Determinants of Physical Activity Maintenance in Breast Cancer Survivors After a Community-Based Intervention
Purpose/Objectives: To determine whether empirically selected and social cognitive theory-based factors, including baseline characteristics and modifiable behavioral and psychosocial factors, were determinants of physical activity (PA) maintenance in breast cancer survivors (BCSs) six months after a PA intervention.
Design: Single-group longitudinal study.
Setting: The Breast Health Centre in Winnipeg, Manitoba, Canada.
Sample: 42 survivors with stage 0–III breast cancer who completed chemotherapy and/or radiation therapy.
Methods: The community-based PA intervention included six weekly education and practice sessions on home-based aerobic, resistance, balance, and flexibility exercises.
Main Research Variables: The dependent variable, PA maintenance, was determined based on PA level measurement at six months postintervention. The independent variables of baseline characteristics (age, stage of cancer, and chronic musculoskeletal symptoms) and modifiable behavioral and psychosocial factors (PA level, fatigue, PA self-efficacy in overcoming barriers and performing tasks) were assessed at baseline and postintervention.
Findings: Multivariate regression analyses revealed that baseline fatigue and chronic musculoskeletal symptoms were the only factors significantly associated with PA maintenance.
Conclusions: Baseline fatigue level and chronic musculoskeletal symptoms were significant determinants of PA maintenance in breast cancer survivors who had completed a community-based PA intervention. However, other key factors were considered.
Implications for Nursing: Prior to participation in community-based PA interventions, clinicians should take into account the effects of high baseline fatigue levels and chronic musculoskeletal symptoms on potential PA maintenance, and consider additional assessments and support for BCSs to sustain their PA behavioral change.
Jump to a section
Physical activity (PA), including both occupational and leisure activities, is increasingly recognized as having an important role in breast cancer (BC) outcomes and survivorship. Breast cancer survivors (BCSs) often experience side effects from treatment (surgery, chemotherapy, radiation, adjuvant endocrine therapy), including functional limitations, poor quality of life, fatigue, weight gain, psychosocial distress, and increased risk of other chronic diseases (Schmitz, 2011; Speck, Courneya, Masse, Duval, & Schmitz, 2010). Evidence indicates that PA can help optimize the recovery of physical functioning, decrease deconditioning, improve quality of life, manage treatment side effects, and potentially protect against disease recurrence and mortality among BCSs (Kim, Choi, & Jeong, 2013; Lahart, Metsios, Nevill, & Carmichael, 2015). However, the benefit of PA for BCSs depends on the extent of their PA maintenance (Stull, Snyder, & Demark-Wahnefried, 2007), which is defined as the ability to sustain initial improvement in PA six months or more after a PA intervention (White, McAuley, Estabrooks, & Courneya, 2009).
BCSs may find it challenging to be physically active after cancer treatments and even more difficult to maintain the recommended level of 150 minutes of moderate physical activity per week (Bourke et al., 2014). Based on a population-based survey, about 16% of BCSs participate in a sufficient amount of PA (Smith & Chagpar, 2010). PA interventions are effective in improving PA level and managing cancer treatment side effects in BCSs after cancer treatment (Bluethmann, Vernon, Gabriel, Murphy, & Bartholomew, 2015; Kim et al., 2013). Specifically, community-based PA interventions (geographically located in a community institution in which interventions are implemented, such as health centers) (McLeroy, Norton, Kegler, Burdine, & Sumaya, 2003) have demonstrated efficacy in PA improvement and reduction in cancer treatment–related side effects (Cheifetz et al., 2014; Knobf, Thompson, Fennie, & Erdos, 2014; McLeroy et al., 2003) and are cost effective (Roux et al., 2008).
Adherence (participation in the PA behavior) during supervised PA interventions among BCSs is shown to be as high as 89% (Courneya et al., 2008; Swenson, Nissen, & Henly, 2010); however, this rate was not sustained over time. Only 42%–58% maintained the recommended levels at six months after completion of the PA intervention (Courneya et al., 2009; Vallance, Courneya, Plotnikoff, Dinu, & Mackey, 2008). Reports on longer-term PA maintenance (six months after completion of interventions) in BCSs are scarce (Spark, Reeves, Fjeldsoe, & Eakin, 2013; White et al., 2009).
To date, only two randomized, controlled trials (RCTs) have examined PA maintenance and its potential determinants among BCSs (Courneya et al., 2009; Vallance, Plotnikoff, Karvinen, Mackey, & Courneya, 2010). Age, PA level, and fatigue were baseline characteristics and modifiable behavioral and psychosocial factors that have been consistently assessed across these studies. However, the findings were inconclusive, and the factors examined were limited. Based on the current empirical literature and guided by social cognitive theory (SCT) (Bandura, 1986), the authors of the current article proposed that baseline characteristics, including stage of cancer (Courneya et al., 2008, 2009; Irwin et al., 2004) and chronic musculoskeletal symptoms (Brown et al., 2014), and the modifiable psychosocial factor of PA self-efficacy (confidence and belief of one’s own ability to perform particular activities or overcome barriers to PA) (Pinto, Rabin, & Dunsiger, 2009; Vallance et al., 2010) should also be taken into account in the investigation of potential determinants of PA maintenance in BCSs, particularly within the context of a community-based PA intervention (see Figure 1).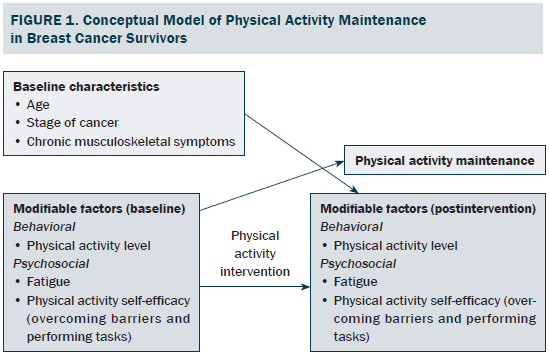
Age was considered as a potential determinant of PA maintenance in both RCTs (Courneya et al., 2009; Vallance et al., 2010). Courneya et al. (2009) reported negative association between age and PA maintenance in 201 BCSs who had completed a supervised gym-based PA intervention while on active treatment. In contrast, Vallance et al. (2010) did not find any association between age and PA maintenance in 266 BCSs who completed an unsupervised home-based PA intervention after treatment completion. However, the findings were equivocal. Both studies have yielded inconsistent findings on the influence of age on PA maintenance, and these have not been tested in BCSs who have completed a community-based PA intervention.
