Advances in Melanoma: The Rationale for the Melanoma Nursing Initiative
This article provides an overview of this supplement, outlining the needs assessment process the Melanoma Nursing Initiative (MNI) used to determine the immunotherapy and targeted therapy topics for discussion as well as the process for developing the consensus statements. The article provides specific discussion of a unique feature of the MNI, the care step pathways (CSPs) for management of adverse events (AEs) associated with melanoma therapies, and looks to the future in terms of the potential benefits of engaging and enabling oncology nurses to adopt a standardized approach to AE management and adherence promotion for melanoma therapies.
AT A GLANCE
- Nurses have an essential role in managing AEs and promoting adherence in patients receiving newer melanoma therapies.
- This supplement outlines nursing assessment and AE management for melanoma immunotherapies and targeted therapies.
- Early and comprehensive management of AEs by oncology nurses is likely to lead to improved outcomes for patients with melanoma.
Jump to a section
Metastatic melanoma is a complex and challenging malignancy. Until recently, treatment options for this tumor were limited and, for the most part, ineffective. However, a virtual explosion of new scientific knowledge has given rise to an impressive number of novel therapeutics. The U.S. Food and Drug Administration’s (FDA’s) approval of nine drugs and drug combinations since 2011 is the concrete bounty of these endeavors (National Cancer Institute, 2015). These advances have transformed this from a disease that, once metastatic, was uniformly fatal, to one in which control, if not cure, is the new expectation (Achkar & Tarhini, 2017). Patients have derived tremendous benefits from these advances, and the survival statistics for melanoma have improved dramatically (Achkar & Tarhini, 2017; Grossmann & Margolin, 2015; Hodi et al., 2016).
As the treatment options for melanoma have evolved, so has the role of the oncology nurse. This revolution in treatment has also been accompanied by the appearance of new treatment-related toxicities, some of which can be difficult to diagnose, complex to manage, and potentially persistent throughout the patient’s life. These toxicities are completely different from those associated with chemotherapy, with which oncology nurses are very familiar. In addition, oncology nurses often have little experience with managing these regimens for patients with melanoma because they rarely see this tumor type.
Genesis of the Melanoma Nursing Initiative
This supplement consists of a series of articles describing the novel therapies now considered standard for high-risk and advanced melanoma, an overview of associated toxicities, and the nursing role in the provision of care. The Melanoma Nursing Initiative (MNI) was spearheaded by Valerie Guild, co-founder and president of the AIM at Melanoma Foundation, a not-for-profit foundation dedicated to increasing education, research, and support related to melanoma, and Lisa Tushla and her staff at Terranova Medica, LLC, a medical education company specializing in the delivery of education content to healthcare professionals. They envisioned a comprehensive initiative developed for oncology nurses and other allied health professionals in community and academic melanoma centers. The goal of the initiative is to educate and engage healthcare providers to address adverse events associated with melanoma therapies, adherence issues, and patient education, thereby improving therapeutic outcomes for patients with melanoma.
The content was developed by members of the MNI, a group of advanced practice providers and other members of the healthcare community. The MNI members have extensive knowledge about melanoma, targeted therapies, immunotherapies, and their associated toxicity profiles. This nurse-centric educational effort began in fall 2016 when the invited group of multidisciplinary professionals met for brainstorming and discussion regarding the significance of unmet education needs identified in the nursing community. The group included the advanced practice providers of the MNI, a physician expert in melanoma, a patient, and patient advocacy experts. After the meeting, members continued discussions via interactive teleconferences to finalize the materials for this supplement and the MNI online portal (www.themelanomanurse.org).
For the most part, optimal management of immune-related adverse events is based on clinical experience. To date, there have been no prospective trials conducted to evaluate best treatment strategies to manage immune-related adverse events for immune checkpoint inhibitors, and this applies to targeted therapies as well. To develop consensus statements regarding supportive care, members of the MNI reviewed the literature and provided insight, shared their clinical experiences, and assessed the benefits and limitations of prior educational endeavors. They described necessary components of patient assessment and emphasized the need for tailored intervention. In sum, members endeavored to describe what nurses need to know to have the greatest impact and positively influence patient outcomes.
