A Systematic Review of Cognitive Impairment in Individuals With Colorectal Cancer
Problem Identification: Patients with colorectal cancer (CRC) encounter varying degrees of objective and subjective cognitive impairment. The prevalence of objective and subjective cognitive impairment, factors affecting cognitive impairment, and interventions are presented in this review.
Literature Search: The CINAHL Plus®, Cochrane Library, Embase®, PsycINFO®, PubMed®, and CNKI databases were systematically searched from the time of the database’s establishment to May 2023. Manual searches for the relevant articles in the literature’s references were also conducted.
Data Evaluation: The results were independently assessed by two reviewers.
Synthesis: 25 studies were included. The prevalence of cognitive impairment in individuals with CRC was measured differently according to study designs. A model of factors contributing to cognitive impairment guided the integration of factors, including cancer treatments, psychosocial factors, and physical and emotional health conditions. Incorporated intervention programs could be integrated between objective and subjective aspects. Interventions relieved cognitive impairment in individuals with CRC.
Implications for Nursing: The results of this review supported enhanced assessment and monitoring of cognitive impairment, particularly subjective cognitive impairment.
Jump to a section
Colorectal cancer (CRC) is the third most prevalent cancer and the second most fatal cancer (Siegel et al., 2023). The five-year relative survival rate for CRC has risen to around 65% because of the advances in early screening, diagnosis, and treatment (Li et al., 2021). The five-year survival rates for rectal and colon cancer are reported to be 67% and 64%, respectively (Miller et al., 2022). During the diagnosis and treatment of CRC, patients exhibit certain physical and emotional symptoms, such as cognitive function alteration or impairment (El-Shami et al., 2015; Hess & Insel, 2007).
Cognitive impairment occurs within the central nervous system, and patients with non–central nervous system cancer have also reported experiencing cognitive impairment (Wefel et al., 2014). The influence of chemotherapy on cognitive function (e.g., specific drug, dosage) has been termed “chemotherapy-related cognitive impairment,” also known as “chemobrain” or “chemofog” (Dwek et al., 2015; Hermelink, 2015; Wefel et al., 2014; Winocur et al., 2018). Data from a systematic review reported that about 30%–40% of patients with cancer experienced cognitive impairment before treatment and 50%–75% had cognitive impairment during treatment (Janelsins et al., 2014). About 35% of patients reported cognitive impairment for months or even years after treatment (Janelsins et al., 2014). Cognitive impairment is not only a side effect of chemotherapy, but also can occur at any stage during the cancer trajectory (Cerulla Torrente et al., 2020; Mayo et al., 2021).
The International Cognition and Cancer Task Force (ICCTF) has recommended neuropsychological (NP) testing, also known as objective cognitive impairment (OCI) evaluations, as the gold standard for detecting cognitive impairment (Wefel et al., 2014). Attention, memory, information processing speed, and executive function were all measured by NP tests in the corresponding studies (Joly et al., 2015; Wefel et al., 2011). Subjective cognitive impairments (SCIs), or cognitive reports, were collected using multi-item self-report questionnaires (Lange et al., 2019; Richardson-Vejlgaard et al., 2009). In cohort studies of breast cancer, cognitive impairment was assessed by patient-reported outcome questionnaires or interviews, and the results showed that 46%–60% of survivors of breast cancer had SCI for an average of five to seven years postdiagnosis (Janelsins et al., 2017; Kim et al., 2022; Rodríguez Martín et al., 2020). However, the lack of acceptable measurements makes it difficult to standardize SCI assessments.
One systematic review on cognitive impairment presented a novel synthesis on patients with CRC postchemotherapy by using different cognitive assessment scales. The findings identified that OCI was less prevalent than SCI in patients with CRC (Chan et al., 2021). However, in a longitudinal cohort study of patients with CRC postchemotherapy, OCI was more commonly reported than SCI (Dhillon et al., 2018). This inconsistency may be related to the discrepancies in study design, scale selection, and cognitive impairment definitions (Chan et al., 2021). ICCTF has recommended larger samples to explore factors contributing to cognitive impairment and to apply interventions to improve cognitive function (Wefel et al., 2011). Therefore, researchers have a tendency to analyze the latent risk factors for cognitive impairment to understand the associations between OCI and SCI, and to investigate the effects of rehabilitation programs on cognitive function (Chan et al., 2021; Fernandes et al., 2019; Hwang et al., 2021; Oldacres et al., 2023; Országhová et al., 2021). For patients with CRC, it is useful to explore the factors that influence OCI and SCI across the CRC trajectory (Chan et al., 2021; Hwang et al., 2021).
To better understand the interrelationship of the factors contributing to OCI and SCI for patients with CRC, and to provide more evidence for future intervention, Green et al. (2005) proposed a model of factors contributing to cognitive impairment (MFCCI). As shown in Figure 1, four factors (cancer treatments, psychosocial factors, physical health, and emotional health) are interrelated to each other. These factors directly or indirectly contribute to cognitive impairment. There is a unique correlation among cancer treatments, psychosocial factors, and physical health. In this pattern, physical health putatively correlates with cancer treatments and psychosocial factors. Subsequently, these three correlated factors (cancer treatments, psychosocial factors, physical health) further influence emotional health. As proposed, cancer treatments, physical health, and emotional health are recognized as predictors of OCI, whereas emotional health and OCI are related to SCI (Green et al., 2005). In addition, the MFCCI suggests that the interventions for managing cognitive impairment may incorporate remediating the possible factors contributing to cognitive impairment and vulnerabilities in individuals (Green et al., 2005).
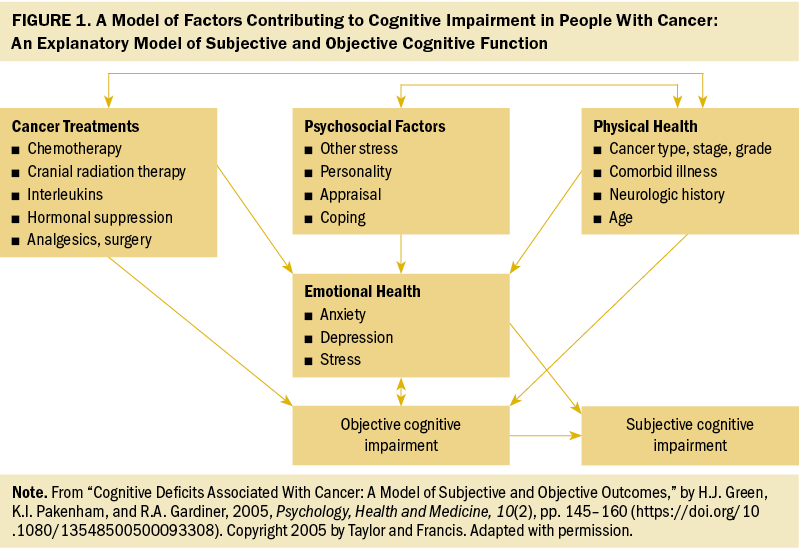
Based on ICCTF recommendations on OCI and SCI in patients with CRC, the present review aimed to (a) summarize the prevalence of cognitive impairment (including OCI and SCI) and analyze the factors influencing cognitive impairment based on the MFCCI, and (b) draw conclusions about the available interventions on cognitive impairment and identify directions for future interventions in CRC.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) tool was adopted in guiding this review (Page et al., 2021). PRISMA provides the updated reporting guidelines for systematic reviews. PRISMA checklists have the advantages of optimizing the quality of reports and increasing the efficiency of the peer review process (Page et al., 2021).