Stage of cancer is a baseline characteristic that has been explored as a potential determinant of PA maintenance in BCSs (Courneya et al., 2008, 2009; Irwin et al., 2004). Irwin et al. (2004) and Courneya et al. (2008) found a paradoxical positive association between stage of cancer and PA level (i.e., individuals with higher stage had greater PA adherence) in their cross-sectional studies of 806 and 242 BCSs, respectively. Conversely, Courneya et al. (2009) reported a small negative association between stage of cancer and PA level in their longitudinal study of 201 BCSs who completed a PA intervention while on active treatment (i.e., individuals with higher stage had lower levels of PA adherence).
Chronic musculoskeletal symptoms (e.g., joint or muscle pain) are commonly experienced by BCSs (23%–48%) (Brown et al., 2014; Menas et al., 2012; Park et al., 2013). However, the impact of this baseline characteristic on PA maintenance is rarely studied. Brown et al. (2014) reported that the 139 BCSs who experienced musculoskeletal symptoms were significantly more likely to have reduced PA levels compared to the other 161 BCSs in the cross-sectional study. The effects of chronic musculoskeletal symptoms on long-term PA maintenance remains to be determined.
PA level and fatigue often are the main targets of behavioral and psychosocial outcomes of PA interventions (Cramp & Byron-Daniel, 2012). Evidence suggests these behavioral and psychosocial outcomes could also be potential determinants of PA maintenance (Courneya et al., 2009; Pinto et al., 2009; Vallance et al., 2010). Courneya et al. (2009) reported that only baseline PA level and postintervention fatigue were significant determinants of PA maintenance in 201 BCSs who had completed a supervised gym-based PA intervention. On the contrary, Vallance et al. (2010) identified both baseline and postintervention PA levels and postintervention fatigue as significant predictors of PA maintenance among 377 BCSs who had completed an unsupervised home-based PA intervention. The discrepancies in the association between PA level, fatigue, and PA maintenance among BCSs appeared to depend on the delivery approach of the PA interventions. The impact of these modifiable behavioral and psychosocial factors on PA maintenance has not been studied in PA interventions delivered in the community.
Self-efficacy is important in PA intervention. Bandura’s (1986) SCT framework suggested that PA self-efficacy could be improved with SCT-based PA intervention and is essential for PA maintenance. However, research in the role of PA self-efficacy on PA maintenance among BCSs is lacking and equivocal. Vallance et al. (2010) found that higher postintervention PA self-efficacy in performing tasks was strongly associated with PA maintenance among 266 BCSs who had completed a home-based PA intervention. However, Pinto et al. (2009) reported that baseline PA self-efficacy in overcoming barriers was significantly associated with PA levels during a home-based PA intervention among 43 BCSs. Additional research is needed to determine the influence of PA self-efficacy on PA maintenance in BCSs, and also within the context of community-based PA interventions.
Overall, a limited understanding exists about the effects of baseline characteristics (age, stage of cancer, and chronic musculoskeletal symptoms) and modifiable PA intervention-targeted behavioral and psychosocial factors (PA level, fatigue, and PA self-efficacy) on PA maintenance among BCSs. The evidence also has been primarily drawn from RCTs, which often have limited reporting on external validity of their findings (White et al., 2009). Therefore, whether these findings on determinants of PA maintenance could be translated into the context of community-based PA interventions is unclear. To address this gap, the current study focused on the role of baseline characteristics and modifiable PA intervention–targeted behavioral and psychosocial factors that may influence PA maintenance after BCSs completed a community-based PA intervention. This study was part of a larger longitudinal study that examined the effects of a community-based PA intervention on PA maintenance (Lee, Von Ah, Szuck, & Lau, 2015). The current authors hypothesized that all baseline characteristics and postintervention behavioral and psychosocial factors would be significantly associated with PA maintenance at six months postintervention.
Methods
The current article reports on a secondary analysis of a longitudinal single-group study (Lee et al., 2015). Informed consent was obtained from the participants. The University of Manitoba’s Human Research Ethics Board approved the study protocol.
Women diagnosed with stage 0–III breast cancer who had completed their chemotherapy and/or radiation treatments at least one month prior to the PA intervention and had received physician clearance to participate were recruited. Those with metastatic disease were excluded. Recruitments were through healthcare professionals, flyer distributions at outpatient breast cancer clinics, and a local newsletter for cancer survivors.
