Clinical Trial Participation: A Pilot Study of Patient-Identified Barriers
Background: Clinical trial enrollment in the United States is lacking, particularly among older adult and ethnic and racial minority populations.
Objectives: The aim of the current study was to identify patient-related barriers to clinical trial participation using a mixed-methods patient survey and to offer insights to develop evidence-based implementation strategies to address these barriers.
Methods: A retrospective survey was conducted of patients who were not interested in participating in a clinical trial to quantify the reasons these patients chose not to participate. Directed qualitative content analysis was used to identify themes that emerged from the write-in responses.
Findings: The greatest patient-reported barriers were misperceptions about placebos, a desire to not feel like a human guinea pig, uncertainty surrounding clinical trial treatment effectiveness compared to standard care, and concerns about additional appointments or tests. Oncology nurses can address patient enrollment barriers by providing targeted education and participating in the informed consent process.
Jump to a section
Progress in cancer care requires ongoing clinical trials. Only 3%–5% of adult cancer treatment in the United States is provided within a clinical trial (Hallquist Viale, 2016). Little progress has been made in the past decade in terms of improving clinical trial enrollment in the United States, particularly for older adults and those in ethnic and racial minority groups (Sedrak et al., 2021). Barriers to cancer trial enrollment have been explored and reported extensively in the literature. The most common obstacles have been broadly categorized as structural, clinical, and physician or patient related (Lara et al., 2001; Mills et al., 2006; Nipp et al., 2019; Sedrak et al., 2021; Unger et al., 2019). Hillyer et al. (2020) reported that there remains a wide disparity in provider versus patient attitudes and beliefs regarding clinical trials. Oncology nurses play a pivotal role in identifying and addressing patient concerns about clinical trials.
In a systematic review and meta-analysis, structural and clinical barriers accounted for more than 77% of patients not enrolling in a clinical trial (Unger et al., 2019). Structural barriers include the absence of an available trial at an institution and other factors, such as limited clinical research staff support. The institution may not offer clinical trials at all or may not offer a trial that is appropriate for the patient’s cancer type or stage. More than 85% of patients in the United States receive cancer care in a community setting, where there are fewer opportunities for trial participation than at an urban academic center (American Cancer Society Cancer Action Network, 2018).
Clinical barriers include restrictive clinical trial eligibility criteria. Trial eligibility criteria help to ensure a defined population to address the research question and protect the safety of trial participants because of the potential impact the study may have on patients with more serious health issues. Despite recommendations from the American Society of Clinical Oncology to update trial eligibility to be more representative of the health of patients with cancer, stringent eligibility criteria persist (Kim et al., 2017). Ineligibility rates in the United States are between 18.5% and 25.4% (Unger et al., 2019). In particular, the expansion of personalized medicine (i.e., the use of patients’ genetic or other biomarker information to make treatment decisions) has led to an increase in biomarker-specific trials that limit eligibility to a small group of patients (Janiaud et al., 2019).
Physicians play a critical role in presenting trials to patients and helping them to understand the role of a clinical trial in their treatment. When eligible patients are presented with a trial by their physician, they agree to participate more than 50% of the time (Unger et al., 2019). Physicians may decide not to discuss trials with patients because of time constraints or treatment preference. They may also be unaware of trial options or have concerns about the complex nature of protocols (Mills et al., 2006). This may be an area where oncology nurses can help physicians through assisting in identifying trial candidates and educating patients about the value of clinical trials.
Patient-related barriers can include personal factors and beliefs that affect patient willingness to participate. Other factors identified include concerns of a negative impact on their relationship with their physician (Mills et al., 2006), patient and family dynamics (Hillyer et al., 2020), fear of placebo, loss of control, time required to participate, and fear of side effects (Hillyer et al., 2020; Mills et al., 2006; Nielsen & Berthelsen, 2019; Nipp et al., 2019; Sedrak et al., 2021).
The aims of this study were to (a) identify patient-related barriers to clinical trial participation and (b) present oncology nurses with evidence-based strategies to address these barriers.
Methods
Sample and Setting
This study was a retrospective mixed-methods analysis of patients not interested in participating in a clinical trial. Quantitative analysis was used for closed-ended survey questions, and directed qualitative content analysis was used to identify themes that emerged from the write-in responses. The survey was conducted from January 24 to June 30, 2019. The Roswell Park Institutional Review Board approved this study, and participants signed a written consent form to participate.