Search Strategy
A systematic search was conducted using six electronic databases (CINAHL Plus®, Cochrane Library, Embase®, PsycINFO®, PubMed®, and CNKI) for articles published between the time of the database’s establishment and May 2023. To ensure that the included studies reflected a variety of timelines, no date limits were placed on the search process. Search terms included colorectal or colon or rectal or colostomy AND cancer or tumor or neoplasm or carcinoma AND cognitive impairment or cognitive dysfunction or cognitive disorder or cognitive decline or cognitive complaint. Additional eligible articles from the literature’s references were manually searched. The detailed search strategy is presented online in Supplemental Table 1.
Inclusion and Exclusion Criteria
Inclusion criteria for the review were as follows: (a) patients diagnosed with CRC without central nervous system disorders, (b) studies focused on cognitive impairment in patients with CRC, and (c) research published in peer-reviewed journals in English or Chinese. Articles were excluded for the following reasons: (a) Studies involved drugs, anesthesia, or surgery modalities as control variables; (b) studies did not use a cognitive function assessment scale; (c) studies included mice models or clinic experiments; and (d) studies were reviews, degree dissertations, conference proceedings, case reports, editorials, or commentaries.
Quality Assessment
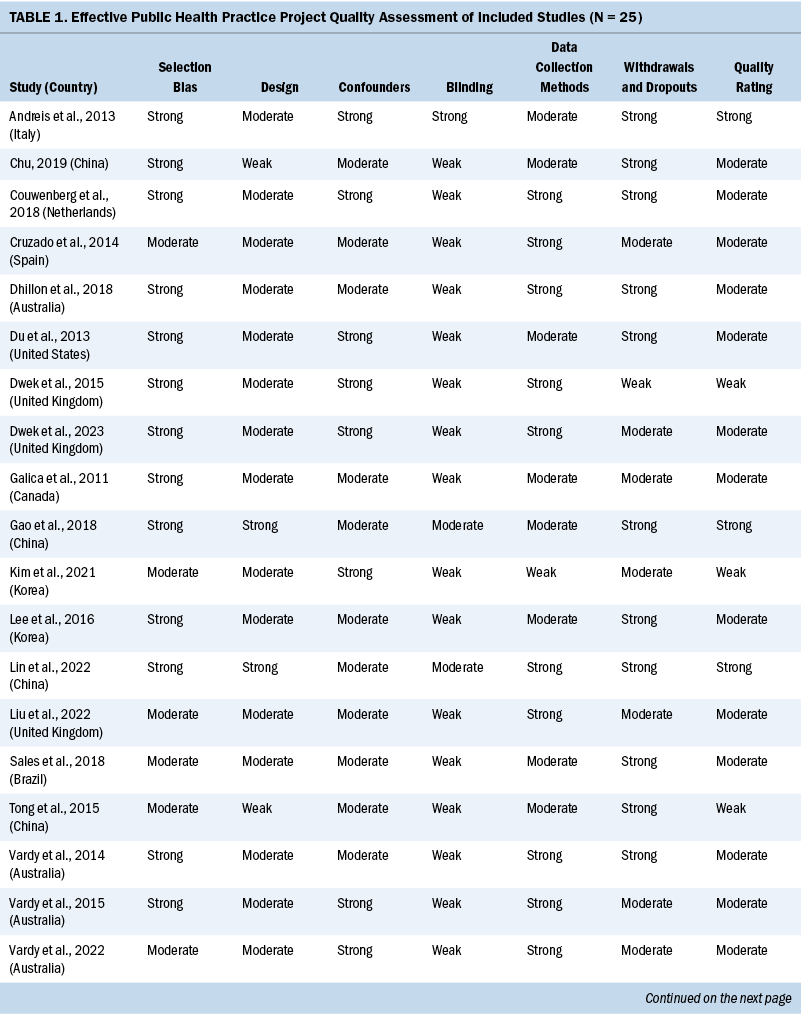
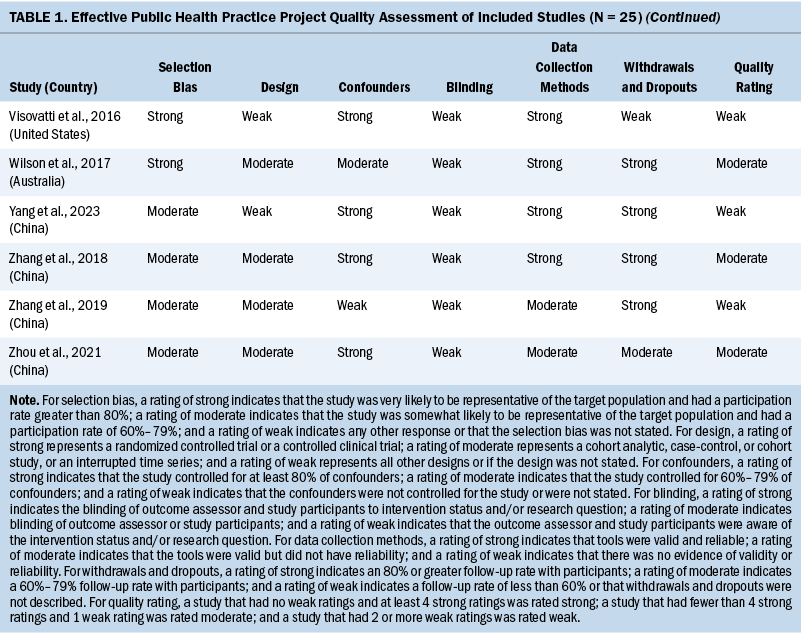
Two independent reviewers evaluated the methodologic quality of eligible studies using the Effective Public Health Practice Project quality assessment tool (Thomas et al., 2004). The checklist assessed the validity of each study as strong, moderate, or weak by analyzing selection bias, study design, confounders, data collection, withdrawals and dropouts, and blinding of outcome assessors and participants. The details of the quality evaluation are summarized in Table 1.
Data Extraction and Synthesis
Data extracted from the included studies contained the names of the authors, year of publication, country of study, study design, targeted populations, time points of assessment, measures of cognition outcome, prevalence, and results related to cognitive impairment. Two reviewers conducted the data extraction procedure and engaged in conversation to resolve disagreements. The details are provided online in Supplemental Table 2.