Measurements
The independent variables were baseline characteristics of age, stage of cancer, and chronic musculoskeletal symptoms, as well as modifiable behavioral and psychosocial factors of PA level, fatigue, and PA self-efficacy. The self-reported baseline characteristics were obtained as part of the intake assessment. Stages of cancer were categorized into four groups, stage 0–III. Self-reported presence of chronic musculoskeletal symptoms at baseline had four categories— none, upper body only, lower body only, or upper and lower body. Musculoskeletal symptoms included reported joint and/or muscle pain and aches with or without limited range of motions. These symptoms were considered chronic when individuals had experienced them for more than six weeks (Cimmino, Ferrone, & Cutolo, 2011).
Modifiable behavioral and psychosocial factors including PA level, fatigue, and PA self-efficacy were assessed at baseline and postintervention. PA level was assessed using the interviewer-based Seven-Day Physical Activity Recall (PAR) (Blair et al., 1985; Sallis et al., 1985). The Seven-Day PAR used interview questions that prompted participants to recall the amount of time spent in sleeping and physical activities that were moderate, hard, and very hard intensity for each of the previous seven days prior to the interview. Any activity that added at least 10 minutes in one intensity category was counted. PA level in the past week was represented as weekly energy expenditure, and was estimated based on the metabolic equivalents tasks (METs) for the different intensity categories: moderate (4 METs), hard (6 METs), and very hard (10 METs). The MET-weighted total time spent on each of the PA categories of at least moderate intensity in all seven days were summed, normalized to individuals’ measured body mass index, and converted to be expressed in kcal per week. The instrument has an excellent test-retest (inter-class correlation = 0.99) and inter-rater reliability (r = 0.86) (Gross, Sallis, Buono, Roby, & Nelson, 1990), as well as good validity (r = 0.66–0.77) (Dishman & Steinhardt, 1988; Taylor et al., 1984).
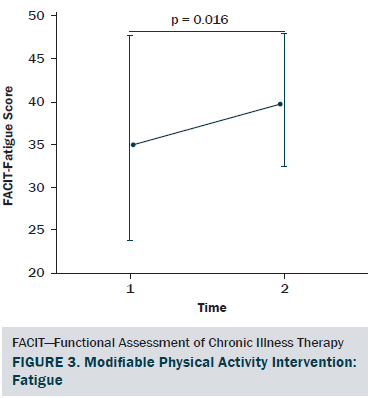
Fatigue was assessed using the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue) scale, version 4 (Yellen, Cella, Webster, Blendowski, & Kaplan, 1997). Individuals rate how accurately each of the 13 fatigue-related items describes them. The total scale score ranges from 0–52, with higher scores representing better quality of life as a result of less fatigue. The scale has strong test-retest reliability (r = 0.9) and internal consistency (a = 0.93–0.95), and it has been validated with good convergent/divergent and discriminant validity (Yellen et al., 1997).
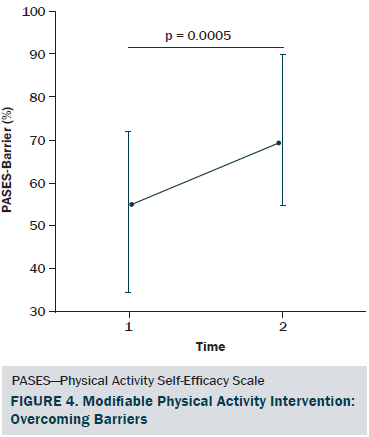
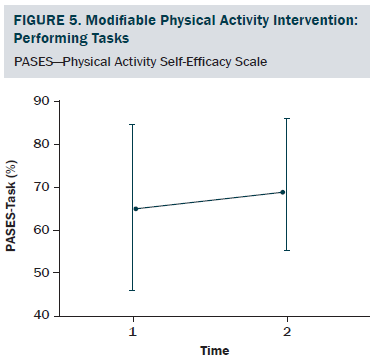
PA self-efficacy in performing tasks and overcoming barriers was assessed using the PA Self-Efficacy Scale (PASES) (Rogers et al., 2006; Rogers, McAuley, Courneya, & Verhulst, 2008). The scale has two distinct subscales that are scored separately: the PA self-efficacy in overcoming barriers subscale assesses an individual’s confidence in his or her ability to exercise when encountering nine different barriers to PA, and the PA self-efficacy in performing tasks subscale assesses an individual’s confidence in his or her ability to complete four different PA tasks. All items are rated on a 0%–100% scale. Each subscale score is calculated based on the mean of corresponding subscale items. Both subscales have demonstrated excellent internal consistency (a = 0.92 and 0.96, respectively) and good test-retest reliability (r = 0.83 and 0.89, respectively) (Rogers et al., 2005, 2006).
The primary dependent outcome was PA maintenance. PA maintenance in this study was defined as PA level at six months postintervention. It was determined based on the PA level assessed using the Seven-Day PAR at six months postintervention.
Intervention
The six-week PA intervention was based on Bandura’s SCT framework (Bandura, 1986) that focuses on improving PA self-efficacy and outcome expectations so as to enhance PA behavior (Pinto & Ciccolo, 2011). The intervention was conducted at the Breast Health Centre in Winnipeg, Manitoba, Canada. Six weekly 2.5-hour structured classes consisted of education and practice sessions on aerobic, resistance, balance, and flexibility exercises that could be performed at home. The education sessions on self-management and goal setting were aimed at improving the understanding of PA outcome expectations, whereas the supervised, hands-on practice PA sessions were aimed at enhancing PA self-efficacy. The aerobic exercise included a progressive walking program that used pedometers for self-monitoring to reach a weekly goal of 150 minutes of moderate-intensity PA. The resistance exercises included warm-ups and one to three sets of 8–12 repetitions of nine core and upper and lower extremity progressive routines using TherabandsTM, or weights, starting with the least resistance. The flexibility exercises included four upper and lower limb gentle stretches that were performed after the aerobic and resistance exercises. The level of difficulty of the exercises was tailored according the participants’ baseline ability. The classes were co-facilitated by a certified exercise instructor, a registered dietitian, and a registered social worker. Physical therapists and lymphedema therapists were consulted as necessary to individualize exercises for participants who had musculoskeletal symptoms that affected their exercise or physical activity participation during the program.
