Fear of Progression in Outpatients With Chronic Myeloid Leukemia on Oral Tyrosine Kinase Inhibitors
Purpose/Objectives: To assess fear of progression (FoP) in outpatients with chronic myeloid leukemia (CML) on oral tyrosine kinase inhibitors (TKIs).
Design: Prospective and descriptive.
Setting: A university-based outpatient cancer clinic in Wuerzburg, Germany.
Sample: 37 outpatients with CML on oral TKIs.
Methods: FoP was assessed with a questionnaire. Clinical data were extracted from the medical charts.
Main Research Variables: Frequency and contents of FoP.
Findings: Sum scores and levels of FoP in the sample population (N = 37) were as high as in cancer populations with more unfavorable life expectancies. Regarding single items, fear that medication may harm the body was most prevalent, regardless of group affiliation. The actual fear of disease progression was only ranked sixth out of 12 items for the total sample and was ranked second by the second-generation TKI group.
Conclusions: In a sample of outpatients with CML, FoP was frequent and most often generated by fears of treatment side effects.
Implications for Nursing: Nurses should be vigilant about FoP in this population. Established questionnaires may help to identify and evaluate this frequent source of distress. Specific communication could reveal unmet informational needs and may help to initiate interventions. Additional studies are needed to confirm the numbers in a larger cohort of patients, to examine the prevalence during the course of disease, to search for potential influences on the outcome (i.e., via adherence), and to extract the best interventions.
Jump to a section
Chronic myeloid leukemia (CML) is a malignant hematologic disease caused by genetic mutation in hematopoietic stem cells in the bone marrow (Apperley, 2015; Jabbour, Bixby, & Akard, 2012; Jabbour & Kantarjian, 2014). The growth of malignant cells leads to a number of unspecific symptoms, such as fatigue, weight loss, night sweats, or fever. As a result of the clonal proliferation of malignant cells in the bone marrow, the suppressed normal hematopoiesis may lead to hepatomegaly, easy bleeding, and frequent infections (Apperley, 2015; Jabbour, Bixby, et al., 2012; Jabbour & Kantarjian, 2014). Three phases of CML can be described. Most patients present in the chronic phase with fairly stable symptoms and pathologic blood counts. Without treatment, CML will progress into an accelerated phase and, eventually, into an acute leukemic-like stage or so-called blast crisis (Apperley, 2015). Reports of medicinal treatment alleviating symptoms date back to the 19th century (Thompson, 1877), and treatment options showing improved survival were available in the 1970s when interferon alpha and allogeneic stem cell transplantation were implemented (Baccarani et al., 2002; Bonifazi et al., 2001; Guilhot et al., 1997). The median survival time with interferon alpha rose from 4–5 years to 6–7 years (Apperley, 2015). The price for the survival benefit was a wide range of side effects, which rendered treatment impossible for many patients (Apperley, 2015). Allogeneic stem cell transplantation promised a cure for as many as 80% of patients diagnosed with CML in the chronic phase and, therefore, became one of the primary treatments for this population (Jabbour, Bixby, et al., 2012). However, even in the early 2000s, treatment-related mortality was at least 10%–20% because of high toxicity and complications such as graft-versus-host disease (Jabbour, Bixby, et al., 2012). In addition, patients in the accelerated or the blast phase could only expect a cure rate of 35%–45% or 10%–20%, respectively (Jabbour, Bixby, et al., 2012).
Preclinical trials in the early 1970s provided the first evidence for the pathognomonic BCR-ABL1 gene and its encoded protein which instantly represented biomarkers for diagnosis and treatment monitoring of CML. However, the first targeted therapy was not available until 2001. The approval of the oral tyrosine kinase inhibitor (TKI) imatinib (Gleevec®) marked the start of targeted therapies in CML because of its impact on the tyrosine kinase encoded by the BCR-ABL gene and, at the same time, represented a paradigm shift in cancer medicine (Kris et al., 2010). The direct approach turned out to be so effective that the latest guidelines (Baccarani, Castagnetti, Gugliotta, Palandri, & Rosti, 2014; Jabbour & Kantarjian, 2014) propose the following treatment milestones for CML:
• Blood cell counts should be normalized by three months (representing hematologic remission).
• A complete absence of genetic abnormalities (cytogenetic remission [CyR] or two log reduction in tumor load or molecular remission [MR]2) should be reached after one year.