Results
Study Selection
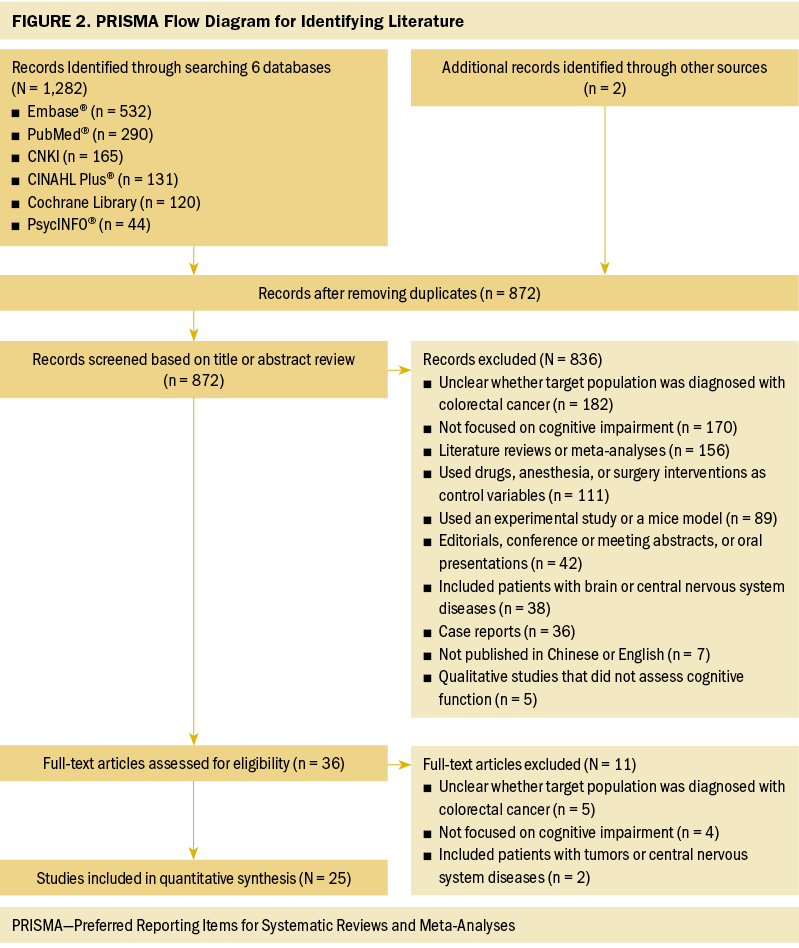
An initial search using the six databases recognized 1,282 articles. Among these articles, 412 duplicates were identified by EndNote X9 and were removed. The remaining 872 articles were screened for title and abstract. After preliminary screening, 36 articles were retrieved for full-text review. Ultimately, after eliminating 11 articles based on the exclusion criteria, 25 articles were included in this review. The selection and evaluation process is outlined in Figure 2.
Study Quality
Three studies were rated strong, 16 were rated moderate, and the remaining 6 were rated weak. The most common reason for studies receiving a moderate or weak rating was related to study design because the included observational studies usually did not use blinding. Considering the fact that the findings of the included observational studies met the review aims (e.g., summarizing the prevalence, attributing the factors of cognitive impairment in patients with CRC), the studies rated weak were not excluded.
Study Characteristics
The 25 eligible studies were published between 2011 and 2023 in 10 countries: China (n = 8), Australia (n = 5), the United Kingdom (n = 3), Korea (n = 2), the United States (n = 2), Brazil (n = 1), Canada (n = 1), Italy (n = 1), the Netherlands (n = 1), and Spain (n = 1). The following study designs were adapted: longitudinal (n = 9), cross-sectional (n = 6), cohort (n = 5), case-control (n = 3), and randomized controlled trial (n = 2). The randomized controlled trials were performed using postoperative psychological interventions or cognitive behavioral therapy (CBT) coupled with Baduanjin exercise (a traditional Chinese moderate intensity aerobic exercise based on body movement, respiration, and psychological adjustment). The sample sizes ranged from 25 to 95,303 participants, the majority of whom were male participants. However, not every study had more than 50% male participants, as six studies had less than 50% male participants (Andreis et al., 2013; Du et al., 2013; Dwek et al., 2015, 2023; Gao et al., 2018; Yang et al., 2023).
Prevalence of Cognitive Impairment and Association Between OCI and SCI
ICCTF has recommended the following study designs to measure cognitive impairment in patients with cancer: longitudinal studies with repeated assessments, cross-sectional studies with control groups, and randomized controlled trials with follow-up (Wefel et al., 2011). This review explores the prevalence of cognitive impairment based on the different study designs. Objective cognitive assessments visibly indicated the degree of cognitive decline. Subjective measures revealed mild or moderate cognitive impairment from the patients’ cognitive reports. Associations of cognitive impairment in OCI and SCI were also presented.
Prevalence of cognitive impairment based on study design: For nine longitudinal studies, researchers conducted repeated cognitive assessments to evaluate cognitive impairment in individuals with CRC (Andreis et al., 2013; Couwenberg et al., 2018; Cruzado et al., 2014; Dwek et al., 2015; Lee et al., 2016; Sales et al., 2019; Vardy et al., 2014, 2015, 2022). Most patients were evaluated before surgery or chemotherapy, during treatment, after chemotherapy or radiation therapy completion, and at follow-up periods. NP tests were used to measure OCI and to detect the cognitive impairment domains simultaneously. Four longitudinal studies explicated the prevalence of OCI in patients with CRC (ranging from 20% to 52% of the patient population) (Cruzado et al., 2014; Vardy et al., 2014, 2015, 2022). Six studies revealed the domains of cognitive impairment among patients undergoing adjuvant chemotherapy, mainly with attention, memory, and information processing speed being highly impaired at each assessment time point (p = 0.000) (Andreis et al., 2013; Cruzado et al., 2014; Sales et al., 2019; Vardy et al., 2014, 2015, 2022). The European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (Couwenberg et al., 2018; Lee et al., 2016; Vardy et al., 2022) and the Functional Assessment of Cancer Therapy–Cognitive Function (FACT-Cog) evaluation (Dwek et al., 2015; Vardy et al., 2014, 2015, 2022) were used to assess SCI. The rate of SCI in patients with CRC was reported in three articles and ranged from 20% to 32% (Couwenberg et al., 2018; Vardy et al., 2014, 2015).
In six cross-sectional studies, researchers estimated the cognitive impairment of patients by setting up a healthy control group or by grouping participants together (Chu, 2019; Galica et al., 2012; Liu et al., 2022; Tong et al., 2015; Visovatti et al., 2016; Yang et al., 2023). The results showed that 25% of patients with CRC experienced OCI (Galica et al., 2012). Participants within two years of receiving chemotherapy had a significant decrease in attention and processing speed compared to the group two years after completing chemotherapy (p = 0.017 and 0.045, respectively) (Yang et al., 2023). Participants showed poorer behaviors in attention and cognitive control as compared to long-term memory (Tong et al., 2015). Attention impairment also appeared in individuals with rectal cancer undergoing postoperative chemotherapy (Visovatti et al., 2016). One study used the FACT-Cog to assess and compare SCI before and after chemotherapy. The results indicated that before chemotherapy, the cognitive impairment of patients was 66%, which increased to 90% after chemotherapy (p < 0.05) (Chu, 2019).
For five cohort studies, researchers used multiple cognitive assessments to accurately capture cognitive impairment in patients at different treatment stages (surgery, neoadjuvant or adjuvant chemotherapy, radiation therapy) (Dhillon et al., 2018; Du et al., 2013; Dwek et al., 2023; Kim et al., 2021; Zhang et al., 2019). Chemotherapy had a negative impact on the cognition of patients in two large-sample cohort studies (hazard ratio = 0.88, 95% confidence interval [0.75, 1.04]) (Du et al., 2013; Kim et al., 2021). Three studies used NP tests to assess OCI, and the corresponding prevalence of OCI ranged from 24% to 55% (Dhillon et al., 2018; Dwek et al., 2023; Zhang et al., 2019).
