Malakoplakia After Allogeneic Hematopoietic Stem Cell Transplantation
A 32-year-old woman named A.C. was diagnosed five years ago with stage IIIB nodular sclerosing Hodgkin lymphoma. Following initial chemotherapy, she had refractory disease as evidenced by hypermetabolic activity on positron-emission tomography scan. A.C. was treated with a short course of salvage chemotherapy, stem cell collection, and autologous HSCT about 11 months after initial diagnosis. Her post-transplantation course was complicated by pneumonia and interstitial lung disease secondary to chemotherapy that rapidly improved with high-dose steroids.
Jump to a section
A 32-year-old woman named A.C. was diagnosed five years ago with stage IIIB nodular sclerosing Hodgkin lymphoma (HL). Following initial chemotherapy, she had refractory disease as evidenced by hypermetabolic activity on positron-emission tomography (PET) scan. A.C. was treated with a short course of salvage chemotherapy, stem cell collection, and autologous HSCT about 11 months after initial diagnosis. Her post-transplantation course was complicated by pneumonia and interstitial lung disease secondary to chemotherapy that rapidly improved with high-dose steroids.
Three months later, A.C. was feeling well, tapered off prednisone (Deltasone®), and was free of symptoms. A repeat PET scan showed persistent hypermetabolic activity treated with a radical course of radiotherapy with complete resolution; however, another small area of concern emerged in the supraclavicular area. The decision was made to observe and repeat imaging in a few months but, before this could occur, A.C. had recurrence with supraclavicular lymphadenopathy. She was now six weeks pregnant and opted to continue her pregnancy, a goal she had prior to embarking on autologous HSCT. She gave birth to a healthy baby girl, began salvage chemotherapy, and was referred back to the transplantation program for consideration of allogeneic transplantation. She was initially not interested in pursuing another transplantation, but as a new mother she did not want to risk not being able to fulfill this role.
On completion of treatment, PET imaging again showed disease progression. At this point, 37 months from initial diagnosis and 2 years after autologous HSCT, A.C. agreed to an allogeneic mismatched unrelated donor HSCT in an attempt to cure her HL. She would be at risk for disease relapse, lung toxicity (secondary to her prior pneumonitis), graft-versus-host disease (GVHD), and infectious complications. She was counselled on the risks and benefits of transplantation and given a 20%–40% chance for long-term, disease-free survival. Lack of complete remission at the time of transplantation, prior autologous transplantation (less than 100 days), and a human leukocyte antigen (HLA)-mismatched unrelated donor transplantation would increase her risk of GVHD and infections in the immediate post-transplantation period.
The immediate post-transplantation course was complicated by acute GVHD of the skin (grade 2) and upper and lower gastrointestinal (GI) tract (grade 2), which required significant immunosuppressive therapy to control. A.C. was discharged from hospital (day 35) for close outpatient monitoring of her GVHD and potential for infectious complications. On day 82, A.C. presented to the outpatient clinic with a 10-day history of diarrhea, nausea, vomiting, dehydration, and weakness. She was readmitted for investigation and management of these new and debilitating symptoms. She was diagnosed with Escherichia coli (E. coli) gram-negative bacteremia in the blood and urine. A colonoscopy was conducted to assess her watery and bloody diarrhea and A.C. was diagnosed of malakoplakia of her GI tract. She was treated with IV antibiotics and attempts to change to oral antibiotics resulted in relapse of infection. A month after combination IV therapy, her blood cultures cleared but she continued with IV therapy for an additional two months to prevent recurrence. A follow-up computed tomography (CT) scan of the abdomen showed progressive bowel thickening necessitating the addition of daily ertapenem (Invanz®) therapy (see Table 1).