• BCR-ABL mRNA molecules should be undetectable by 18 months (MR or three log reduction in tumor load or MR3).
For patients who are adherent and respond to treatment as described, life spans are considered comparable to the general population (Apperley, 2015; Gambacorti-Passerini et al., 2011). Even patients showing resistances have profited enormously from modern TKI alternatives (Kantarjian et al., 2006, 2010, 2012; Talpaz et al., 2006), such as the second-generation (dasatinib [Sprycel®], nilotinib [Tasigna®], bosutinib [Bosulif®]) or third-generation (ponitinib [Iclusig®]) TKIs, depending on their varying levels of inhibition of so-called off-target kinases (Apperley, 2015). The basic spectrum of side effects is similar in all TKIs according to their mutual mechanism of action. Most prevalent are fatigue, rash, myalgia and arthralgia, diarrhea, nausea, edema, impaired pancreatic or liver function, and changes in blood chemistry and blood counts (Apperley, 2015). Second- and third-generation TKIs have infrequently been shown to cause serious side effects, such as cardiac toxicity, pleural effusions, pulmonary hypertension, or arterial occlusive disease, because of their off-target kinase inhibition (Apperley, 2015). However, TKIs are generally considered to be well tolerated and convenient because they are orally administered (Hochhaus, 2011). Because oncologists are in pursuit of perfection (Jayakar, 2012) regarding modern CML treatment, it might be expected that patients would show low levels of distress. However, in contrast to the fast-growing literature on biochemical and clinical aspects of oral TKIs in CML, little is known about patient-related outcomes in this population (Efficace et al., 2012; Trask et al., 2013). Data show impaired health-related quality of life (HRQOL) in patients with CML on oral TKIs compared to healthy populations (Phillips et al., 2013). In a study on HRQOL aspects rated the most important by patients, somatic symptoms were at the top of the list (Efficace et al., 2012). In fact, 6 of the top 10 items in Efficace et al.’s (2012) study were related to symptoms, three were related to psychosocial function, and one to satisfaction. Unfortunately, groups of patients in that report were heterogenic regarding different TKIs, and it remains unclear whether they assessed the actual extent of symptoms or their fear of symptoms (Efficace et al., 2012). Data on distress in outpatients diagnosed with CML are scarce. To the authors’ knowledge, fear of progression (FoP) in the context of CML was only mentioned in a single Chinese report (Mo et al., 2014) and was not conceptualized or specifically investigated. Mo et al. (2014) only assumed that FoP was present and possibly related to incalculable costs of lifelong medication, which are not covered by the local health system.
Cancer-related worries about disease recurrence or progression are found in 24%–70% of patients with different cancer entities, whereas anxiety disorders detected with ICD-10– or DSM-IV–based interviews have a prevalence of about 10% (Herschbach & Dinkel, 2014; Holland et al., 2013; Mehnert, Koch, Sundermann, & Dinkel, 2013). Of note, FoP may cause the patient to become dysfunctional, compromising decision making and adherence to treatment (Herschbach & Dinkel, 2014; Mehnert et al., 2013). Considering the rising number of CML survivors and the fact that FoP is one of the most prevalent symptoms of distress in patients with cancer, more information is essential. Therefore, the authors conducted an explorative study on the intensity and contents of fear of cancer progression with validated instruments in a typical sample of outpatients diagnosed with CML and treated with oral TKIs.
For the first time and within the framework of a pilot study, the authors investigated the intensity and contents of FoP in outpatients with CML treated with oral TKIs to generate results and innovative hypotheses for future assessments and interventions.
Methods
A prospective and descriptive design was adopted for this explorative study. All assessments were performed at the Medizinische Klinik und Poliklinik II of the University of Wuerzburg in Germany. At that institution, patients with all types of hematologic diseases are treated and supported by a multidisciplinary team (specialists in hematology/oncology and psychosomatic medicine). Eligible patients were informed about the study’s aims during their regular visit to the institution’s outpatient cancer clinic and were told about the psycho-oncologic support program at the hospital. After obtaining informed consent, the participants were asked to complete their questionnaires. The assessments had a mean duration of 15 minutes and were performed in a separate room at the outpatient clinic to provide privacy and confidentiality.
The research project was approved by the Ethics Committee for Medical Research in Wuerzburg in accordance with the Declaration of Helsinki. Patients were informed about the aims of the study and gave their informed consent in writing. They were free to withdraw from the study at any time. The data collection procedure, as well as the storage of documents, ensured the anonymity of the participants and the confidentiality of the data collected.