For three case-control studies, through retrospective analysis, survivors of CRC experienced mild cognitive decline after treatments (Wilson et al., 2018), and patients with cognitive impairment had lower assessment scores (as compared with the non–cognitive impairment group) (p < 0.05) (Zhang et al., 2018; Zhou et al., 2021).
In this review, the prevalence of cognitive impairment varied according to study designs. In summary, OCI was commonly measured by NP tests, with prevalence ranging from 20% to 55%, and attention, memory, and processing speed were the most susceptible cognitive domains. Patients reported cognitive decline, and the prevalence of SCI ranged from 20% to 90%, as measured using different self-report scales.
Association Between OCI and SCI
For the association between OCI and SCI, some findings showed that there was a weak association between the NP test outcomes and the SCI reports (Dhillon et al., 2018; Vardy et al., 2014, 2015). For the poor attention domain, the results of NP tests showed that patients with CRC had poorer attention and cognitive control. However, the self-reported memory in patients was irrelevant (Visovatti et al., 2016). When it came to the special cognitive domain in NP tests, there was a moderate association between OCI and SCI (Yang et al., 2023). In summary, the association between OCI and SCI (including the total scale and its domains) was inconclusive. These inconsistent findings (irrelevant, weak, or moderate associations between OCI and SCI) suggest that there is a need to develop a new procedure to assess OCI and SCI to fully evaluate cognitive impairment.
Factors Influencing Cognitive Impairment in Patients With CRC
The MFCCI proposed four factors contributing to cognitive impairment: cancer treatments, psychosocial factors, physical health, and emotional health (Green et al., 2005). The factors that influence cognitive impairment were summarized based on the MFCCI and on OCI and SCI in individuals with CRC.
Cancer treatments: It was demonstrated that cancer treatments could increase the possibility of cognitive deterioration and, therefore, could be associated with cognitive impairment (Green et al., 2005). In this review, cancer treatments were specified as chemotherapy or surgery.
Chemotherapy: Four studies found no sufficient evidence to support the existence of an association between cognitive impairment and chemotherapy (Andreis et al., 2013; Du et al., 2013; Galica et al., 2012; Kim et al., 2021). On the contrary, other studies suggested that chemotherapy is associated with cognitive impairment. In one study, chemotherapy increased the risk of cognitive impairment (Chu, 2019). Chemotherapy with a specific regimen was associated with cognitive impairment in patients with colon cancer, particularly in terms of verbal memory (Cruzado et al., 2014). Two studies found that cognitive function was vulnerable to the neurotoxic side effects of chemotherapy drugs and was impaired postchemotherapy (p = 0.041) (Lee et al., 2016; Liu et al., 2022). Other studies found that cognitive impairment showed no tendency to improve over time in patients undergoing chemotherapy and no trend of improvement for 12–24 months of follow-up (Dwek et al., 2015, 2023; Liu et al., 2022; Sales et al., 2019; Vardy et al., 2015). Compared with a nonchemotherapy group, patients who received chemotherapy exhibited higher rates of deterioration in cognitive impairment (Dhillon et al., 2018; Liu et al., 2022; Tong et al., 2015).
Surgery: Three studies stated that individuals with CRC who received surgery could have an increased risk of cognitive impairment (Dwek et al., 2015; Zhang et al., 2019; Zhou et al., 2021). Without psychological intervention, the postoperative patients tended to experience mild cognitive impairment (Gao et al., 2018).
Although inconsistent findings regarding the association between chemotherapy and cognitive impairment were revealed in the current review, some studies reported that chemotherapy is associated with cognitive impairment. Cancer treatments appear to have a persisting effect on cognitive function because there was no discernible trend toward the improvement of cognitive impairment during the post-treatment or follow-up period. This suggests cognitive impairment should be monitored during and after cancer treatments.
Psychosocial factors: Psychosocial factors correlated with cognitive impairment in patients with cancer based on the MFCCI (Green et al., 2005). Because of individual personality and assessment differences, patients experienced variable aspects of psychosocial coping, including fatigue, changes in quality of life (QOL), and cognitive self-efficacy.
Fatigue is defined as sustained subjective sensory exhaustion or cognitive fatigue, which is associated with cancer (Berger et al., 2010). After a 12-month follow-up, compared to the general population, patients with cognitive impairment had a considerably higher incidence of fatigue (Couwenberg et al., 2018). When individuals with CRC experienced fatigue, their cognitive impairment worsened (Tong et al., 2015). However, one of the studies (Visovatti et al., 2016) reported that fatigue was not a solid predictor of OCI. In addition, it was suggested that NP tests were not as sensitive to the patients’ impairments (e.g., attentional capacity, cognitive control), although patients with SCI reported fatigue (Visovatti et al., 2016).
QOL is described as a subjective awareness of the functional state-of-life domains (mental, physical, social, and spiritual well-being) (Vearncombe & Pachana, 2009). QOL worsened and was accompanied by SCI in patients with CRC who had received six cycles of chemotherapy (Lee et al., 2016). Six months after rectal cancer treatments, QOL decreased significantly, as did cognitive impairment (Couwenberg et al., 2018). In a 24-month follow-up, QOL and cognitive impairment scores remained lower for patients with CRC than scores in the general population (Couwenberg et al., 2018).
Cognitive self-efficacy is a measure of the confidence or perception of the efficiency of cognitive function (Cherry et al., 2019). One study revealed that the incidence of cognitive impairment was negatively correlated with self-efficacy levels (Chu, 2019).
In summary, there is an interaction between psychosocial factors and cognitive impairment. Poorer psychosocial scores lead to more severe cognitive impairment in patients with CRC. Therefore, monitoring cognitive function requires identifying subtle changes in psychosocial status.
Physical health: According to the MFCCI, physical health includes cancer stage, comorbid illness, age, and gender, and is closely related to cognitive impairment. Physical health in the included studies presented inconsistent findings. However, there was evidence to support that physical health (e.g., later disease stage, presence of a comorbid illness, older age, female gender) contributed to the severity of cognitive impairment.
Cancer stage: The degree of cognitive impairment was similar at different cancer stages (Du et al., 2013). A predictive model suggested that the later clinical stages (stages III and IV) might be a risk indicator for cognitive impairment postchemotherapy in patients with CRC (Zhou et al., 2021). In this case, SCI could be used to reveal the effects of cancer stage. For patients with stage III cancer, NP test scores were significantly worse than for those with stage I or II cancer (Yang et al., 2023).
Comorbid illness: One study found that if comorbidity scores are higher, the incidence of cognitive impairment will be higher as well (Du et al., 2013). Three studies identified that the comorbidity of diabetes history was a hazard factor for cognitive impairment in older adult patients after surgery or chemotherapy (Zhang et al., 2018, 2019; Zhou et al., 2021).
Age: Cognitive impairment did not change significantly and there was no evidence of a correlation between accelerated aging and cognitive impairment (as many as 6–12 years postdiagnosis of CRC) (Vardy et al., 2022). However, two studies suggested that older age and a lower education level could increase the risk of cognitive impairment (Visovatti et al., 2016; Zhou et al., 2021). Adverse cognitive consequences of chemotherapy were pronounced in older adult patients with CRC (Kim et al., 2021).
Gender: At the early stage of CRC, women presented with higher levels of cognitive impairment than men or those in the control group (Vardy et al., 2014, 2015). However, one study showed that women have a slightly lower risk of cognitive impairment than men (Du et al., 2013).