Data from the point-of-care clinical oncology pathway system at the Roswell Park Comprehensive Cancer Center in Buffalo, New York, were leveraged to help identify patients who were eligible for trials based on cancer type, staging, and relevant biomarkers and other clinical characteristics. In the clinical oncology pathway, the medical oncology provider shares information on the patient’s cancer type, stage, biomarkers, and clinical situation. If a clinical trial at the center is open for accrual and matches the basic clinical situation, it is presented to the provider as the first treatment choice. The provider must then either select a trial or select from a list of reasons why a trial was not selected (patient eligibility, provider preference, insurance or cost, patient preference, other reason). If the provider selects the trial, the clinical oncology pathway immediately sends an automatic message to the clinical research coordinator to complete full eligibility screening for that trial.
Clinical research coordinators and physicians at Roswell Park Comprehensive Cancer Center review a patient’s medical history to ascertain whether basic eligibility is met. Physicians introduce the standard of care and the clinical trial to the patient as treatment options. If the patient chooses the clinical trial, the clinical research coordinator reviews the research study consent in depth to ensure that the patient understands the trial. Once consent is obtained, study-related tests are initiated to determine final eligibility.
Patient Eligibility and Recruitment
The study population consisted of adult English- and Spanish-speaking patients receiving a clinical oncology pathway recommendation for a cancer type and circumstance (e.g., adjuvant therapy, metastatic or recurrent cancer) where a clinical trial was presented to the provider for consideration. Eligible patients were identified using data from the clinical oncology pathway. The data were filtered to identify patients who declined to participate and who had a solid tumor cancer: breast, gastrointestinal, genitourinary, gynecologic, head and neck, and thoracic cancers, and melanoma.
If the provider documented in the clinical oncology pathway that the patient was not interested in any trial or this trial, and if the patient met the other basic eligibility criteria for study participation, a research staff member contacted the patient to join the study. The researcher also verbally confirmed with patients that they were not interested in participating in a trial. Eligible patients were consented for this study, and a hard copy survey was given to the patient to complete and collected immediately.
Data Collection
The mixed-methods survey was based on a questionnaire used in a similar study at the Royal Marsden Hospital in London, England (Moorcraft et al., 2016). The questionnaire, licensed under a Creative Commons Attribution 4.0 International License with unrestricted permissions, was developed based on a review of the literature and the authors’ experiences of trial recruitment. The modified mixed-methods survey used for this study was expanded to include open-ended write-in responses to the following questions for richer qualitative analysis:
• How do you view being asked to participate in cancer research? Please elaborate on this question—why?
• Please explain the reasons that you decided not to enroll in the clinical trial; underline or circle the most important reason to you.
• Please let us know any changes you would have made to make you more interested in participating in the clinical trial.
• Please explain what a clinical trial means to you, in your own words.
The survey includes Likert-type (n = 6), multiple-choice (n = 19), and open-ended responses (n = 3), as well as demographic questions. An oncology and nursing research team reviewed the modified version for face and content validity. The open-ended survey responses and their analysis were intended to be an adjunct to the primary survey research, with the intention of enhancing the analysis of closed-ended survey responses. Survey study data were collected and managed using REDCap, a secure, web-based software platform designed to support data capture for research studies (Harris et al., 2019).
Mixed-Methods Analysis
A mixed-methods approach was used to analyze the survey data. Statistical analysis was performed to determine the mean and range for continuous responses, and counts and percentages for categorical responses. Directed qualitative content analysis was used to identify concepts and themes that emerged from the open-ended responses. The directed qualitative content analysis approach is generally used to describe a phenomenon that would benefit from further description (Assarroudi et al., 2018) and was used here to probe patients’ perceptions of participating in a clinical trial. The primary author and research assistant coded the data, and the expert panel reached consensus about the final themes that emerged.
Results
Trials Presented for Prescreening
During the study time period, there were 272 cases of trials offered to 164 unique patients that were categorized in the clinical oncology pathway as the patient not being interested in clinical trial participation. Of these patients, 23 were deceased before being approached about study participation, and 75 were determined to be ineligible because the patient did not recall being offered clinical trial participation when approached by the researcher, or the patient was hospitalized or too ill to approach. In addition, 36 patients did not have a scheduled appointment at the cancer center within the enrollment window or were missed. Of the 30 patients who were approached to take the survey, 9 declined and 21 completed the survey, for a 70% participation rate.