Patients diagnosed with CML and treated with oral TKIs at the local outpatient cancer clinic were considered eligible to take part in the study. Eligible patients were assessed for inclusion or exclusion criteria by one investigator (E-JC). Sociodemographic data, including age, gender, and marital status, were obtained using an established questionnaire (Deck & Röckelein, 1999).
Current TKI side effects and CML remission status were assessed during the patients’ visits. Side effects were defined as cytopenia grade 3/4 and all grades of fatigue, fluid retention, cardiopulmonary abnormalities, myalgia and arthralgia, nausea, vomiting, diarrhea, or rash. Medical history was obtained from the medical charts. Oral TKIs were categorized into first-generation (imatinib), second-generation (dasatinib, nilotinib, bosutinib), and third-generation (ponatinib) TKIs.
Instruments
FoP was assessed by the 12-item short form of the FoP Questionnaire (FoP-Q-SF) (Herschbach et al., 2005; Mehnert, Herschbach, Berg, Henrich, & Koch, 2006). The standard FOP-Q and FOP-Q-SF are reliable instruments developed by Herschbach et al. (2005) to measure FoP in chronically ill individuals. Using the 12-item FoP-Q-SF, patients were invited to rate levels of anxiety on a five-point Likert-type scale, ranging from 1 (never) to 5 (very often), with higher values indicating higher levels of anxiety. The FoP-Q-SF showed high internal consistency (Crohnbach alpha = 0.87) and has been used in a variety of cancer settings (Herschbach & Dinkel, 2014; Mehnert et al., 2013).
The FoP-Q-SF also allows single-item analyses. Typical items address concerns, such as “being nervous prior to doctors’ appointments or periodic examinations” or “worrying about the family if something happens to me.” Items like “feeling anxious about the course of the disease” or “being afraid that medication can harm my body” were of primary importance for the study. The first item refers to the threat of suffering from cancer or leukemia. The latter item focuses on the medication as a possible threat without a direct connection to actual side effects. Ongoing treatment costs are not a topic in this questionnaire, and they were not additionally assessed because of the full financial coverage by the German healthcare system.
For the classification of low, moderate, and high levels of FoP, the authors used the cut-off value based on the mean value of 1 standard deviation (SD), as described by Mehnert et al. (2013). For single-item analysis, ratings of 4 (often) or 5 (very often) were classified as high levels of anxiety.
Statistics
All analyses were performed using SPSS®, version 22. For descriptive analyses, data are expressed as median or mean and SD. For tests of significance, mean differences of continuous variables among two subgroups were examined by t test for independent samples. Pearson correlation was used when appropriate. A p value of less than 0.05 was considered statistically significant.
Hypotheses
The authors’ hypotheses were that (a) FoP is less frequent in this population compared to other cancer types because long-term outcomes are excellent for responding patients; (b) that the item “feeling anxious about the course of the disease” is most prevalent because patients still fight a malignant disease; (c) that the item “being afraid that medication can harm my body” is not very prevalent and mostly concerns patients suffering from side effects at the time point of assessment; and, as different generations of TKIs very much resemble each other, the authors assumed that (d) no differences can be seen between the groups of TKIs.
Findings
Demographic and Medical Variables
The sociodemographic and medical characteristics of the 37 participants were analyzed. Mean age was 59 years (range = 22–87), and 21 were male and 16 female. Most patients (n = 30) were married and lived in multiple-person households. Mean time since CML diagnosis was 73 months (SD = 53, range = 4–236 months) and mean time since start of TKI treatment was 66 months (SD = 41, range = 4–139 months).
TKI side effects at assessment were present in nine patients, and treatment adaption or cessation was not necessary. Past TKI side effects were documented in 27 patients, and side effects were present at both time points in seven patients. Eight patients never experienced TKI side effects. Eight patients in the total sample did not reach molecular remission, and one patient did not reach cytogenetic remission.