An Overview of the Supplement
The editorial in this supplement defines the clinical context for MNI (Kirkwood & Ribas, 2017). Czupryn and Cisneros (2017) illustrate how to recognize and manage adverse events associated with molecularly targeted oral therapies (i.e., cobimetinib, dabrafenib, trametinib, and vemurafenib). In addition, the article notes the importance of recognizing and managing the potential for drug–drug interactions with BRAF/MEK regimens. An article by Madden and Hoffner (2017) reviews the often challenging tasks of recognizing and managing immune-related adverse events associated with ipilimumab-based therapy in the adjuvant and metastatic settings, and discusses monotherapy regimens and combination therapy with nivolumab. McGettigan and Rubin (2017) review the use and immune-related adverse event management of PD-1 monotherapy, including pembrolizumab and nivolumab. Seery (2017) discusses the necessary considerations and logistics for providing care to patients receiving intratumoral therapy with talimogene laherparepvec (T-VEC), a first-in-class oncolytic virus approved for use in melanoma. The author walks the reader through best practices based on experience for pre- and postadministration assessment as well as actual administration, while keeping the role of the nurse at the forefront. Kottschade and Lehner Reed (2017) describe ways to increase adherence and promote patient engagement to oral therapies, an increasingly important issue in oncology.
The Care Step Pathways
The MNI created the care step pathways (CSPs), an innovative but practical tool developed to address notable toxicities unique to targeted therapies or immunotherapies. The CSPs include specialized formats and components designed to provide comprehensive nursing assessment and management strategies in a visually appealing and easy-to-use format (see Figure 1). Each CSP outlines essential components of the nursing functions specific to that adverse event. 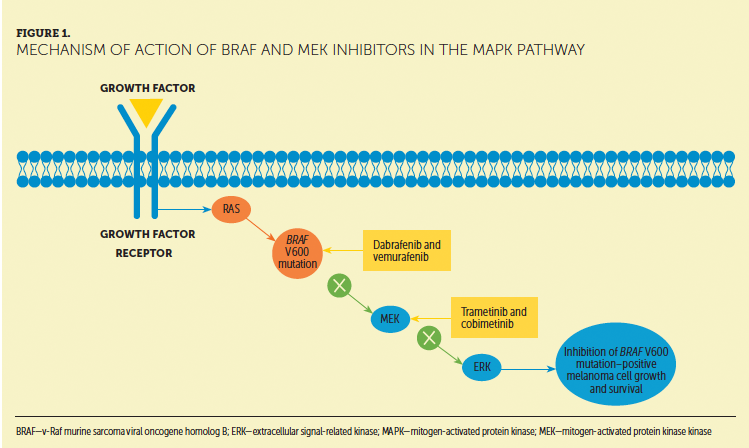
The nursing assessment section includes look, listen, and recognize categories that guide oncology nurses to a specific set of symptom-related questions to ask the patient and/or caregiver and additional information to consider or review. The information obtained from this focused assessment will direct the appropriate management and nursing-specific interventions. CSPs also include grading information. Sharing common assessment terms is fundamental to proper toxicity. In developing this component of the CSP, the MNI members were cognizant that toxicity grading requires a focus on consistency while maintaining flexibility to address evolving clinical scenarios. In academic medical centers, where clinical research is regularly conducted, providers use common terminology regarding adverse events. This terminology originates from the National Cancer Institute (2010) and its standard definitions and grading of adverse event severity, and the Common Terminology Criteria for Adverse Events (CTCAE) allows for interpretation of subjective symptoms in an objective manner. This process uses a grading system from 1 (mild) to 5 (death) to represent that toxicity defined by specific parameters according to the organ system involved (National Cancer Institute, 2010). Although providers in academia use this language, members of the MNI acknowledge that the language is not universal, recognizing that many nurses and other healthcare providers outside of academic centers may not be familiar with CTCAE grading. The relevant CTCAE grading descriptions are included within each CSP, where appropriate. In addition, MNI members also recognize that the CTCAE grading criteria are evolving, particularly for the assessment of immune-mediated endocrinopathies, for which detail is lacking in the current criteria. For this reason, MNI members relied on more practical, clinically oriented grading systems for disorders like thyroiditis and type 1 diabetes.
The CSPs also address management of adverse events. The focus of the MNI is on the practical, nurse-led interventions that oncology nurses can and should undertake as well as anticipatory guidance regarding drug holds or discontinuations, dosage adjustments, and management strategies. The CSP shows overarching management strategies by grade, particularly when examining the immune-related adverse events associated with immune checkpoint inhibitors. The CSP also shows detail about use of corticosteroids for immune-related adverse events and when urgent intervention is needed. Such detail is important because MNI members have encountered much confusion regarding the proper use and dosage of corticosteroids for immune-related adverse events among colleagues. Noting the red flags serves as a reminder to intervene rapidly at the recognition of signs and symptoms of more serious or life-threatening manifestations of these adverse events.