Emotional health: According to the MFCCI, emotional health is associated with SCI, which was demonstrated by the cognitive symptoms (anxiety and depression) reported in the reviewed studies. One survey found that more than half of the patients had anxiety or depression, and the frequency of comorbid anxiety and depression reached 40%, indicating that anxiety and depression were widespread in patients with CRC undergoing chemotherapy (Tong et al., 2015). A case-control research study found that low SCI was associated with increased depression, as measured by the FACT-Cog (Wilson et al., 2018). In addition, a longitudinal study of patients with colon cancer demonstrated a trivial association between emotional factors and OCI (Cruzado et al., 2014). However, the correlation between emotional status and SCI could not be ruled out completely. Another study revealed no connection between general cognitive function and anxiety or depression (Vardy et al., 2015).
Although some findings indicate that emotions influence SCI, there are conflicting conclusions for the relationship between emotional health and cognitive impairment. Therefore, the current review was unable to confirm the connection between emotional health and cognitive impairment.
Interventions for Cognitive Impairment in Patients With CRC
According to the MFCCI, individual vulnerabilities can potentially cause cognitive impairment in patients. Interventions improve cognitive impairment through remedial affecting elements such as cancer treatments, psychosocial factors, physical health, and emotional health (Green et al., 2005).
This review includes two interventions that targeted patients with CRC (Gao et al., 2018; Lin et al., 2022). Gao et al. (2018) performed standard preoperative visits, postoperative follow-up interviews, and concurrent psychological support (six times) for the psychological intervention group (N = 15). The intervention focused on psychotherapy and family support for patients undergoing surgery, such as adjusting patients’ attitude through emotional therapy, clarifying the features of the operation, listening to soothing music, and talking with caregivers and family for support. After the intervention, there was a marked decline in the prevalence of cognitive impairment in the older adult patients. However, perioperative psychological interventions had effects on the patients’ anxiety. Multiple psychological therapies helped patients have a better understanding of CRC and, therefore, reduced the effects of negative emotions toward disease outcomes. This perioperative psychological intervention effectively alleviated postoperative anxiety and reduced the effects of psychological stress on cognitive impairment compared with the control group (p < 0.05) (Gao et al., 2018).
Another study found that CBT combined with Baduanjin exercise significantly improved cognitive impairment in individuals with CRC (Lin et al., 2022). CBT focused on psychological factors (e.g., fatigue, QOL) and emotional states (e.g., anxiety, depression) for implementing positive behavior strategies. Health professionals (psychotherapists, doctors, and exercise coaches) directed the experimental group (N = 30) to regulate and master Baduanjin exercise to minimize cognitive impairment. The experimental group’s improvement in cognitive impairment was substantially greater than that in the control group after the three and six courses (total FACT-Cog score: p = 0.002, scores of corrected cognitive impairments: p < 0.001). The postintervention fatigue scores in the experimental group were significantly lower than those in the control group (p < 0.001). QOL tended to improve and minimize cognitive impairment in the intervention group. The emotional status of the patients was effectively relieved after the intervention. Together, CBT and Baduanjin exercise stimulate a healthy boost in patients with CRC (Lin et al., 2022).
Discussion
This review described the prevalence and associations of cognitive impairment in patients with CRC, investigated the influencing factors via the MFCCI, and explored the effects of interventions on cognitive impairment in patients with CRC.
Prevalence of and Association Between OCI and SCI in Patients With CRC
The prevalence of cognitive impairment varies according to criteria and definitions (Vardy et al., 2014). This review showed that the prevalence of cognitive impairment varied across the study designs, ranging from 20% to 90% (20%–55% for OCI, and 20%–90% for SCI). To some extent, the prevalence of cognitive impairment in patients with CRC was higher than in patients with breast cancer, as reported in a meta-analysis of participants with breast cancer (Whittaker et al., 2022). The total rate of cognitive impairment ranged from 21% to 34% (Whittaker et al., 2022). This discrepancy could be attributed to sample size, control population selection, study design, assessment, and analysis approaches (Lange et al., 2019; Wefel et al., 2011). Future studies should explore the prevalence of cognitive impairment in populations with different cancer types and study designs, and using different assessments and analysis approaches.
The association between OCI and SCI (including the total scale and its domains) in this review was inconclusive, and varied from irrelevant to weak or moderate. This is inconsistent with another finding in which cognitive impairment measured by NP tests had a moderate to high correlation between OCI and SCI (Harder et al., 2002). These inconsistent findings suggest that there is a need to assess OCI and SCI to fully evaluate cognitive impairment.
For assessing SCI, researchers enrolled survivors of CRC and their spouses as participants, interviewed their experiences of cognitive impairment before and after treatment, and validated the survivors’ recollections via their spouses. The findings revealed that SCI in survivors was potentially influenced by biased recollection but was validated independently by their spouses (Wilson et al., 2018). Although there were no significant scores on objective measurements, the spousal caregivers spotted the changes in cognitive impairment for their spouse with cancer (Lin et al., 2022). In addition, a review for survivors of cancer discussed the link between the characteristics of caregivers and the cognitive performance of patients. Caregivers with positive coping styles affected the SCI of the patient under their care, and the corresponding patient reported less regarding their ability to pay attention and their memory loss (Yang et al., 2020). More than half of the family caregivers noticed the cognitive changes in patients’ memory after therapy completion (Hutchinson et al., 2012). Therefore, future research should focus on the performance on SCI among patients with CRC, and on caregivers’ perceptions of patients’ cognitive impairment.
Factors Influencing Cognitive Impairment in Patients With CRC
Four factors contributed to cognitive impairment directly or indirectly. Cancer treatments affected cognitive impairment directly and persistently, as revealed in this review. Consistently, patients’ physical conditions also influenced cognitive impairment. For patients with CRC, their physical health (e.g., cancer stage, comorbid illness, age, gender), could determine their treatment regimen. For health professionals, it is necessary to conduct CRC knowledge sessions and education after patients are diagnosed with CRC (King & Green, 2015). As CRC treatments advance, it is vital to conduct repeated, long-term, and comprehensive assessments of cognitive function to detect and capture the impairments in the cognitive domain as well as self-reported SCI (Couwenberg et al., 2018; Vardy et al., 2022).
For psychosocial factors, negative coping toward a diagnosis of CRC is harmful for patients’ emotional state and leads to SCI (Tong et al., 2015, Vardy et al., 2022), as shown in this review. However, few memory problems were observed when patients with cancer used acceptance coping (Yang et al., 2020). In addition, few patients self-reported cognitive impairment when their caregiver actively and resiliently coped with cancer-related stress (Yang et al., 2020). Therefore, future studies should encourage patients to cope positively. The patients’ psychosocial variables should be incorporated into the development of new cognitive intervention programs.
According to this review, there was no conclusive evidence of emotional health affecting cognitive impairment, although emotions (anxiety and depression) influenced SCI noticeably in patients with CRC (Tong et al., 2015). In other cancer types, negative emotions affected cognitive function and patients tended to report SCI (e.g., lack of concentration, loss of perceptual memory, slow brain response) (Player et al., 2014). In addition, emotional health could be affected by psychosocial factors. Individuals with high psychosocial distress (e.g., fatigue, low QOL, negative coping) experienced somber moods such as deep depression, which in turn led to poor SCI (including forgetfulness, shortened response time, and memory loss) (Vega et al., 2022). This is evidence that nursing providers should assess, manage, and intervene in cognitive impairment based on patients’ vulnerabilities and psychosocial factors.