Statistical Methods
Multivariate stepwise regression analyses were used to identify factors that could be significant predictors of PA maintenance. Stepwise procedure was chosen because it combines the advantages of forward and backward solutions to allow for the reassessment of contributions of each variable when other variables are added at each step (Munro, 2005). The PA maintenance factors included baseline characteristics (age, stage of cancer, and chronic musculoskeletal symptoms) and modifiable behavioral and psychosocial factors (baseline and postintervention PA levels, fatigue, and PA self-efficacy in overcoming barriers and performing tasks). All factors were treated as continuous data, except stage of cancer (ordinal data) and chronic musculoskeletal symptoms (categorical data). The chronic musculoskeletal symptoms category was dummy-coded using two vectors, upper and lower body musculoskeletal symptoms. The proposed factors were entered in a stepwise fashion. The stepping criteria were set at an alpha of 0.05 for entry and 0.1 for removal of independent variables.
All statistical analyses were performed using SPSS®, version 22.0. Assumptions of multiple stepwise regression analysis were checked and met. Outliers were handled according to Tabachnick and Fidell’s (2001) approach; that is, outliers (more than three interquartile ranges) were reassigned to the next highest or lowest values. Collinearity of all independent variables was within an acceptable range. The variance inflation factors (VIF) for all independent variables (1.7 or less) were below the expected VIF cutoff of 10 (Kleinbaum, Kupper, Nizam, & Rosenberg, 2013).
Results
A total of 46 participants agreed to take part in the six-week PA intervention and six-month postintervention assessment. Four dropped out during the study for various reasons, including family death (n = 1), minor surgery (n = 1), injury unrelated to PA intervention (n = 1), and newly diagnosed bone metastasis (n = 1). Therefore, the final analysis included a total of 42 participants.
The sociodemographic and clinical characteristics of the participants are presented in Table 1. The participants were generally middle-aged women (mean age = 53.8 years), mostly diagnosed with stage I or II breast cancer (81%, n = 34), and 26% (n = 11) of the participants reported having chronic musculoskeletal symptoms that impact their physical activity. The modifiable PA intervention–targeted behavioral and psychosocial factors, including PA level, fatigue, and PA self-efficacy in overcoming barriers, had significant improvement from baseline to postintervention (p < 0.03). The descriptive statistics of the modifiable PA intervention-targeted behavioral and psychosocial factors are presented in Table 2 and Figures 2–5. The primary dependent outcome of this study, PA maintenance (PA level at six months postintervention), was 1,002.8 kcal (SD = 664.7 kcal) per week.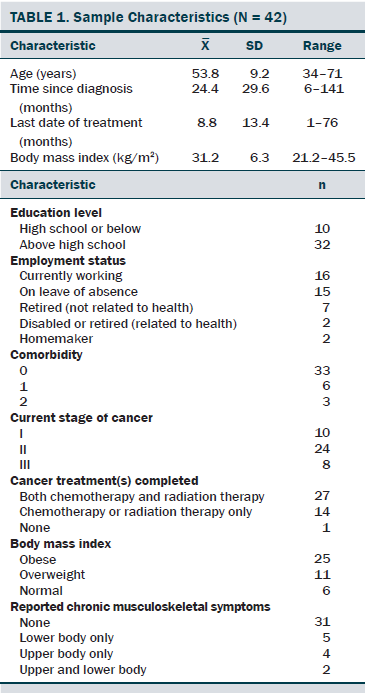
Regression analyses resulted in two models. The first model included only baseline fatigue as a significant factor. The second model included two significant factors, baseline fatigue and chronic musculoskeletal symptoms (see Table 3). In model 1, baseline fatigue accounted for only 12% of the variance of PA maintenance. In model 2, baseline fatigue and chronic musculoskeletal symptoms were included and accounted for 20% of the variance in PA maintenance. Baseline fatigue (alpha = 0.32, p = 0.029) and chronic musculoskeletal symptoms (alpha = –0.3, p = 0.046) were significant determinants of PA maintenance, with an observed power of 82%. The power was calculated based on the observed variance of 0.203 of the two predictors at an alpha of 0.05 (one-tailed) in model 2.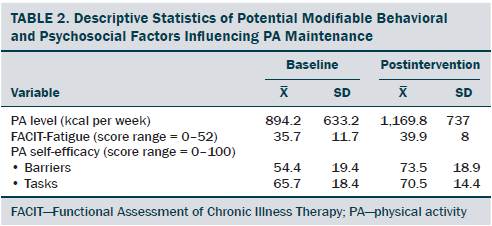
Discussion
The aim of this secondary analysis was to determine if empirically selected and SCT-based factors, including baseline characteristics (age, cancer staging, and chronic musculoskeletal symptoms) and modifiable PA intervention–targeted behavioral and psychosocial factors (fatigue, PA level, and PA self-efficacy at baseline and postintervention), would be significant determinants of PA maintenance at six months post-intervention. The study addressed the gaps in the understanding of the determinants of PA maintenance for BCSs who have completed a community-based PA intervention program. This study demonstrated that the modifiable psychosocial factor of fatigue and the baseline characteristic of chronic musculoskeletal symptoms were the only significant predictors for PA maintenance. This finding is particularly important given that current evidence already suggests that increased PA improves quality of life, specifically fatigue (Kim et al., 2013; Schmitz, 2011), and PA maintenance is crucial for the benefits of PA to be sustained. The challenge, however, faced by the clinicians is the high prevalence of fatigue (56%–95%) (de Jong, Candel, Schouten, Abu-Saad, & Courtens, 2004) and musculoskeletal symptoms (23%–48%) (Menas et al., 2012; Park et al., 2013). Given that maintenance of PA behavior is one of the critical components for the success and effectiveness of a PA intervention provided in the community (Gaglio, Shoup, & Glasgow, 2013), it would be essential for clinicians to consider additional strategies to address the impact of baseline fatigue and chronic musculoskeletal symptoms on long-term PA maintenance.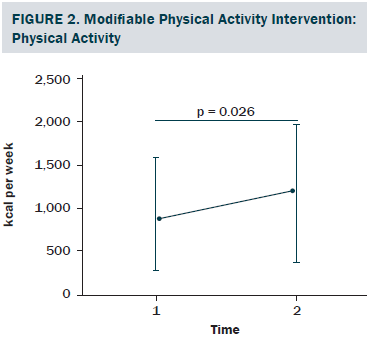