Quantitative Survey Data
Sample characteristics can be found in Table 1. Most participants (n = 17) had a clinical oncology pathway treatment decision for metastatic solid tumors. Most participants were female (n = 14) and non-Hispanic White (n = 17) and reported their education level as college (n = 11). The mean age of participants was 64 years (range = 41–69 years). 
The majority of participants had not previously participated in a clinical trial (n = 17). Travel time to get to the cancer center varied; 11 participants stated it took more than 30 minutes to reach the cancer center, and 6 said it took one to two hours. Most participants (n = 15) had someone else drive them to the cancer center (e.g., friend, family member, public transportation service).
Participants reported receiving the most information about clinical trials from their oncologist (n = 9) or the clinical research coordinator (n = 9). The family and friends that participants reported discussing treatment with most were spouses (n = 10) and their children (n = 9).
Most participants surveyed (n = 16) saw being asked to participate in cancer research as positive. No participants felt that it was negative, with the remainder of respondents (n = 5) seeing it as neither positive nor negative. Most (n = 19) responded positively (agree or strongly agree) to the following statement: “I believe clinical trials associated with cancer research will help doctors better understand and treat cancer.” A few (n = 2) were concerned about the use and storage of blood and tissue samples for research, and one participant reported being concerned about incurring additional costs because of clinical trial participation. Overall, participants reported that the amount of information provided about the clinical trial and the time spent discussing the clinical trial were adequate (see Table 2). The most frequently reported barriers in the multiple-choice response section were concerns about receiving a placebo (n = 11) and not wanting to feel like a human guinea pig (n = 9). 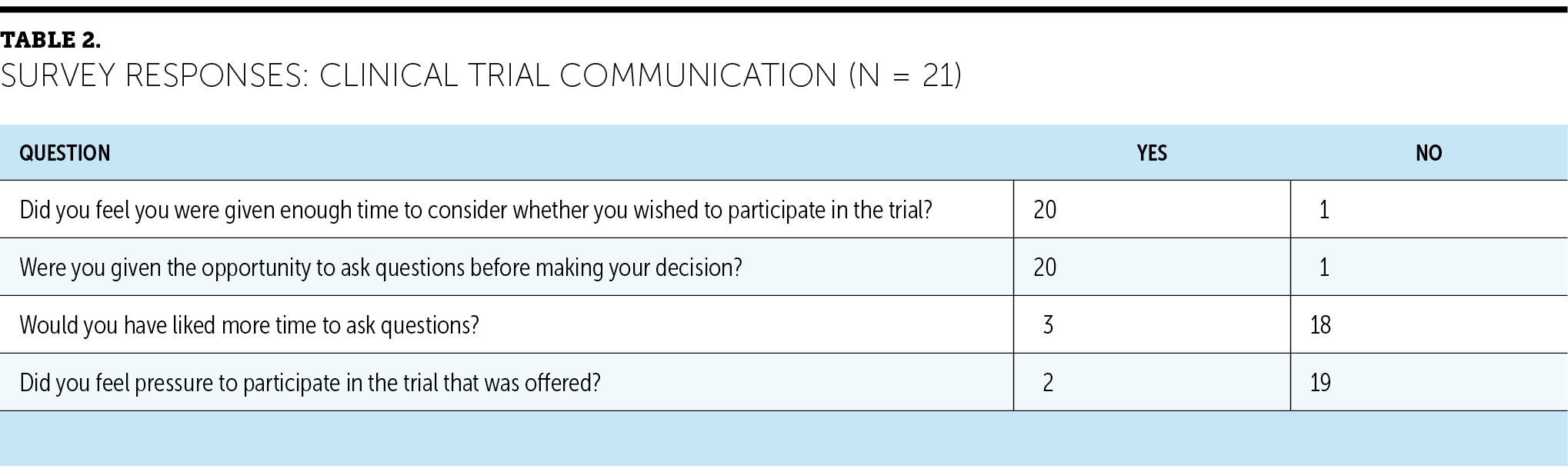
Qualitative Survey Data
When asked about changes that would make them more interested in participating in a clinical trial, 10 participants wanted more supporting evidence for the trial, indicating a perceived risk about the quality of trial outcomes compared to standard-of-care outcomes. Response themes for the question concerning reasons that participants decided not to enroll in a clinical trial indicated concerns about additional appointments or tests and the perceived risk of participating in a clinical trial versus receiving the standard of care, including concerns related to receiving a placebo and the uncertainty of the effectiveness of the treatment. When asked to explain what a clinical trial was in their own words, three participant responses included the word “placebo.” In write-in responses, there was no correlation between the word “placebo” appearing in the trial name and participants voicing their concerns about receiving a placebo. Figure 1 summarizes the participant topics, themes, and quotes. 