Nursing Foundation of Care
The current armamentarium for high-risk and advanced melanoma has evolved to include treatments that will offer cure or life prolongation for a subset of patients. Oncology nurses are essential members of the medical team and are in a pivotal position to positively influence treatment outcomes through their roles as patient advocates and educators to patients, families, staff, and other members of the healthcare team. To have the greatest impact, nurses need to have ready strategies to identify and manage the adverse events associated with these life-saving therapies and the ability to communicate the rationale for such approaches to members of the care team. It is the hope of MNI that the information provided in this supplement, in conjunction with the CSPs and the ancillary nurse and patient materials available online, will provide a strong foundation for ongoing learning.
The realm of management of immune checkpoint inhibitor– and targeted therapy–related toxicity will be an area in which oncology nurses excel. A concerted effort to support nurses in their central adverse event management role is likely to yield life-saving results. Future research is needed to look specifically at nurse-influenced outcomes: decreased toxicity, decreased associated costs, improved adherence, and, ultimately, improved patient outcomes.
For this supplement, the author gratefully acknowledges Samantha R. Guild, Esq., for her insights and reviews from the patient advocacy perspective, Paula M. Muehlbauer, RN, MSN, AOCNS®, for her initial input into optimal educational design for this initiative, Tom Davis, BS, for expert administrative coordination and assistance with graphical development, Jill Maria Weberding, MPH, BSN, RN, OCN®, for reviewing the manuscript from the community oncology nursing perspective, and Lois J. Loescher, PhD, RN, FAAN, for reviewing the content from a melanoma nursing educational perspective.
About the Author(s)
Krista M. Rubin, RN, MS, FNP-BC, is an advanced nurse practitioner in the Center for Melanoma at the Massachusetts General Hospital Cancer Center in Boston. The author takes full responsibility for this content. This supplement was funded by the AIM at Melanoma Foundation, with support via unrestricted grants from Amgen, Array Biopharma, Bristol-Myers Squibb, Incyte Corporation, Merck and Co., and Novartis Pharmaceuticals. Writing and editorial support was provided by Lisa A. Tushla, PhD, H(ASCP), at Terranova Medica. Rubin has served as a consultant for Merck, EMD-Serono, and Novartis, for which she received honoraria and related travel expense reimbursement. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Rubin can be reached at kmrubin@mgh.harvard.edu, with copy to CJONEditor@ons.org. (Submitted April 2017. Accepted May 23, 2017.)
References
Achkar, T., & Tarhini, A.A. (2017). The use of immunotherapy in the treatment of melanoma. Journal of Hematology and Oncology, 10, 88. doi:10.1186/s13045-017-0458-3
Czupryn, M., & Cisneros, J. (2017). BRAF/MEK inhibitor therapy: Consensus statement from the faculty of the Melanoma Nursing Initiative on managing adverse events and potential drug interactions. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 11–29. doi:10.1188/17.CJON.S4.11-29
Grossmann, K.F., & Margolin, K. (2015). Long-term survival as a treatment benchmark in melanoma: Latest results and clinical implications. Therapeutic Advances in Medical Oncology, 7(3), 181–191. doi:10.1177/1758834015572284
Hodi, F.S., Kluger, J., Sznol, M., Carvajal, R., Lawrence, D., Atkins, M., . . . Toaplian, S. (2016, April). Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab monotherapy in a phase I trial. Paper presented at the American Association for Cancer Research Annual Meeting, New Orleans, LA.
Kirkwood, J.M., & Ribas, A. (2017). Collaborative care in melanoma: The essential role of the nurse. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 4–6. doi:10.1188/17.CJON.S4.4-6
Kottschade, L.A., & Lehner Reed, M. (2017). Promoting oral therapy adherence: Consensus statements from the faculty of the Melanoma Nursing Initiative on oral melanoma therapies. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 87–96. doi:10.1188/17.CJON.S4.87-96
Madden, K.M., & Hoffner, B. (2017). Ipilimumab-based therapy: Consensus statement from the faculty of the Melanoma Nursing Initiative on managing adverse events with ipilimumab monotherapy and combination therapy with nivolumab. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 30–41. doi:10.1188/17.CJON.S4.30-41
McGettigan, S., & Rubin, K.M. (2017). PD-1 inhibitor therapy: Consensus statement from the faculty of the Melanoma Nursing Initiative on managing adverse events. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 42–51. doi:10.1188/17.CJON.S4.42-51
National Cancer Institute. (2015). Drugs approved for melanoma. Retrieved from https://www.cancer.gov/about-cancer/treatment/drugs/melanoma
National Cancer Institute. (2010). Common terminology criteria for adverse events (CTCAE) [v4.03]. Retrieved from https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference…
Seery, V. (2017). Intralesional therapy: Consensus statements for best practices in administration from the Melanoma Nursing Initiative. Clinical Journal of Oncology Nursing, 21(Suppl. 4), 76–86. doi:10.1188/17.CJON.S4.76-86




