clep.S47150
Spoelstra, S.L., Given, C.W., Sikorskii, A., Majumder, A., Schueller, M., & Given, B.A. (2015). Treatment with oral anticancer agents: Symptom severity and attribution, and interference with comorbidity management. Oncology Nursing Forum, 42, 80–88. https://doi.org/10.1188/15.ONF.42-01P
Stange, D., Kriston, L., von-Wolff, A., Baehr, M., & Dartsch, D.C. (2013). Reducing cardiovascular medication complexity in a German university hospital: Effects of a structured pharmaceutical management intervention on adherence. Journal of Managed Care Pharmacy, 19, 396–407.
Sun, V., Grant, M., Wendel, C.S., McMullen, C.K., Bulkley, J.E., Herrinton, L.J., . . . Krouse, R.S. (2016). Sexual function and health-related quality of life in long-term rectal cancer survivors. Journal of Sexual Medicine, 13, 1071–1079. https://doi.org/10.1016/j.jsxm.2016.05.005
Tawfik, B., Pardee, T.S., Isom, S., Sliesoraitis, S., Winter, A., Lawrence, J., . . . Klepin, H.D. (2016). Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML). Journal of Geriatric Oncology, 7, 24–31. https://doi.org/10.1016/j.jgo.2015.10.182
Tran, V.T., Barnes, C., Montori, V.M., Falissard, B., & Ravaud, P. (2015). Taxonomy of the burden of treatment: A multi-country web-based qualitative study of patients with chronic conditions. BMC Medicine, 13, 115. https://doi.org/10.1186/s12916-015-0356-x
Tran, V.T., Montori, V.M., Eton, D.T., Baruch, D., Falissard, B., & Ravaud, P. (2012). Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Medicine, 10, 68. https://doi.org/10.1186/1741-7015-10-68
Trivedi, D., Landsman-Blumberg, P., Darkow, T., Smith, D., McMorrow, D., & Mullins, C.D. (2014). Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. Journal of Managed Care and Specialty Pharmacy, 20, 1006–1015. https://doi.org/10.18553/jmcp.2014.20.10.1006
van Leersum, N.J., Janssen-Heijnen, M.L., Wouters, M.W., Rutten, H.J., Coebergh, J.W., Tollenaar, R.A., & Lemmens, V.E. (2013). Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995-2010. Internatinal Journal of Cancer, 132, 2157–2163. https://doi.org/10.1002/ijc.27871
Verbrugghe, M., Duprez, V., Beeckman, D., Grypdonck, M., Quaghebeur, M., Verschueren, C., . . . Van Hecke, A. (2016). Factors influencing adherence in cancer patients taking oral tyrosine kinase inhibitors: A qualitative study. Cancer Nursing, 39, 153–162. https://doi.org/10.1097/ncc.0000000000000250
Williams, G.R., Mackenzie, A., Magnuson, A., Olin, R., Chapman, A., Mohile, S., . . . Holmes, H. (2016). Comorbidity in older adults with cancer. Journal of Geriatric Oncology, 7, 249–257.
Winn, A.N., Keating, N.L., & Dusetzina, S.B. (2016). Factors associated with tyrosine kinase inhibitor initiation and adherence among medicare beneficiaries with chronic myeloid leukemia. Journal of Clinical Oncology, 34, 4323–4328.
Zerillo, J.A., Goldenberg, B.A., Kotecha, R.R., Tewari, A.K., Jacobson, J.O., & Krzyzanowska, M.K. (2017). Interventions to improve oral chemotherapy safety and quality: A systematic review. JAMA Oncology, 4, 105–117.