Interventions on Cognitive Impairment for Patients With CRC
With developments in cancer therapies (surgery and chemotherapy), it is vital to focus on the improvement of psychological factors in patients and to relieve psychological stress and minimize cognitive impairment and anxiety through postoperative psychological interventions. Because trivial cognitive impairment was not easily recognized by the patients who practiced Baduanjin exercise and their family members tended to concentrate on the recovery from disease, cognitive impairment was easily overlooked (Lin et al., 2022).
One review revealed the associations between the cognitive function of the patients and the features of the caregivers. Seven categories were identified for the caregivers’ characteristics, which provides additional guidelines on formulating interventions for integrating the influences of caregivers (Yang et al., 2020). The characteristic of caregivers mediated the relationship between the emotional status of patients and OCI (memory domain) in patients with breast cancer (Yang et al., 2019). Future studies should provide deep insight into the role of caregivers on SCI in patients with CRC. Therefore, the development of interventions targeting factors of cognitive impairment and focusing on the patient–caregiver relationship is expected to improve cognitive impairment in patients.
Limitations
This review has certain limitations. First, to discover cognitive impairment in patients, ICCTF suggests repeating cognitive tests or establishing control groups; however, there is no standardized practice for measuring SCI. Second, the qualified articles have language limitations (e.g., only Chinese or English) and may lack representation. Third, this review did not find a consistent association between OCI and SCI. This is largely because of a lack of standard procedures for the measurement of SCI.
Implications for Nursing
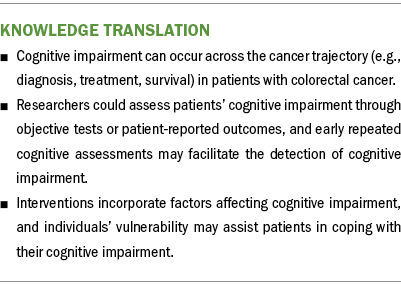
Once patients are diagnosed with CRC, they may experience psychosocial maladjustment or cognitive impairment. The findings of this review suggest that in caring for patients with CRC, healthcare professionals (particularly nursing providers) should pay special attention to the following three aspects: (a) obtaining reports of cognitive impairment, (b) monitoring cognitive impairment across the cancer care continuum, and (c) integrating supportive interventions to improve QOL and cognitive impairment in patients with CRC. It is important to understand the cognitive perception experienced by individuals with CRC. To do so, thoughts or opinions of caregivers (e.g., family, spousal) about the patient’s SCI and the patient’s self-reported SCI should be integrated. Cognitive impairment should be monitored across the cancer trajectory (e.g., diagnosis, treatment, survival), particularly when patients with CRC are undergoing treatments. In addition, supportive interventions and strategies should be provided to improve the daily function, psychosocial health, and emotional health of patients.
Conclusion
This review summarizes cognitive impairment in patients with CRC who are undergoing treatment or are post-treatment. Because there are no confirmed consistent associations between OCI and SCI, NP tests can effectively detect only OCI. Therefore, more attention needs to be paid to the determinations of SCI. Based on the MFCCI, cognitive impairment is under the influence of cancer treatments, psychological factors, physical health, and emotional health. Although incorporating intervention programs has been demonstrated to have a positive improvement on cognitive impairment, specific interventions for SCI in patients with CRC are rare. In addition, there is a lack of engagement from CRC caregivers in dyadic coping on patients’ SCI. Future studies should combine CRC cognitive reports and individual vulnerabilities, incorporate influencing factors, and focus on interventions to ameliorate cognitive impairment in patients with CRC.
About the Authors
Ye Wang, BN, Zhiming Wang, BN, Rongyu Li, BN, Zheng Sun, BN, and Yi Zhang, BN, are all students in the Wuxi School of Medicine at Jiangnan University in Jiangsu, China, and Qiuping Li, PhD, FAAN, is a professor at the Wuxi School of Medicine at Jiangnan University and at Affiliated Hospital of Jiangnan University, Wuxi, both in Jiangsu, China. This research was funded, in part, by the National Natural Science Foundation of China (No. 82172844). Y. Wang, Z. Wang, R. Li, and Zhang contributed to the conceptualization and design. Sun completed the data collection. Y. Wang and Zhang provided the analysis. Y. Wang, Sun, and Q. Li contributed to the manuscript preparation. Q. Li can be reached at liqp@163.com, with copy to ONFEditor@ons.org. (Submitted September 2023. Accepted January 13, 2024.)
References
Andreis, F., Ferri, M., Mazzocchi, M., Meriggi, F., Rizzi, A., Rota, L., . . . Zaniboni, A. (2013). Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients. A pilot study. Supportive Care in Cancer, 21(2), 583–590.
Berger, A.M., Pickar Abernethy, A., Atkinson, A., Barsevick, A.M., Breitbart, W.S., Cella, D., . . . Wagner, L.I. (2010). Cancer-related fatigue: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 8(8), 904–931. https://doi.org/10.6004/jnccn.2010.0067
Cerulla Torrente, N., Navarro Pastor, J.-B., & de la Osa Chaparro, N. (2020). Systematic review of cognitive sequelae of non-central nervous system cancer and cancer therapy. Journal of Cancer Survivorship, 14(4), 464–482. https://doi.org/10.1007/s11764-020-00870-2
Chan, Y.-N., Leak Bryant, A., Conklin, J.L., Girdwood, T., Piepmeier, A., & Hirschey, R. (2021). Systematic review of cognitive impairment in colorectal cancer survivors who received chemotherapy. Oncology Nursing Forum, 48(6), 634–647. https://doi.org/10.1188/21.ONF.634-647
Cherry, K.E., Lyon, B.A., Boudreaux, E.O., Blanchard, A.B., Hicks, J.L., Elliott, E.M., . . . Jazwinski, S.M. (2019). Memory self-efficacy and beliefs about memory and aging in oldest-old adults in the Louisiana healthy aging study (LHAS). Experimental Aging Research, 45(1), 28–40.