Twenty-five of the patients in the total sample were treated with imatinib and 12 were treated with second-generation TKIs (dasatinib or nilotinib). No significant group differences were noted regarding age, gender, or time course of disease or treatment (see Table 1). Four patients had to change to second-generation TKIs because of imatinib resistance. With another four patients, previous side effects were the reason for change. Four others received second-generation TKIs as first-line treatment.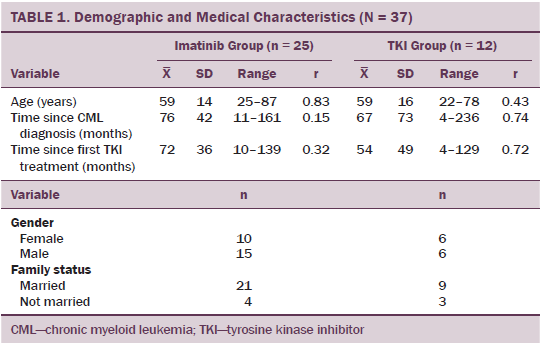
Fear of Progression Sum Score
Mean score of FoP in the total sample was 28.7 (SD = 10.12). FoP sum score was not significantly associated with age, gender, or marital status. In addition, the FoP sum score was not significantly associated with elapsed time since first diagnosis of CML or elapsed time since the start of TKI therapy. FoP sum score was significantly higher in the group of patients with current side effects (p = 0.011) and was not associated with remission status. FoP was significantly higher in patients on second-generation TKIs (mean = 34, SD = 12.95) than in the group treated with imatinib (mean = 26.16, SD = 7.49, p = 0.025).
Fear of Progression Levels
By using the cut-off value based on the mean value of 1 SD, six patients were classified as having high FoP. Two of those patients were on imatinib, and four were on second-generation TKIs. In addition, 27 patients were classified as having moderate FoP and 4 patients as having low FoP. In the group with high FoP, side effects related to TKI were present in four of six patients at the assessment time point. Treatment adaption or cessation was not necessary. All patients with high FoP were at least in complete CyR or MR2 and four patients were in MR or MR3.
Single-Item Analyses
The item, “worrying that medication can harm my body” (item 10) described the fear that was, by far, the most relevant (mean = 3.03, SD = 1.24) in the total sample (see Table 2). Thirteen patients worried often or very often about possible harms to their body and were classified as having high fear regarding that item. Six of those patients were in the imatinib group, and seven were in the second-generation TKI group.
Of the patients stating high fear, six expressed TKI side effects during their visit. Change or cessation of medication was not necessary in any of those cases. In another four patients, side effects were present in the past. Three patients with high fear of side effects never experienced any adverse events.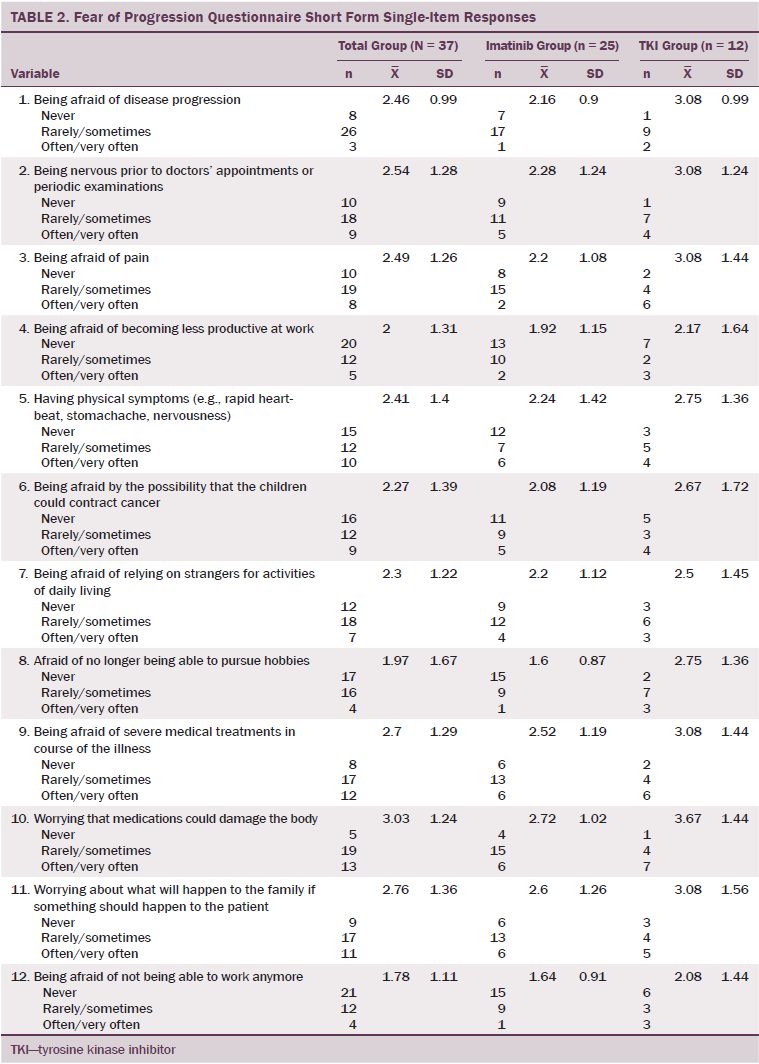
Item 1 (“being afraid of disease progression”) only ranked sixth out of 12 items (mean = 2.46, SD = 0.99) for the total sample. Three patients worried often or very often about disease progression and were classified as having high fear concerning that item. One patient was on imatinib, and two were on second-generation TKIs. All three patients with high fear in that item were not in MR. One of those three patients was not in CyR.