Discussion
Participants who declined clinical trial participation primarily cited concerns related to the ambiguity of effectiveness versus standard of care, the time required for participation in a trial, and lack of control of treatment choice. Findings from the current study support those in the literature regarding patient barriers to enrollment (American Cancer Society Cancer Action Network, 2018; Dias et al., 2016; Hillyer et al., 2020; Lara et al., 2001; Manne et al., 2015; Mills et al., 2006). The survey data suggest that designers of clinical trials should consider factors affecting the patient’s burden of participation. This has been examined extensively in the literature (Manne et al., 2015; Mills et al., 2006; Naidoo et al., 2020; Nielsen & Berthelsen, 2019; Sedrak et al., 2021). Making the frequency of clinic visits and time commitment equivalent to standard-of-care treatment should be considered a goal when designing trials. Findings from the current study support that time can be a significant burden.
Although cost was not reported as a barrier by most participants, indirect costs, such as additional requirements for time away from work or family care (Nusbaum et al., 2017), could result in a financial burden to patients (Nipp et al., 2019; Winkfield et al., 2018).
Wright et al. (2004) showed that perceived personal benefit was the most significant patient-related predictor of clinical trial enrollment. The current authors’ analysis of write-in responses expands on this by providing additional perspective on the paradoxical concern about the risk of participation because, as one participant stated, it is “unknown if it would be better than standard of care—too risky.”
Participant comments on the study survey indicated confusion about the availability of efficacy data for the trial treatment offered. Some participants did not appear to understand that efficacy data are not yet available for most phases of a clinical trial. For example, one participant stated that they would be more willing to participate in a trial in the future by “knowing the results of testing in the past.” The provision of efficacy data to the patient depends on the phase of the trial offered.
Patient-related barriers can be addressed through communication and education. Implementation of patient-level interventions, such as PRE-ACT (Preparatory Education About Clinical Trials) (Meropol et al., 2016), has been shown to be useful in a prospective multisite randomized clinical trial and should be considered for broad dissemination. The study by Meropol et al. (2016) is the largest randomized controlled trial to date that has looked at an intervention using a series of patient-facing educational videos specifically designed to address patient-level barriers. Topics address many of the barriers identified in the current study, including the following questions:
• What is a placebo?
• Will taking part in a clinical trial help me?
• Are there ways to deal with transportation and financial issues?
Patients can access this free series of educational videos on Cancer.Net, a patient information website managed by the American Society of Clinical Oncology (n.d.).
Work that addresses nursing interventions includes a National Institutes of Health–funded study, Oncology Nurse IMPACT: Improving Communication with Patients about Clinical Trial, testing the value of a tailored video-based educational intervention designed to increase oncology nurse intention to discuss clinical trials with patients. This study was built on work by Flocke et al. (2019) that measured the attitudes, subjective norms, and perceived behavioral control using survey data from more than 1,900 Oncology Nursing Society members.
A potential solution to the barrier of patient knowledge deficit suggested by Nipp et al. (2019) is the integration of patient navigators into the clinical trial accrual process. Navigation has been shown to improve accrual to clinical trials in multiple studies, and particularly to increase participation among African American individuals (Fouad et al., 2016; Winkfield et al., 2018). In addition, the Education Network to Advance Cancer Clinical Trials program recommends that documented prescreening of all patients for clinical trial eligibility and the inclusion of clinical trial navigators be mandated (Nipp et al., 2019).
Limitations
The current study was conducted using a convenience sample at a single cancer center. Methodologic limitations included a small sample size (n = 21) that was 7% of the eligible patient population (N = 272). In addition, the study ratio of females to males was 2:1. Because of the small sample size, there were few non-White participants. The survey, although used previously in a similar research context, was not a validated tool and was modified for the practice setting. Therefore, findings from the study may not be generalizable.