This study is the first to demonstrate that chronic musculoskeletal symptoms are negatively associated with PA maintenance. One key factor could have been the inclusion of BCSs with chronic musculoskeletal symptoms, who were often excluded in RCTs (Pinto et al., 2008; Vallance et al., 2008, 2010). With an intention to have a broader reach to BCSs in the community who needed PA intervention, this community-based PA intervention program had allowed the authors to include about 26% of BCSs who had chronic musculoskeletal symptoms. The proportion of participants with musculoskeletal symptoms in this study seemed to corroborate with published prevalence among BCSs (23%–48%) (Menas et al., 2012; Park et al., 2013). Although ad-hoc physical therapy consultations were provided to adapt exercises for individuals with musculoskeletal symptoms, it did not seem to be sufficient. Early screening of chronic musculoskeletal symptoms that affect daily activities and, therefore, referral for further musculoskeletal assessments and interventions may minimize their negative impact on longer-term PA maintenance among BCSs. Differential predictors of PA maintenance could exist between BCSs with and without chronic musculoskeletal symptoms, but stratified analyses were not performed in this study because of the small sample size. However, the current findings highlight the significant influence of musculoskeletal symptoms on longer-term PA maintenance among BCSs who have completed a community-based PA intervention.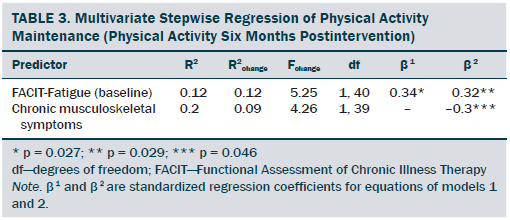
The important role of baseline fatigue, rather than postintervention fatigue, in predicting PA maintenance among BCSs was an unexpected finding given that the authors’ primary analyses showed that fatigue and PA level improved significantly from baseline to postintervention (Lee et al., 2015). Fatigue can be reduced by increasing PA among BCSs (Pinto et al., 2008; Vallance et al., 2008, 2010; Voskuil et al., 2010), and Courneya et al.’s (2009) study indicated that postintervention fatigue predicted PA maintenance. The discrepancies between the current authors’ findings and Courneya et al.’s (2009) findings might have been caused by the differences in study type, sample size, and PA intervention implementation timing. The current authors’ PA intervention included a single small sample group of BCSs who had received the PA intervention after they had completed chemotherapy and radiation therapy, but Courneya et al.’s (2009) study was an RCT with a follow-up sample of 201 BCSs who had received PA intervention while they were receiving chemotherapy. The current study was similar to Vallance et al.’s (2010) home-based PA intervention that also targeted BCSs who had completed treatments, and both studies found baseline fatigue as an independent predictor for PA maintenance. However, Vallance et al. (2010) also identified postintervention fatigue as a significant predictor, and the authors of the current article did not. The differences may be attributed to the dissimilar analyses approach, study type, and sample size between the studies. Vallance et al. (2010) was an RCT with 377 participants and the PA level at six months was analyzed as categorical variable and used logistic regression analyses. On the other hand, the current article had a much smaller sample size in a single-group study, and the authors also treated the same dependent variable as continuous data and used multivariate regression analyses. In addition, both studies had different intervention effects on fatigue. Vallance et al. (2010) had smaller positive change in fatigue (about 5%) than the current article (about 11%).
Taken together, strong evidence suggests that baseline fatigue is significantly associated with PA maintenance for BCSs who have completed cancer treatments. This has important clinical implications because 68% of cancer survivors never receive advice on fatigue management (Blaney, Lowe-Strong, Rankin-Watt, Campbell, & Gracey, 2013). PA intervention should emphasize the importance of maintaining PA as an effective way to combat fatigue. Additional targeted support may be required for BCSs with high baseline fatigue levels so as to facilitate maintenance of their PA behavior after completion of a community-based PA intervention.