Regarding subgroups, the fear of TKI side effects (item 10) was ranked first in the imatinib group (mean = 2.72, SD = 1.02) and in the second-generation TKI group (mean = 3.67, SD = 1.44). The differences between the two groups were not statistically significant. Fear of disease progression (item 1) was ranked only eighth (mean = 2.16, SD = 0.9) in the imatinib group. In the second-generation TKI group, that item was ranked second (mean = 3.08, SD = 0.99). The difference was not statistically significant.
Discussion
In contrast to a fast-growing literature base on biologic mechanisms and somatic variables, much less is known about distress in outpatients with CML receiving oral TKIs; FoP as a frequent quality of distress has not been investigated in this population before. The authors’ main goal was to examine the frequency and content of FoP in a typical, single-center sample of outpatients diagnosed with CML on oral TKIs. And, for the first time, the authors demonstrated pronounced FoP with established instruments. Financial aspects could be ruled out because financing was provided by the local health system. The main result was a mean score of 28.7 (SD = 10.12) on the FoP-Q-SF. In contrast to the authors’ first hypothesis, the score is comparable to other populations where patients suffer from much more unfavorable hematologic neoplasms (Hefner et al., 2014; Mehnert et al., 2013). According to the cutoff method, 6 patients stated high FoP and 27 stated moderate FoP. These numbers also are comparable with percentages of cancer rehabilitation patients with different solid and hematologic tumors (Mehnert et al., 2013). The result from the current study is unique and opposes the authors’ hypothesis that responding patients with excellent life expectancies might suffer less from FoP than patients with other cancers. Sociodemographic variables or time spans since primary diagnosis or treatment start were not associated with FoP. The latter findings are in line with much larger samples of patients with various cancers, which show that FoP is an issue even in long-term survivorship (Koch, Jansen, Brenner, & Arndt, 2013).
In single-item analyses, the distinct fear that leukemia progresses (item 1) was outscored by the fear that medication may harm the body (item 10), regardless of group affiliation or side effects. The results also contradict the authors’ hypothesis and agree with Efficace et al. (2012), where somatic symptoms were rated more burdensome than psychosocial issues in patients on different TKIs. Data from the current study indicate differences regarding TKI groups. FoP sum scores in patients on second-generation TKIs were significantly higher than in patients on imatinib. The distinct fear of disease progression in the group on second-generation TKIs was ranked second compared to the imatinib group, where it was only ranked sixth. These figures may result from the fact that eight of the patients on second-generation TKIs had to switch medication because of former imatinib side effects or resistances and do show that psychosocial issues gain importance, at least in subgroups of patients. Another major aspect determined from study results pertains to somatic symptoms. As suggested earlier, somatic symptoms and FoP seem to be linked closely. However, in the current study, only 6 of 13 patients worrying often or very often about TKI side effects showed typical symptoms at the assessment time point without the necessity of therapy modification.
Limitations
Major conclusions cannot be drawn from this study, particularly because of the small sample size during subgroup analysis. Newer TKIs, such as bosutinib or ponatinib, as well as previous therapies with interferon alpha or chemotherapy, were not monitored and, as a result, eligible caveats may arise against some of the authors’ conclusions. However, the investigation was designed as an explorative study, and the data do show that FoP is an important problem in typical outpatients with CML on oral TKIs. Only six of the patients who reported very high fear that medication might harm the body had to deal with current side effects. The data suggest, therefore, that the fear of side effects is more troubling than the actual side effect.