A.C. spent more than 200 days in the hospital following her transplantation because of infectious complications and supportive care. Her home was four hours from the hospital, and her husband relocated to be closer while her parents took on a large role in parenting her daughter. A.C. experienced numerous complications and suffered many losses, including her role as a mother and her independence. She experienced frequent urinary incontinence as well as explosive, bloody, fecal incontinence and required narcotics to ease severe abdominal and lower back pain; she remained largely bedridden. Efforts to ambulate often precipitated incontinence and exacerbated her pain; she became withdrawn from medical and nursing staff and seemed distant from family. A.C. was malnourished and deconditioned. She experienced steroid myopathy, weakness, dehydration, malabsorption and, therefore, malnutrition. She refused physiotherapy and rehabilitation services. Nurses were frustrated with their inability to provide care and connect with A.C. and to facilitate improvement in her well-being. Despite visitors, A.C. was isolated and remained withdrawn and passive in her care.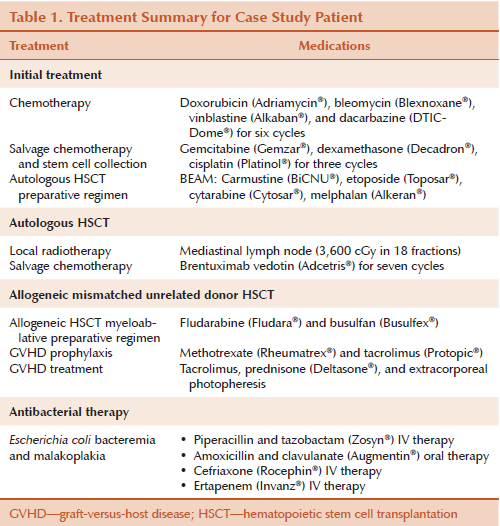
Background
HL accounts for about 0.5% of all new cancer cases and 0.2% of all cancer deaths (National Cancer Institute, 2015). The majority of patients can be cured with standard first-line chemotherapy, with or without radiation therapy; however, a subset will have primary refractory disease (Kako et al., 2015). About 25%–33% of patients with advanced-stage HL experience relapse after conventional chemotherapy, with a three-year overall survival rate of 70% (Kako et al. 2015).
Autologous HSCT is a common treatment for relapsed or refractory HL, whereas allogeneic HSCT is reserved for patients with disease relapse following autologous HSCT (Perales et al., 2015). The main benefit allogeneic HSCT offers is the graft-versus-lymphoma effect, whereby donor T-lymphocytes eliminate malignant residual host T-cells (Perales et al., 2015). In addition to the side effects of the preparative chemotherapy regimens, common post-allogeneic transplantation complications also include infection, acute and chronic GVHD, organ damage (heart, lungs, liver, or kidney), early menopause, cataracts, hypothyroidism, secondary malignancy, and bone health issues (Leger & Neville, 2004; McAdams & Burgunder, 2013).
Malakoplakia
Malakoplakia was first described in the literature more than a century ago and is derived from the Greek malakos (soft) and plakos (plaques) (Graves, Texler, Manning, & Kulkarni, 2014). Malakoplakia is a rare finding in the setting of HL or stem cell transplantation, with few published articles or case reports (Yang, Huang, Tsung, & Han, 1983). Slightly more publications have been cited in the renal transplantation setting (Biggar, Keating, & Bear, 1981; Graves et al., 2014).
Malakoplakia is a rare granulomatous disease that commonly occurs in the urinary and GI tracts but has been reported in the head and neck, lungs, lymph nodes, prostate, and cervix (Graves et al., 2014; Yousef & Naghibi, 2007). Malakoplakia is characterized by the microscopic accumulation of tissue macrophages containing unique inclusion bodies, known as Michaelis-Gutmann bodies, and grossly appears as soft, tan, yellow plaques and nodules or extensive bands (McClure, 1981; Yousef & Haghibi, 2007). Malakoplakia has been reported in both pediatric and adult populations with no gender, racial, or age predominance (McClure, 1981; Yousef & Naghibi, 2007). The cause is not fully understood, but pathogenesis is thought to be a result of abnormal macrophage response, microorganisms, or abnormal or altered immune response (Yousef & Naghibi, 2007). Several organisms have been implicated, including E. coli (the most common organism found in about 66% of cases), Proteus, and Staphylococcus aureus (Yousef & Naghibi, 2007).
Malakoplakia is characterized by an inflammatory disorder with diminished bactericidal action of leukocytes present in hosts with an impaired immune response. In the setting of HSCT, the immunosuppressed host lacks the ability to mount a normal protective immune response and, therefore, the infection that has caused the malakoplakia to develop can be difficult to treat.