Limitations
Interpretation of the findings should be limited within the strengths and weaknesses of this study. As previously mentioned, the strengths of the study included a theory-based PA intervention program that was implemented in a community setting, a broader inclusion criteria of BCSs who have chronic musculoskeletal symptoms, and a longer-term time period after completion of the intervention. The limitations of this study included the self-reported nature of the PA outcome and the independent variables (age, stage of cancer, and chronic musculoskeletal symptoms), the lack of severity indicator for the musculoskeletal symptoms, and the small sample size. In addition, the sample size restricted the inclusion of other potential determinants in this study; therefore, only the key factors from the current empirical literature and Bandura’s (1986) SCT framework were selected for analyses. Only 20% of the variance in PA maintenance was accounted for by the two factors of baseline fatigue and chronic musculoskeletal symptoms. Other potential factors for PA maintenance, such as intentions, personality, and depression, could be included in future studies (Andrykowski, Beacham, Schmidt, & Harper, 2006; Courneya, Friedenreich, Sela, Quinney, & Rhodes, 2002; Rhodes, Martin, & Taunton, 2001). Larger studies could also address differences in determinants of PA maintenance between stratified groups of BCSs with and without chronic musculoskeletal symptoms, and could examine the effects of hormonal therapy that could produce or exacerbate musculoskeletal symptoms. In addition, stratified analysis on determinants of PA maintenance could be performed in BCSs who are obese, overweight, or normal weight in a larger sample size.
Implications for Nursing
According to Karvinen, McGourty, Parent, and Walker (2012), a substantial proportion (67%) of oncology nurses provide physical activity recommendations to BCSs. To facilitate longer-term PA maintenance, these findings suggest clinicians should consider the level of fatigue and the presence of chronic musculoskeletal symptoms in BCSs who will be starting a PA intervention. Brief screening assessment on fatigue level and the duration and extent of musculoskeletal symptoms may help clinicians identify BCSs with high levels of fatigue and chronic musculoskeletal symptoms, such as upper and/or lower body joint pain that affect daily activities. Additional assessments (e.g., musculoskeletal-specific) and additional support and interventions (e.g., individualized exercise adaptations) could then be recommended and integrated into the community-based PA interventions for BCSs. Additional research is needed to develop practical clinical tools for screening high levels of fatigue and chronic musculoskeletal symptoms. Studies also are necessary to determine the best evidence-based and cost-effective approach to integrating additional targeted assessments and support into existing community-based PA interventions. The feasibility and efficacy of collaborative programs between oncology and community health professionals that could be integrated into community-based PA interventions could be explored.
Conclusion
This study found that baseline fatigue and chronic musculoskeletal symptoms were significant determinants of PA maintenance six months after BCSs completed a community-based PA intervention. The data will provide further information to guide the planning and design of future community-based PA intervention programs for BCSs to best facilitate longer-term maintenance of PA.
The authors gratefully acknowledge the continued support of the community-based physical activity intervention program from Tania Sloan, Ethel MacIntosh, MD, Lorena Gerl, BA, Paul J. Daeninck, MD, MSc, Jill Taylor-Brown, MSW, RSW, Joel Gingrich, MD, and Debjani Grenier, MD. The authors also thank the program staff for their commitment to the study: Margaret Samsoondar, Kat Rother, MSc, Nancy Ryan-Arbez, MSc, BMR(PT), Maureen Walker, MSc, BMR(PT), Karen Dobbin, MSc, BMR(PT), and Srikesavan Sabapathy, MSc. Most of all, the authors thank the breast cancer survivors who participated in the program evaluation to help establish the evidence necessary to improve the quality of the program delivery.
References
Andrykowski, M.A., Beacham, A.O., Schmidt, J.E., & Harper, F.W. (2006). Application of the theory of planned behavior to understand intentions to engage in physical and psychosocial health behaviors after cancer diagnosis. Psycho-Oncology, 15, 759–771. doi:10.1002/pon.1007
Bandura, A. (1986). Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall.
Blair, S.N., Haskell, W.L., Ho, P., Paffenbarger, R.S., Jr., Vranizan, K.M., Farquhar, J.W., & Wood, P.D. (1985). Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology, 122, 794–804.
Blaney, J.M., Lowe-Strong, A., Rankin-Watt, J., Campbell, A., & Gracey, J.H. (2013). Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psycho-Oncology, 22, 186–194. doi:10.1002/pon.2072
Bluethmann, S.M., Vernon, S.W., Gabriel, K.P., Murphy, C.C., & Bartholomew, L.K. (2015). Taking the next step: A systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Research and Treatment, 149, 331–342. doi:10.1007/s10549-014-3255-5
Bourke, L., Homer, K.E., Thaha, M.A., Steed, L., Rosario, D.J., Robb, K.A., . . . Taylor, S.J. (2014). Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: A systematic review. British Journal of Cancer, 110, 831–841. doi:10.1038/bjc.2013.750
Brown, J.C., Mao, J.J., Stricker, C., Hwang, W.T., Tan, K.S., & Schmitz, K.H. (2014). Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast Journal, 20, 22–28. doi:10.1111/tbj.12202
Cheifetz, O., Park Dorsay, J., Hladysh, G., Macdermid, J., Serediuk, F., & Woodhouse, L.J. (2014). CanWell: Meeting the psychosocial and exercise needs of cancer survivors by translating evidence into practice. Psycho-Oncology, 23, 204–215. doi:10.1002/pon.3389
Cimmino, M.A., Ferrone, C., & Cutolo, M. (2011). Epidemiology of chronic musculoskeletal pain. Best Practice and Research: Clinical Rheumatology, 25, 173–183. doi:10.1016/j.berh.2010.01.012
Courneya, K.S., Friedenreich, C.M., Reid, R.D., Gelmon, K., Mackey, J.R., Ladha, A.B., . . . Segal, R.J. (2009). Predictors of follow-up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Research and Treatment, 114, 179–187. doi:10.1007/s10549-008-9987-3
Courneya, K.S., Friedenreich, C.M., Sela, R.A., Quinney, H.A., & Rhodes, R.E. (2002). Correlates of adherence and contamination in a randomized controlled trial of exercise in cancer survivors: An application of the theory of planned behavior and the five factor model of personality. Annals of Behavioral Medicine, 24, 257–268.