Implications for Nursing Practice and Research
Oncology nurses are the healthcare professionals interacting most frequently with patients (Mann, 2011); therefore, they are in a prime position to screen not only for physical symptoms, but also for symptoms of anxiety. They may also evaluate levels of distress by implementing existing screening tools, such as the FoP-Q-SF. Future studies in larger populations may help to determine levels of FoP in a fast-growing population. Modified designs with screening tools for anxiety and depression would help to approximate the actual level of distress in these patients because anxiety may lead to depression and symptoms of anxiety and depression may be present independently from one another (Brintzenhofe-Szoc, Levin, Li, Kissane, & Zabora, 2009; Burgess et al., 2005). In addition, more data are needed on distress in the trajectory of CML because different phases may lead to different levels and appearances of distress.
If FoP is present, oncology nurses may instruct patients in relaxation training or mindfulness-based stress reduction techniques (Henderson et al., 2012). If distress is very high or not responding to unspecific distress management, oncology nurses could act as gatekeepers and enable access to professional psychological support. Regarding FoP, group therapy has been shown to be beneficial for other patients with cancer (Herschbach et al., 2010) and could be initiated by nurse specialists as part of a comprehensive therapeutic approach.
Research on other cancer populations has shown that less-educated patients suffer more from anxiety (Garcia, 2014). The current authors’ results suggest that outpatients with CML on oral TKIs are not well informed about side effects and their management. In addition to assessing information needs, oncology nurses also are effective providers of patient education and, as such, could alleviate FoP in this population (D’Souza, Blouin, Zeitouni, Muller, & Allison, 2013; Garcia, 2014; Polat, Arpaci, Demir, Erdal, & Yalcin, 2014). Education has been shown to be most effective before the start of treatment in other cancer populations (Garcia, 2014; McClellan et al., 2013; Polat et al., 2014). However, informational needs and distress may vary and require tailored information in the trajectory of CML. Future research may not only reveal different informational needs, but also provide information on the best setting for intervention. Modern means of telecommunication could be used and help nurses to support distressed outpatients with CML (Siekkinen, Pyrhonen, Ryhanen, Vahlberg, & Leino-Kilpi, 2015). If barriers of communication between patients and physicians are detected, oncology nurses should work to improve communication (Shields et al., 2010).
Oncology nurse navigators may represent a model of comprehensive cancer care for a growing number of outpatients with CML. They have been shown to alleviate distress in other chronic and complex cancer settings (Ludman et al., 2015; McPhillips et al., 2015; Swanson & Koch, 2010). Nurse navigators can stay in close contact to outpatients with CML and regularly reevaluate distress. Because distress, such as fear, anxiety, and depression, may cause patients to become dysfunctional, future studies should investigate possible links between patients’ fears and adherence, which is reported to be low in outpatients with CML (Gater et al., 2012; Herschbach & Dinkel, 2014; Jabbour, Kantarjian, Eliasson, Cornelison, & Marin, 2012; Noens et al., 2009). By addressing those fears and supporting patients and physicians to closely collaborate over time, nurse navigators may contribute to optimizing survival.
Conclusion
Modern biochemical insights and TKI treatment may represent a paradigm shift in diagnostics and therapy of CML, but data from the current article support the idea that a considerable number of patients still suffer from FoP. As more oral cancer therapies emerge, a rising number of patients with cancer will be confronted with higher standards of self-observation, self-responsibility, and self-management. Oncology nurses will play an increasingly important role in supporting highly distressed outpatients with cancer on oral agents.
References
Apperley, J.F. (2015). Chronic myeloid leukaemia. Lancet, 385, 1447–1459.
Baccarani, M., Castagnetti, F., Gugliotta, G., Palandri, F., & Rosti, G. (2014). Treatment recommendations for chronic myeloid leukemia. Mediterranean Journal of Hematology and Infectious Diseases, 6, e2014005.
Baccarani, M., Rosti, G., de Vivo, A., Bonifazi, F., Russo, D., Martinelli, G., . . . Tura, S. (2002). A randomized study of interferon-alpha versus interferon-alpha and low-dose arabinosyl cytosine in chronic myeloid leukemia. Blood, 99, 1527–1535.