The use of the directed qualitative content analysis method to analyze the open-ended survey responses presents limitations. The directed approach can lead to confirmation bias, meaning that researchers are likely to find evidence that is supportive of a particular hypothesis (Hsieh & Shannon, 2005). There is also the potential that contextual features that may have influenced participant responses were not recognized. To reduce the amount of bias, study team members reviewed the data independently to confirm trustworthiness of the responses.
Implications for Nursing
When addressing educational barriers, the entire healthcare team should help patients understand the purpose of clinical trials and the potential value of trial participation. Nurses have many roles in their facilities and often have extended contact with patients; therefore, they are in a unique position to support patients in their decision-making regarding clinical trial participation. Nurses can provide targeted education, address patient-identified concerns, and participate in the informed consent process. Understanding and assimilating themes identified in this study may enhance nurses’ ability to identify, teach, and proactively discuss terms such as “placebo” and the idea of receiving “extra treatment,” as well as help patients explore concerns about the effectiveness of trial treatments.
Nurses should maintain proficiency through continuing education related to the design and importance of clinical trials. Nurses can also benefit from watching the PRE-ACT patient video series to enhance their knowledge; the videos provide an example of how to present complex concepts in a concise, understandable way to patients (American Society of Clinical Oncology, n.d.). Nurses can address patient concerns about the availability of efficacy data for the trial treatment by reassuring patients that although they cannot predict whether a trial will be more beneficial than the standard of care, trials are based on scientific evidence that the new treatment has promise (American Cancer Society Cancer Action Network, 2018).
Nurses may also be involved in providing informed consent. As noted in the Oncology Nursing Society’s (2016) Oncology Clinical Trial Nurse Competencies, nurses play a role in providing leadership and ensuring patient comprehension and safety during the informed consent process (Ness & Royce, 2017). The use of evidence-based nursing interventions, such as the teach-back method to verify patient understanding during informed consent discussions, is recommended (Brega et al., 2015). A key component of the teach-back method is putting the responsibility of patient understanding on the nurse.
Studies such as that by Regan (2018) have demonstrated the effectiveness of the teach-back method, an evidence-based health literacy intervention, during informed consent. Nurses are provided with examples of teach-back scripts that can be used with patients in the informed consent process. Regan (2018) demonstrated that after receiving teach-back training, nurses had high research knowledge scores and demonstrated statistically significant improvement in post-test conviction and confidence. 
Conclusion
The results of the current study can be used by all stakeholders to develop multifaceted interventions that include evidence-based education programs for nurses and patients, as well as accommodations to support patients in minimizing the time and effort required to participate in a clinical trial. In addition, these findings demonstrate key gaps in patient understanding of clinical trials; they also support the need to conduct more extensive implementation studies on the feasibility and acceptability of evidence-based nursing interventions that have been shown to help address patient-reported concerns about enrolling in clinical trials. Given their central role in oncology care, nurses should be considered integral members of the clinical research education program. 
The authors gratefully acknowledge Nessa Stefaniak, MA, for data collection and research coordination; Monica Murphy, BSMT, for analytics support, and Lu Liu, PhD, for demographic data, as well as Joanne Abbotoy, MSN, RN, and Laurie Musial, PhD, MSN, RN, who both provided guidance with the protocol formation and study design.
About the Author(s)
Mishellene McKinney, MHA, RN, OCN®, was, at the time of this writing, the director of clinical pathways and implementation science at Roswell Park Comprehensive Cancer Center in Buffalo, NY; Rose Bell, PhD, AOCNP®, ARNP-C, is a clinical oncology nurse practitioner at Roswell Park Care Network in Buffalo, NY, and an associate professor in the Department of Nursing at Daemen College in Amherst, NY; Cindy Samborski, MSN, MHA, RN, CCRC, is a senior clinical research coordinator and clinical research educator, and Kristopher Attwood, PhD, is an assistant professor of oncology, biostatistics, and bioinformatics, both at Roswell Park Comprehensive Cancer Center; Grace Dean, PhD, RN, is an associate professor in the School of Nursing at the University at Buffalo in New York; Katherine Eakle, PharmD, is the medical science director, and Wei Yu, PhD, is the head of oncology biostatistics and a data and statistical sciences leader, both at Genentech in South San Francisco, CA; and Stephen B. Edge, MD, FACS, FASCO, is a professor of oncology and the vice president of system quality and outcomes at Roswell Park Comprehensive Cancer Center. The authors take full responsibility for this content. This work was supported by Genentech and a grant (P30CA016056) from the National Cancer Institute involving the use of Roswell Park Comprehensive Cancer Center’s Pathology Network, Genomic, and Clinical Data Network shared resources. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. McKinney can be reached at mishellene.x.mckinney@kp.org, with copy to CJONEditor@ons.org. (Submitted February 2021. Accepted July 12, 2021.)