Courneya, K.S., Segal, R.J., Gelmon, K., Reid, R.D., Mackey, J.R., Friedenreich, C.M., . . . McKenzie, D.C. (2008). Predictors of supervised exercise adherence during breast cancer chemotherapy. Medicine and Science in Sports and Exercise, 40, 1180–1187.
Cramp, F., & Byron-Daniel, J. (2012). Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews, 11, CD006145. doi:10.1002/14651858.CD006145.pub3
de Jong, N., Candel, M.J., Schouten, H.C., Abu-Saad, H.H., & Courtens, A.M. (2004). Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Annals of Oncology, 15, 896–905.
Dishman, R.K., & Steinhardt, M. (1988). Reliability and concurrent validity for a 7-d recall of physical activity in college students. Medicine and Science in Sports and Exercise, 20, 14–25.
Gaglio, B., Shoup, J.A., & Glasgow, R.E. (2013). The RE-AIM framework: A systematic review of use over time. American Journal of Public Health, 103, e38–e46. doi:10.2105/AJPH.2013.301299
Gross, L.D., Sallis, J.F., Buono, M.J., Roby, J.J., & Nelson, J.A. (1990). Reliability of interviewers using the Seven-Day Physical Activity Recall. Research Quarterly for Exercise and Sport, 61, 321–325.
Irwin, M.L., McTiernan, A., Bernstein, L., Gilliland, F.D., Baumgartner, R., Baumgartner, K., & Ballard-Barbash, R. (2004). Physical activity levels among breast cancer survivors. Medicine and Science in Sports and Exercise, 36, 1484–1491.
Karvinen, K.H., McGourty, S., Parent, T., & Walker, P.R. (2012). Physical activity promotion among oncology nurses. Cancer Nursing, 35, E41–E48. doi:10.1097/NCC.0b013e31822d9081
Kim, J., Choi, W.J., & Jeong, S.H. (2013). The effects of physical activity on breast cancer survivors after diagnosis. Journal of Cancer Prevention, 18, 193–200.
Kleinbaum, D.G., Kupper, L.L., Nizam, A., & Rosenberg, E.S. (2013). Applied regression analysis and other multivariable methods (5th ed.). Independence, KY: Cengage Learning.
Knobf, M.T., Thompson, A.S., Fennie, K., & Erdos, D. (2014). The effect of a community-based exercise intervention on symptoms and quality of life. Cancer Nursing, 37, E43–E50. doi:10.1097/NCC.0b013e318288d40e
Lahart, I.M., Metsios, G.S., Nevill, A.M., & Carmichael, A.R. (2015). Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncologica, 54, 635–654. doi:10.3109/0284186x.2014.998275
Lee, C.E., Von Ah, D., Szuck, B., & Lau, Y.K.J. (2015, March 27). Physical activity maintenance and associated factors in breast cancer survivors after a community-based lifestyle intervention program. Paper presented at the University of Cincinnati Cancer Institute’s Cancer Survivorship Program Consortium, Cincinnati, OH.
McLeroy, K.R., Norton, B.L., Kegler, M.C., Burdine, J.N., & Sumaya, C.V. (2003). Community-based interventions. American Journal of Public Health, 93, 529–533.
Menas, P., Merkel, D., Hui, W., Lawton, J., Harper, A., & Carro, G. (2012). Incidence and management of arthralgias in breast cancer patients treated with aromatase inhibitors in an outpatient oncology clinic. Journal of Oncology Pharmacy Practice, 18, 387–393. doi:10.1177/1078155211434853
Munro, B.H. (2005). Statistical methods for health care research (5th ed.). Philadelphia, PA: Lippincott Williams and Wilkins.
Park, J.Y., Lee, S.K., Bae, S.Y., Kim, J., Kim, M.K., Kil, W.H., . . . Nam, S.J. (2013). Aromatase inhibitor-associated musculoskeletal symptoms: Incidence and associated factors. Journal of the Korean Surgical Society, 85, 205–211. doi:10.4174/jkss.2013.85.5.205
Pinto, B.M., & Ciccolo, J.T. (2011). Physical activity motivation and cancer survivorship. Recent Results in Cancer Research, 186, 367–387. doi:10.1007/978-3-642-04231-7_16
Pinto, B.M., Rabin, C., & Dunsiger, S. (2009). Home-based exercise among cancer survivors: adherence and its predictors. Psycho-Oncology, 18, 369–376. doi:10.1002/pon.1465
Pinto, B.M., Rabin, C., Papandonatos, G.D., Frierson, G.M., Trunzo, J.J., & Marcus, B.H. (2008). Maintenance of effects of a home-based physical activity program among breast cancer survivors. Supportive Care in Cancer, 16, 1279–1289. doi:10.1007/s00520-008-0434-0
Rhodes, R.E., Martin, A.D., & Taunton, J.E. (2001). Temporal relationships of self-efficacy and social support as predictors of adherence in a 6-month strength-training program for older women. Perceptual and Motor Skills, 93, 693–703.