Bonifazi, F., de Vivo, A., Rosti, G., Guilhot, F., Guilhot, J., Trabacchi, E., . . . Baccarani, M. (2001). Chronic myeloid leukemia and interferon-alpha: A study of complete cytogenetic responders. Blood, 98, 3074–3081.
Brintzenhofe-Szoc, K.M., Levin, T.T., Li, Y., Kissane, D.W., & Zabora, J.R. (2009). Mixed anxiety/depression symptoms in a large cancer cohort: Prevalence by cancer type. Psychosomatics, 50, 383–391.
Burgess, C., Cornelius, V., Love, S., Graham, J., Richards, M., & Ramirez, A. (2005). Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ, 330, 702.
Deck, R., & Röckelein, E. (1999). [On the elicitation of socio-demographic and socio-medical Indicators in research associations of rehabilitation sciences]. DRV-Schriften, 16, 84–102.
D’Souza, V., Blouin, E., Zeitouni, A., Muller, K., & Allison, P.J. (2013). An investigation of the effect of tailored information on symptoms of anxiety and depression in head and neck cancer patients. Oral Oncology, 49, 431–437.
Efficace, F., Breccia, M., Saussele, S., Kossak-Roth, U., Cardoni, A., Caocci, G., . . . Sprangers, M. (2012). Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Annals of Hematology, 91, 1371–1381.
Gambacorti-Passerini, C., Antolini, L., Mahon, F.X., Guilhot, F., Deininger, M., Fava, C., . . . Kim, D.W. (2011). Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. Journal of the National Cancer Institute, 103, 553–561.
Garcia, S. (2014). The effects of education on anxiety levels in patients receiving chemotherapy for the first time: An integrative review. Clinical Journal of Oncology Nursing, 18, 516–521.
Gater, A., Heron, L., Abetz-Webb, L., Coombs, J., Simmons, J., Guilhot, F., & Rea, D. (2012). Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leukemia Research, 36, 817–825.
Guilhot, F., Chastang, C., Michallet, M., Guerci, A., Harousseau, J.L., Maloisel, F., . . . Tanzer, J. (1997). Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. New England Journal of Medicine, 337, 223–229.
Hefner, J., Kapp, M., Drebinger, K., Dannenmann, A., Einsele, H., Grigoleit, G.U., . . . Mielke, S. (2014). High prevalence of distress in patients after allogeneic hematopoietic SCT. Bone Marrow Transplant, 49, 581–584.
Henderson, V.P., Clemow, L., Massion, A.O., Hurley, T.G., Druker, S., & Hebert, J.R. (2012). The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: A randomized trial. Breast Cancer Research and Treatment, 131, 99–109.
Herschbach, P., Berg, P., Dankert, A., Duran, G., Engst-Hastreiter, U., Waadt, S., . . . Henrich, G. (2005). Fear of progression in chronic diseases: Psychometric properties of the Fear of Progression Questionnaire. Journal of Psychosomtic Research, 58, 505–511.
Herschbach, P., Book, K., Dinkel, A., Berg, P., Waadt, S., Duran, G., . . . Henrich, G. (2010). Evaluation of two group therapies to reduce fear of progression in cancer patients. Supportive Care in Cancer, 18, 471–479.
Herschbach, P., & Dinkel, A. (2014). Fear of progression. Recent Results in Cancer Research, 197, 11–29.
Hochhaus, A. (2011). Educational session: Managing chronic myeloid leukemia as a chronic disease. Hematology, 2011, 128–135.
Holland, J.C., Andersen, B., Breitbart, W.S., Buchmann, L.O., Compas, B., Deshields, T.L., . . . Freedman-Cass, D.A. (2013). Distress management. Journal of the National Comprehensive Cancer Network, 11, 190–209.
Jabbour, E., & Kantarjian, H. (2014). Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management. American Journal of Hematology, 89, 547–556.
Jabbour, E.J., Bixby, D., & Akard, L.P. (2012). Clinical roundtable monograph: Unmet needs in the management of chronic myelogenous leukemia. Clinical Advances in Hematology Oncology, 10(12, Suppl. 22), 1–16.
Jabbour, E.J., Kantarjian, H., Eliasson, L., Cornelison, A.M., & Marin, D. (2012). Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. American Journal of Hematology, 87, 687–691.
Jayakar, V. (2012). Chronic myeloid leukemia: In pursuit of perfection. South Asian Journal of Cancer, 1, 16–24.