References
American Cancer Society Cancer Action Network. (2018). Barriers to patient enrollment in therapeutic clinical trials for cancer: A landscape report. https://www.fightcancer.org/sites/default/files/National%20Documents/Cl…
American Society of Clinical Oncology. (n.d.). Welcome to PRE-ACT! Cancer.Net. https://www.cancer.net/research-and-advocacy/clinical-trials/welcome-pr…
Assarroudi, A., Heshmati Nabavi, F., Armat, M.R., Ebadi, A., & Vaismoradi, M. (2018). Directed qualitative content analysis: The description and elaboration of its underpinning methods and data analysis process. Journal of Research in Nursing, 23(1), 42–55. https://doi.org/10.1177/1744987117741667
Brega A.G., Barnard, J., Mabachi, M.N., Weiss, B.D., DeWalt, D.A., Brach, C., . . . West, D.R. (2015). AHRQ health literacy universal precautions toolkit (2nd ed.). Agency for Healthcare Research and Quality. https://www.ahrq.gov/sites/default/files/publications/files/healthlitto…
Dias, A.L., Chao, J.H., Lee, D., Wu, Y., & Kloecker, G.H. (2016). Patient perceptions concerning clinical trials in oncology patients. Contemporary Clinical Trials Communications, 4, 179–185. https://doi.org/10.1016/j.conctc.2016.09.005
Flocke, S.A., Nock, N.L., Fulton, S., Margevicius, S., Manne, S., Meropol, N.J., & Daly, B.J. (2019). A national study of oncology nurses discussing cancer clinical trials with patients. Western Journal of Nursing Research, 41(12), 1747–1760. https://doi.org/10.1177/0193945919829145
Fouad, M.N., Acemgil, A., Bae, S., Forero, A., Lisovicz, N., Martin, M.Y., . . . Vickers, S.M. (2016). Patient navigation as a model to increase participation of African Americans in cancer clinical trials. Journal of Oncology Practice, 12(6), 556–563. https://doi.org/10.1200/jop.2015.008946
Hallquist Viale, P. (2016). Participation in cancer clinical trials: Researching the causes of low accrual. Journal of the Advanced Practitioner in Oncology, 7(2), 143–144. https://doi.org/10.6004/jadpro.2016.7.2.1
Harris, P.A., Taylor, R., Minor, B.L., Elliott, V., Fernandez, M., O’Neal, L., . . . Duda, S.N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. https://doi.org/10.1016/j.jbi.2019.103208
Hillyer, G.C., Beauchemin, M., Hershman, D.L., Kelsen, M., Brogan, F.L., Sandoval, R., . . . Schwartz, G.K. (2020). Discordant attitudes and beliefs about cancer clinical trial participation between physicians, research staff, and cancer patients. Clinical Trials, 17(2), 184–194. https://doi.org/10.1177/1740774520901514
Hsieh, H.-F., & Shannon, S.E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. https://doi.org/10.1177/1049732305276687
Janiaud, P., Serghiou, S., & Ioannidis, J.P.A. (2019). New clinical trial designs in the era of precision medicine: An overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treatment Reviews, 73, 20–30. https://doi.org/10.1016/j.ctrv.2018.12.003
Kim, E.S., Bruinooge, S.S., Roberts, S., Ison, G., Lin, N.U., Gore, L., . . . Schilsky, R.L. (2017). Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. Journal of Clinical Oncology, 35(33), 3737–3744. https://doi.org/10.1200/jco.2017.73.7916
Lara, P.N., Jr., Higdon, R., Lim, N., Kwan, K., Tanaka, M., Lau, D.H.M., . . . Lam, K.S. (2001). Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. Journal of Clinical Oncology, 19(6), 1728–1733. https://doi.org/10.1200/jco.2001.19.6.1728
Manne, S., Kashy, D., Albrecht, T., Wong, Y.-N., Lederman Flamm, A., Benson, A.B., III, . . . Meropol, N.J. (2015). Attitudinal barriers to participation in oncology clinical trials: Factor analysis and correlates of barriers. European Journal of Cancer Care, 24(1), 28–38. https://doi.org/10.1111/ecc.12180