Rogers, L.Q., Courneya, K.S., Verhulst, S., Markwell, S., Lanzotti, V., & Shah, P. (2006). Exercise barrier and task self-efficacy in breast cancer patients during treatment. Supportive Care in Cancer, 14, 84–90.
Rogers, L.Q., McAuley, E., Courneya, K.S., & Verhulst, S.J. (2008). Correlates of physical activity self-efficacy among breast cancer survivors. American Journal of Health Behavior, 32, 594–603.
Rogers, L.Q., Shah, P., Dunnington, G., Greive, A., Shanmugham, A., Dawson, B., & Courneya, K.S. (2005). Social cognitive theory and physical activity during breast cancer treatment. Oncology Nursing Forum, 32, 807–815.
Roux, L., Pratt, M., Tengs, T.O., Yore, M.M., Yanagawa, T.L., Van Den Bos, J., . . . Buchner, D.M. (2008). Cost effectiveness of community-based physical activity interventions. American Journal of Preventive Medicine, 35, 578–588. doi:10.1016/j.amepre.2008.06.040
Sallis, J.F., Haskell, W.L., Wood, P.D., Fortmann, S.P., Rogers, T., Blair, S.N., & Paffenbarger, R.S., Jr. (1985). Physical activity assessment methodology in the Five-City Project. American Journal of Epidemiology, 121, 91–106.
Schmitz, K. (2011). Physical activity and breast cancer survivorship. Recent Results in Cancer Research, 186, 189–215. doi:10.1007/978-3-642-04231-7_8
Smith, S.G., & Chagpar, A.B. (2010). Adherence to physical activity guidelines in breast cancer survivors. American Surgeon, 76, 962–965.
Spark, L.C., Reeves, M.M., Fjeldsoe, B.S., & Eakin, E.G. (2013). Physical activity and/or dietary interventions in breast cancer survivors: A systematic review of the maintenance of outcomes. Journal of Cancer Survivorship, 7, 74–82.
Speck, R.M., Courneya, K.S., Masse, L.C., Duval, S., & Schmitz, K.H. (2010). Erratum to: An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Journal of Cancer Survivorship, 5, 112.
Stull, V.B., Snyder, D.C., & Demark-Wahnefried, W. (2007). Lifestyle interventions in cancer survivors: Designing programs that meet the needs of this vulnerable and growing population. Journal of Nutrition, 137(1, Suppl.), 243S–248S.
Swenson, K.K., Nissen, M.J., & Henly, S.J. (2010). Physical activity in women receiving chemotherapy for breast cancer: Adherence to a walking intervention. Oncology Nursing Forum, 37, 321–330. doi:10.1188/10.ONF.321-330
Tabachnick, B.G., & Fidell, L.S. (2001). Using multivariate statistics. New York, NY: HarperCollins College Publishers.
Taylor, C.B., Coffey, T., Berra, K., Iaffaldano, R., Casey, K., & Haskell, W.L. (1984). Seven-day activity and self-report compared to a direct measure of physical activity. American Journal of Epidemiology, 120, 818–824.
Vallance, J.K., Courneya, K.S., Plotnikoff, R.C., Dinu, I., & Mackey, J.R. (2008). Maintenance of physical activity in breast cancer survivors after a randomized trial. Medicine and Science in Sports and Exercise, 40, 173–180.
Vallance, J.K., Plotnikoff, R.C., Karvinen, K.H., Mackey, J.R., & Courneya, K.S. (2010). Understanding physical activity maintenance in breast cancer survivors. American Journal of Health Behavior, 34, 225–236. doi:10.5555/ajhb.2010.34.2.225
Voskuil, D.W., van Nes, J.G., Junggeburt, J.M., van de Velde, C.J., van Leeuwen, F.E., & de Haes, J.C. (2010). Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Annals of Oncology, 21, 2094–2101. doi:10.1093/annonc/mdq151
White, S.M., McAuley, E., Estabrooks, P.A., & Courneya, K.S. (2009). Translating physical activity interventions for breast cancer survivors into practice: An evaluation of randomized controlled trials. Annals of Behavioral Medicine, 37, 10–19. doi:10.1007/s12160-009-9084-9
Yellen, S.B., Cella, D.F., Webster, K., Blendowski, C., & Kaplan, E. (1997). Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management, 13, 63–74.
About the Author(s)
Lee is a visiting assistant scientist in the School of Nursing and School of Health and Rehabilitation Sciences, and Von Ah is an associate professor and the Department Chair of the School of Nursing, both at Indiana University in Indianapolis; Szuck is a registered dietitian for the Winnipeg Regional Health Authority Breast Health Centre in Manitoba; and Lau is a medical advisor in Indianapolis, IN. This research was supported by the Canadian Breast Cancer Foundation Community Grants and was funded by a grant (No. R25CA117865) from the National Cancer Institute of the National Institutes of Health. Lee, Szuck, and Lau contributed to the conceptualization and design. Lee and Szuck completed the data collection. Lee and Von Ah provided statistical support. Lee, Von Ah, and Lau contributed to the analysis. All of the authors contributed to the manuscript preparation. Lee can be reached at leece@iupui.edu, with copy to editor at ONFEditor@ons.org. Submitted March 2015. Accepted for publication May 25, 2015.