Malakoplakia is historically associated with poor outcomes and mortality rates greater than 50% at six months, often from the underlying condition (Graves et al. 2014). Morbidity is related to chronicity, and symptoms of malakoplakia of the colon include diarrhea, abdominal pain, rectal bleeding, vomiting, malaise, fever, cough, and constipation with symptom duration varying from weeks to months (McClure, 1981).
GVHD is one of the most serious complications following allogeneic transplantation and is a significant cause of non-relapse mortality and morbidity (Lee, 2004). About 70% of patients after allogeneic unrelated donor transplantation develop some degree of GVHD despite prophylactic strategies (Mitchell, 2013). Symptoms typically present in the first 100 days after allogeneic transplantation and affect the skin, GI tract mucosa, and the liver (Mitchell, 2013). Immunosuppression is required to assist with prevention and management, and to minimize morbidity and mortality, but immunosuppression also increases the host’s risk of infection. Attempts to control GVHD also may eradicate graft-versus-lymphoma effect, leading to disease relapse.
After stem cell transplantation, immunosuppressive medications are tapered to foster normal leukocyte function and immune reconstitution in the host. Impaired leukocyte function reduces the ability of the host to fight bacterial invasion. In the presence of acute GVHD requiring active intervention, treatment of malakoplakia can be complicated. Symptoms of GVHD and malakoplakia of the colon are clinically similar, and active GVHD requires immune suppression to prevent severe organ compromise that can result from uncontrolled GVHD. Poor GVHD control can progress to have devastating and potentially fatal outcomes (Mitchell, 2013). Symptoms of malakoplakia of the GI tract may erroneously be attributed to GVHD in the patient after transplantation.
The risk factors for malakoplakia are related to the degree of immune suppression of the host and the duration of the underlying illness. Factors which increase an individual’s risk of complication post-HSCT include type of transplantation (autologous or allogeneic), time from transplantation, GVHD, the degree of HLA match, disease status, graft type, post-transplantation immunosuppression, intensity of conditioning regimen, and timing of neutrophil engraftment (McAdams & Burgunder, 2013). Treatment is either aimed at eradicating the isolated organism with antibiotics that penetrate the cell membrane and concentrate in macrophages or by correcting the lysosomal defect with a cholinergic agonist. Antibiotics and surgery offer patients the best chance for cure (Yousef & Naghibi, 2007).
Implications for Nursing
The development of malakoplakia poses a challenge for clinicians for two reasons. First, the clinical manifestations may be mild and subtle or multisystem and debilitating depending on the degree of organ involvement. Secondly, malakoplakia presents in the setting of an existing illness. On its own, a stem cell transplantation can result in long hospitalizations, isolation, physical changes, life-threatening toxicities, anxiety, and depression (Sherman, Cooke, & Grant, 2005). Malakoplakia of the colon in the setting of HSCT is a rare finding and may mimic symptoms of GVHD, therefore going unrecognized and untreated. Malakoplakia as a result of microbial involvement can be difficult to treat and eradicate in an immunosuppressed individual already at risk of disease relapse, GVHD, and infection after transplantation.
The challenge for oncology nurses is to support and educate patients and family members through this potentially chronic and unexpected complication. Illness can strip an individual of his or her ability to perform basic self-care tasks, including maintaining food and water intake, elimination, balancing rest and activity, and social interaction, and impairs the ability to maintain well-being. As symptoms and illness progress, so must the level of nursing involvement. Patients who are bedridden rely completely on nurses to carry out basic functions but, throughout the recovery process, nurses engage patients and encourage them to care for themselves once again. Nurses are continually assessing an individual’s ability and desire to participate and learn, providing them small manageable tasks and bits of information that yield success and understanding. Restoration of optimal well-being requires a multidisciplinary approach from a team of clinicians dedicated to being present, patient, and persistent throughout the recovery process.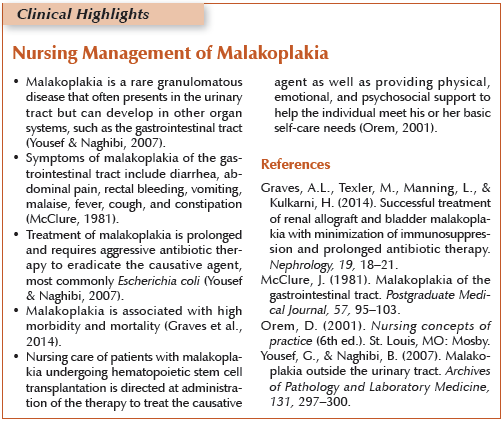
Conclusion
An allogeneic HSCT is an intense treatment option for patients with aggressive, life-threatening disease who are willing to accept the risks to achieve cure. Patients can be counseled about the known risks and complications of transplantation, but they cannot be fully prepared for the impact of rare and potentially fatal complications. Knowledge and awareness of malakoplakia in the setting of HSCT is the first step in early recognition and intervention.