Chu, J. (2019). Chemotherapy-related cognitive obstacle of colorectal cancer patients and their self-efficacy: A relativity analysis. Chinese Journal of Coloproctology, 39(11), 16–18. https://bit.ly/4aOgiUA
Couwenberg, A.M., Burbach, J.P.M., van Grevenstein, W.M.U., Smits, A.B., Consten, E.C.J., Schiphorst, A.H.W., . . . Verkooijen, H.M. (2018). Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clinical Colorectal Cancer, 17(3), e499–e512. https://doi.org/10.1016/j.clcc.2018.03.009
Cruzado, J.A., López-Santiago, S., Martínez-Marín, V., José-Moreno, G., Custodio, A.B., & Feliu, J. (2014). Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Supportive Care in Cancer, 22(7), 1815–1823. https://doi.org/10.1007/s00520-014-2147-x
Dhillon, H.M., Tannock, I.F., Pond, G.R., Renton, C., Rourke, S.B., & Vardy, J.L. (2018). Perceived cognitive impairment in people with colorectal cancer who do and do not receive chemotherapy. Journal of Cancer Survivorship, 12(2), 178–185. https://doi.org/10.1007/s11764-017-0656-6
Du, X.L., Cai, Y., & Symanski, E. (2013). Association between chemotherapy and cognitive impairments in a large cohort of patients with colorectal cancer. International Journal of Oncology, 42(6), 2123–2133. https://doi.org/10.3892/ijo.2013.1882
Dwek, M.-R., Newman, S.P., Brini, S., Holder, P., Machesney, M., Propper, D., . . . Hurt, C.S. (2023). The impact of chemotherapy on cognitive performance post-surgery in patients with colorectal cancer: A prospective cohort study. Psycho-Oncology, 32(7) 1057–1066. https://doi.org/10.1002/pon.6147
Dwek, M.-R., Rixon, L., Simon, A., Hurt, C., & Newman, S. (2015). Examining the effects of adjuvant chemotherapy on cognition and the impact of any cognitive impairment on quality of life in colorectal cancer patients: Study protocol. BMC Psychology, 3, 43. https://doi.org/10.1186/s40359-015-0100-5
El-Shami, K., Oeffinger, K.C., Erb, N.L., Willis, A., Bretsch, J.K., Pratt-Chapman, M.L., . . . Cowens-Alvarado, R.L. (2015). American Cancer Society colorectal cancer survivorship care guidelines. CA: A Cancer Journal for Clinicians, 65(6), 428–455.
Fernandes, H.A., Richard, N.M., & Edelstein, K. (2019). Cognitive rehabilitation for cancer-related cognitive dysfunction: A systematic review. Supportive Care in Cancer, 27(9), 3253–3279. https://doi.org/10.1007/s00520-019-04866-2
Galica, J., Rajacich, D., Kane, D., & Pond, G.R. (2012). The impact of chemotherapy-induced cognitive impairment on the psychosocial adjustment of patients with nonmetastatic colorectal cancer. Clinical Journal of Oncology Nursing, 16(2), 163–169. https://doi.org/10.1188/12.CJON.163-169
Gao, X., Wang, S., Dai, Z., Gao, Y., Zhang, Y., Yu, H., . . . Xing, W. (2018). Effect of psychological intervention on postoperative cognitive function and anxiety in elderly patients with colorectal cancer. China Journal of Modern Medicine, 28(28), 95–101. https://doi.org/10.3969/j.issn.1005-8982.2018.28.017
Green, H.J., Pakenham, K.I., & Gardiner, R.A. (2005). Cognitive deficits associated with cancer: A model of subjective and objective outcomes. Psychology, Health and Medicine, 10(2), 145–160. https://doi.org/10.1080/13548500500093308
Harder, H., Cornelissen, J.J., Van Gool, A.R., Duivenvoorden, H.J., Eijkenboom, W.M.H., & van den Bent, M.J. (2002). Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer, 95(1), 183–192.
Hermelink, K. (2015). Chemotherapy and cognitive function in breast cancer patients: The so-called chemo brain. JNCI Monographs, 2015(51), 67–69. https://doi.org/10.1093/jncimonographs/lgv009
Hess, L.M., & Insel, K.C. (2007). Chemotherapy-related change in cognitive function: A conceptual model. Oncology Nursing Forum, 34(5), 981–994. https://doi.org/10.1188/07.ONF.981-994
Hutchinson, A.D., Hosking, J.R., Kichenadasse, G., Mattiske, J.K., & Wilson, C. (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews, 38(7), 926–934. https://doi.org/10.1016/j.ctrv.2012.05.002
Hwang, S.Y., Kim, K., Ha, B., Lee, D., Kim, S., Ryu, S., . . . Jung, S.J. (2021). Neurocognitive effects of chemotherapy for colorectal cancer: A systematic review and a meta-analysis of 11 studies. Cancer Research and Treatment, 53(4), 1134–1147. https://doi.org/10.4143/crt.2020.1191
Janelsins, M.C., Heckler, C.E., Peppone, L.J., Kamen, C., Mustian, K.M., Mohile, S.G., . . . Morrow, G.R. (2017). Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. Journal of Clinical Oncology, 35(5), 506–514. https://doi.org/10.1200/jco.2016.68.5826
Janelsins, M.C., Kesler, S.R., Ahles, T.A., & Morrow, G.R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. https://doi.org/10.3109/09540261.2013.864260
Joly, F., Giffard, B., Rigal, O., De Ruiter, M.B., Small, B.J., Dubois, M., . . . Castel, H. (2015). Impact of cancer and its treatments on cognitive function: Advances in research from the Paris International Cognition and Cancer Task Force symposium and update since 2012. Journal of Pain and Symptom Management, 50(6), 830–841. https://doi.org/10.1016/j.jpainsymman.2015.06.019
Kim, H.-J., Jung, S.-O., Kim, E., & Abraham, I. (2022). Association of chemotherapy and subjective cognitive impairment in breast cancer patients: Meta-analysis of longitudinal prospective cohort studies. European Journal of Oncology Nursing, 57, 102099. https://doi.org/10.1016/j.ejon.2022.102099
Kim, K., Kim, C.W., Shin, A., Kang, H., & Jung, S.J. (2021). Effect of chemotherapy and radiotherapy on cognitive impairment in colorectal cancer: Evidence from Korean national health insurance database cohort. Epidemiology and Health, 43, e2021093.
King, S., & Green, H.J. (2015). Psychological intervention for improving cognitive function in cancer survivors: A literature review and randomized controlled trial. Frontiers in Oncology, 5, 72. https://doi.org/10.3389/fonc.2015.00072
Lange, M., Joly, F., Vardy, J., Ahles, T., Dubois, M., Tron, L., . . . Castel, H. (2019). Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Annals of Oncology, 30(12), 1925–1940. https://doi.org/10.1093/annonc/mdz410
Lee, S.H., Lee, T.-G., Baek, M.J., Kim, J.J., Park, S.-S., & Lee, S.-J. (2016). Quality of life changes during adjuvant chemotherapy in patients with colon cancer. Korean Journal of Clinical Oncology, 12(1), 60–66. https://doi.org/10.14216/kjco.16010
Li, N., Lu, B., Luo, C., Cai, J., Lu, M., Zhang, Y., . . . Dai, M. (2021). Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Letters, 522, 255–268. https://doi.org/10.1016/j.canlet.2021.09.034
Lin, Z.-G., Li, R.-D., Ai, F.-L., Li, S., & Zhang, X.-A. (2022). Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer. World Journal of Gastrointestinal Oncology, 14(1), 319–333. https://doi.org/10.4251/wjgo.v14.i1.319
Liu, S., Guo, Y., Ni, J., Yin, N., Li, C., Pan, X., . . . Li, X. (2022). Chemotherapy-induced functional brain abnormality in colorectal cancer patients: A resting-state functional magnetic resonance imaging study. Frontiers in Oncology, 12, 900855.