Meropol, N.J., Wong, Y.-N., Albrecht, T., Manne, S., Miller, S.M., Flamm, A.L., . . . Schluchter, M.D. (2016). Randomized trial of a web-based intervention to address barriers to clinical trials. Journal of Clinical Oncology, 34(5), 469–478. https://doi.org/10.1200/jco.2015.63.2257
Mills, E.J., Seely, D., Rachlis, B., Griffith, L., Wu, P., Wilson, K., . . . Wright, J.R. (2006). Barriers to participation in clinical trials of cancer: A meta-analysis and systematic review of patient-reported factors. Lancet Oncology, 7(2), 141–148. https://doi.org/10.1016/s1470-2045(06)70576-9
Moorcraft, S.Y., Marriott, C., Peckitt, C., Cunningham, D., Chau, I., Starling, N., . . . Rao, S. (2016). Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: Results of a prospective patient survey. Trials, 17, 17. https://doi.org/10.1186/s13063-015-1105-3
Naidoo, N., Nguyen, V.T., Ravaud, P., Young, B., Amiel, P., Schanté, D., . . . Boutron, I. (2020). The research burden of randomized controlled trial participation: A systematic thematic synthesis of qualitative evidence. BMC Medicine, 18(1), 6. https://doi.org/10.1186/s12916-019-1476-5
Ness, E.A., & Royce, C. (2017). Clinical trials and the role of the oncology clinical trials nurse. Nursing Clinics of North America, 52(1), 133–148. https://doi.org/10.1016/j.cnur.2016.10.005
Nielsen, Z.E., & Berthelsen, C.B. (2019). Cancer patients’ perceptions of factors influencing their decisions on participation in clinical drug trials: A qualitative meta-synthesis. Journal of Clinical Nursing, 28(13–14), 2443–2461. https://doi.org/10.1111/jocn.14785
Nipp, R.D., Hong, K., & Paskett, E.D. (2019). Overcoming barriers to clinical trial enrollment. American Society of Clinical Oncology Educational Book, 39, 105–114. https://doi.org/10.1200/edbk_243729
Nusbaum, L., Douglas, B., Damus, K., Paasche-Orlow, M., & Estrella-Luna, N. (2017). Communicating risks and benefits in informed consent for research: A qualitative study. Global Qualitative Nursing Research, 4, 2333393617732017. https://doi.org/10.1177/2333393617732017
Oncology Nursing Society. (2016). 2016 oncology clinical trials nurse competencies. https://www.ons.org/sites/default/files/2018-10/Oncology_Clinical_Trial…
Regan, E.M. (2018). Clinical trials informed consent: An educational intervention to improve nurses’ knowledge and communications skills. Clinical Journal of Oncology Nursing, 22(6), E152–E158. https://doi.org/10.1188/18.CJON.E152-E158
Sedrak, M.S., Freedman, R.A., Cohen, H.J., Muss, H.B., Jatoi, A., Klepin, H.D., . . . Dale, W. (2021). Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA: A Cancer Journal for Clinicians, 71(1), 78–92. https://doi.org/10.3322/caac.21638
Unger, J.M., Vaidya, R., Hershman, D.L., Minasian, L.M., & Fleury, M.E. (2019). Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute, 111(3), 245–255. https://doi.org/10.1093/jnci/djy221
Winkfield, K.M., Phillips, J.K., Joffe, S., Halpern, M.T., Wollins, D.S., & Moy, B. (2018). Addressing financial barriers to patient participation in clinical trials: ASCO policy statement. Journal of Clinical Oncology, 36(33), 3331–3339. https://doi.org/10.1200/jco.18.01132
Wright, J.R., Whelan, T.J., Schiff, S., Dubois, S., Crooks, D., Haines, P.T., . . . Levine, M.N. (2004). Why cancer patients enter randomized clinical trials: Exploring the factors that influence their decision. Journal of Clinical Oncology, 22(21), 4312–4318. https://doi.org/10.1200/jco.2004.01.187