Regarding A.C., she continued to be troubled by infections. At the time of submission, A.C. was hospitalized in the intensive care unit for community acquired pneumonia. However, with excellent medical and nursing care, she regained strength and returned home to raise her young daughter.
References
Biggar, W., Keating, A., & Bear, R. (1981). Malakoplakia: Evidence for an acquired disease secondary to immunosuppression. Transplantation, 31, 109–112.
Graves, A.L., Texler, M., Manning, L., & Kulkarni, H. (2014). Successful treatment of renal allograft and bladder malakoplakia with minimization of immunosuppression and prolonged antibiotic therapy. Nephrology, 19, 18–21. doi:10.1111/nep.12194
Kako, S., Isutsu, K., Kato, K., Kim, S.W., Mori, T., Fukuda, T., . . . Suzumiya, J. (2015). The role of hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. American Journal of Hematology, 90, 152–158. doi:10.1002/ajh.23897
Lee, S. (2004). New approaches for preventing and treating chronic graft-versus-host disease. Blood, 105, 4200–4206. doi:10.1182/blood-2004-10-4023
Leger, C.S., & Neville, T.J. (2004). Hematopoietic stem cell transplantation: A primer for the primary care physician. Canadian Medical Association Journal, 170, 1569–1577. doi:10.1503/cmaj.1011625
McAdams, F.W., & Burgunder, M.R. (2013). Transplant treatment course and acute complications. In S.A. Ezzone (Ed.), Hematopoietic stem cell transplantation: A manual for nursing practice (2nd ed., pp. 47–66). Pittsburgh, PA: Oncology Nursing Society.
McClure, J. (1981). Malakoplakia of the gastrointestinal tract. Postgraduate Medical Journal, 57, 95–103. doi:10.1136/pgmj.57.664.95
Mitchell, S.A. (2013). Acute and chronic graft-versus-host-disease. In S.A. Ezzone (Ed.), Hematopoietic stem cell transplantation: A manual for nursing practice (2nd ed., pp. 103–153). Pittsburgh, PA: Oncology Nursing Society.
National Cancer Institute. (2005). SEER stat fact sheets: Hodgkin lymphoma. Retrieved from http://seer.cancer.gov/statfacts/html/hodg.html
Perales, M.A., Ceberio, I., Armand, P., Burns, L.J., Chen, R., Cole, P.D., . . . Carpenter, P.A. (2015). Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: Guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation, 21, 971–983. doi:10.1016/j.bbmt.2015.02.022
Sherman, R.S., Cooke, E., & Grant, M. (2005). Dialogue among survivors of hematopoietic stem cell transplantation: Support group themes. Journal of Psychosocial Oncology, 23(1), 1–24. doi:10.1300/J077v23n01_01
Yang, C., Huang, T., Tsung, S., & Han, D. (1983). Rectal malakoplakia in a patient with Hodgkin’s disease. Report of a case and review of the literature. Diseases of the Colon and Rectum, 26, 129–132. doi:10 .1007/BF02562594
Yousef, G., & Naghibi, B. (2007). Malakoplakia outside the urinary tract. Archives of Pathology and Laboratory Medicine, 131, 297–300.
About the Author(s)
Tracy Robinson, RN, MN, CON(C), is a clinical nurse specialist and Erin Streu, RN, MN, CON(C), is a clinic nurse, both at CancerCare Manitoba in Winnipeg, Canada. No financial relationships to disclose. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Robinson can be reached at trobinson@cancercare.mb.ca, with copy to editor at ONFEditor@ons.org.