Mayo, S.J., Lustberg, M., Dhillon, H.M., Nakamura, Z.M., Allen, D.H., Von Ah, D., . . . Peters, K.B. (2021). Cancer-related cognitive impairment in patients with non-central nervous system malignancies: An overview for oncology providers from the MASCC neurological complications study group. Supportive Care in Cancer, 29(6), 2821–2840. https://doi.org/10.1007/s00520-020-05860-9
Miller, K.D., Nogueira, L., Devasia, T., Mariotto, A.B., Yabroff, K.R., Jemal, A., . . . Siegel, R.L. (2022). Cancer treatment and survivorship statistics, 2022. CA: A Cancer Journal for Clinicians, 72(5), 409–436. https://doi.org/10.3322/caac.21731
Oldacres, L., Hegarty, J., O’Regan, P., Murphy-Coakley, N.M., & Saab, M.M. (2023). Interventions promoting cognitive function in patients experiencing cancer related cognitive impairment: A systematic review. Psycho-Oncology, 32(2), 214–228. https://doi.org/10.1002/pon.6073
Országhová, Z., Mego, M., & Chovanec, M. (2021). Long-term cognitive dysfunction in cancer survivors. Frontiers in Molecular Biosciences, 8, 770413.
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., . . . Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Player, L., Mackenzie, L., Willis, K., & Loh, S.Y. (2014). Women’s experiences of cognitive changes or ‘chemobrain’ following treatment for breast cancer: A role for occupational therapy? Australian Occupational Therapy Journal, 61(4), 230–240.
Richardson-Vejlgaard, R., Dawes, S., Heaton, R.K., & Bell, M.D. (2009). Validity of cognitive complaints in substance-abusing patients and non-clinical controls: The Patient’s Assessment of Own Functioning Inventory (PAOFI). Psychiatry Research, 169(1), 70–74. https://doi.org/10.1016/j.psychres.2008.06.018
Rodríguez Martín, B., Fernández Rodríguez, E.J., Rihuete Galve, M.I., & Cruz Hernández, J.J. (2020). Study of chemotherapy-induced cognitive impairment in women with breast cancer. International Journal of Environmental Research and Public Health, 17(23), 8896. https://doi.org/10.3390/ijerph17238896
Sales, M.V.C., Suemoto, C.K., Apolinario, D., Serrao, V.T., Andrade, C.S., Conceição, D.M., . . . Riechelmann, R.P. (2019). Effects of adjuvant chemotherapy on cognitive function of patients with early-stage colorectal cancer. Clinical Colorectal Cancer, 18(1), 19–27. https://doi.org/10.1016/j.clcc.2018.09.002
Siegel, R.L., Miller, K.D., Wagle, N.S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
Thomas, B.H., Ciliska, D., Dobbins, M., & Micucci, S. (2004). A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews on Evidence-Based Nursing, 1(3), 176–184.
Tong, T., Lan, G., & Tang, X. (2015). A study of cognitive function and its influencing factors in patients with colorectal cancer after chemotherapy. Journal of Psychiatry, 28(06), 429–432.
Vardy, J., Dhillon, H.M., Pond, G.R., Rourke, S.B., Xu, W., Dodd, A., . . . Tannock, I.F. (2014). Cognitive function and fatigue after diagnosis of colorectal cancer. Annals of Oncology, 25(12), 2404–2412. https://doi.org/10.1093/annonc/mdu448
Vardy, J.L., Dhillon, H.M., Pond, G.R., Rourke, S.B., Bekele, T., Renton, C., . . . Tannock, I.F. (2015). Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. Journal of Clinical Oncology, 33(34), 4085–4092. https://doi.org/10.1200/jco.2015.63.0905
Vardy, J.L., Pond, G.R., Cysique, L.A., Gates, T.M., Lagopoulos, J., Renton, C., . . . Dhillon, H.M. (2022). Lack of cognitive impairment in long-term survivors of colorectal cancer. Supportive Care in Cancer, 30(7), 6123–6133.
Vearncombe, K.J., & Pachana, N.A. (2009). Impact of health, treatment and psychological factors on cognitive functioning after chemotherapy for early breast cancer. Australian Psychologist, 44(4), 235–247. https://doi.org/10.1080/00050060903096652
Vega, J.N., Albert, K.M., Mayer, I.A., Taylor, W.D., & Newhouse, P.A. (2022). Subjective cognition and mood in persistent chemotherapy-related cognitive impairment. Journal of Cancer Survivorship, 16(3), 614–623.
Visovatti, M.A., Reuter-Lorenz, P.A., Chang, A.E., Northouse, L., & Cimprich, B. (2016). Assessment of cognitive impairment and complaints in individuals with colorectal cancer. Oncology Nursing Forum, 43(2), 169–178. https://doi.org/10.1188/16.ONF.43-02AP
Wefel, J.S., Kesler, S.R., Noll, K.R., & Schagen, S.B. (2014). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians, 65(2), 123–138.
Wefel, J.S., Vardy, J., Ahles, T., & Schagen, S.B. (2011). International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology, 12(7), 703–708.
Whittaker, A.L., George, R.P., & O’Malley, L. (2022). Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Scientific Reports, 12(1), 2135. https://doi.org/10.1038/s41598-022-05682-1
Wilson, C., Giles, K., Nettelbeck, T., & Hutchinson, A. (2018). Locus of control, optimism, and recollections of depression and self-reported cognitive functioning following treatment for colorectal cancer. Psycho-Oncology, 27(2), 676–682. https://doi.org/10.1002/pon.4567
Winocur, G., Johnston, I., & Castel, H. (2018). Chemotherapy and cognition: International Cognition and Cancer Task Force recommendations for harmonising preclinical research. Cancer Treatment Reviews, 69, 72–83. https://doi.org/10.1016/j.ctrv.2018.05.017
Yang, H.-Y., Chang, Y.-L., Lin, B.-R., Chou, Y.-J., & Shun, S.-C. (2023). Cognitive function in patients at different stages of treatment for colorectal cancer: A comparative cross-sectional study. Seminars in Oncology Nursing, 39(4), 151446. https://doi.org/10.1016/j.soncn.2023.151446
Yang, Y., Pan, W., Allen, D., & Hendrix, C.C. (2019). Caregiver burden as a mediator between emotional distress and concentration problems in patients with cancer. Oncology Nursing Forum, 46(6), E180–E184. https://doi.org/10.1188/19.ONF.E180-E184
Yang, Y., Rushton, S., Park, H.K., Son, H., Woodward, A., Mcconnell, E., & Hendrix, C.C. (2020). Understanding the associations between caregiver characteristics and cognitive function of adults with cancer: A scoping review. Asia-Pacific Journal of Oncology Nursing, 7(2), 115–128. https://doi.org/10.4103/apjon.apjon_3_20
Zhang, Y., Bao, H.-G., Lv, Y.-L., Si, Y.-N., Han, L., Wang, H.-Y., . . . Zhang, C. (2019). Risk factors for early postoperative cognitive dysfunction after colorectal surgery. BMC Anesthesiology, 19(1), 6. https://doi.org/10.1186/s12871-018-0676-4
Zhang, Y., Lu, Y.-L., Si, Y.-N., Han, L., Wang, H.-Y., Zhang, C., . . . Bao, H.-G. (2018). Risk factors for early postoperative cognitive dysfunction after radical resection of colorectal cancer in elderly patients. Practical Geriatrics, 32(11), 1061–1063. https://doi.org/10.3969/j.issn.1003-9198.2018.11.018
Zhou, S.P., Fei, S.D., Han, H.H., Li, J.J., Yang, S., & Zhao, C.Y. (2021). A prediction model for cognitive impairment risk in colorectal cancer after chemotherapy treatment. BioMed Research International, 2021, 6666453. https://doi.org/10.1155/2021/6666453