Kantarjian, H., Giles, F., Wunderle, L., Bhalla, K., O’Brien, S., Wassmann, B., . . . Ottmann, O.G. (2006). Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. New England Journal of Medicine, 354, 2542–2551.
Kantarjian, H., Shah, N.P., Hochhaus, A., Cortes, J., Shah, S., Ayala, M., . . . Baccarani, M. (2010). Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. New England Journal of Medicine, 362, 2260–2270.
Kantarjian, H.M., Shah, N.P., Cortes, J.E., Baccarani, M., Agarwal, M.B., Undurraga, M.S., . . . Hochhaus, A. (2012). Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Two-year follow-up from a randomized phase 3 trial (DASISION). Blood, 119, 1123–1129.
Koch, L., Jansen, L., Brenner, H., & Arndt, V. (2013). Fear of recurrence and disease progression in long-term (>/= 5 years) cancer survivors. Psycho-Oncology, 22, 1–11.
Kris, M.G., Benowitz, S.I., Adams, S., Diller, L., Ganz, P., Kahlenberg, M.S., . . . Petrelli, N.J. (2010). Clinical cancer advances 2010: Annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology, 28, 5327–5347.
Ludman, E.J., McCorkle, R., Bowles, E.A., Rutter, C.M., Chubak, J., Tuzzio, L., . . . Wagner, E.H. (2015). Do depressed newly diagnosed cancer patients differentially benefit from nurse navigation? General Hospital Psychiatry, 37, 236–239.
Mann, K.S. (2011). Education and health promotion for new patients with cancer. Clinical Journal of Oncology Nursing, 15, 55–61.
McClellan, W., Klemp, J.R., Krebill, H., Ryan, R., Nelson, E.L., Panicker, J., . . . Stegenga, K. (2013). Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncology Nursing Forum, 40, 254–262.
McPhillips, D., Evans, R., Ryan, D., Daneshvar, C., Sarkar, S.A., & Breen, D. (2015). The role of a nurse specialist in a modern lung-cancer service. British Journal of Nursing, 24(Suppl. 4), S21–S27.
Mehnert, A., Herschbach, P., Berg, P., Henrich, G., & Koch, U. (2006). [Fear of progression in breast cancer patients—Validation of the short form of the Fear of Progression Questionnaire]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 52, 274–288.
Mehnert, A., Koch, U., Sundermann, C., & Dinkel, A. (2013). Predictors of fear of recurrence in patients one year after cancer rehabilitation: A prospective study. Acta Oncologica, 52, 1102–1109.
Mo, X.D., Jiang, Q., Xu, L.P., Liu, D.H., Liu, K.Y., Jiang, B., . . . Huang, X.J. (2014). Health-related quality of life of patients with newly diagnosed chronic myeloid leukemia treated with allogeneic hematopoietic SCT versus imatinib. Bone Marrow Transplant, 49, 576–580.
Noens, L., van Lierde, M.A., De Bock, R., Verhoef, G., Zachee, P., Berneman, Z., . . . Abraham, I. (2009). Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia. Blood, 113, 5401–5411.
Phillips, K.M., Pinilla-Ibarz, J., Sotomayor, E., Lee, M.R., Jim, H.S., Small, B.J., . . . Jacobsen, P.B. (2013). Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A controlled comparison. Supportive Care in Cancer, 21, 1097–1103.
Polat, U., Arpaci, A., Demir, S., Erdal, S., & Yalcin, S. (2014). Evaluation of quality of life and anxiety and depression levels in patients receiving chemotherapy for colorectal cancer: Impact of patient education before treatment initiation. Journal of Gastrointestinal Oncology, 5, 270–275.
Shields, C.G., Ziner, K.W., Bourff, S.A., Schilling, K., Zhao, Q., Monahan, P., . . . Champion, V. (2010). An intervention to improve communication between breast cancer survivors and their physicians. Journal of Psychosocial Oncology, 28, 610–629.
Siekkinen, M., Pyrhonen, S., Ryhanen, A., Vahlberg, T., & Leino-Kilpi, H. (2015). Psychosocial outcomes of e-feedback of radiotherapy for breast cancer patients: A randomized controlled trial. Psycho-Oncology, 24, 515–522.
Swanson, J., & Koch, L. (2010). The role of the oncology nurse navigator in distress management of adult inpatients with cancer: A retrospective study. Oncology Nursing Forum, 37, 69–76.
Talpaz, M., Shah, N.P., Kantarjian, H., Donato, N., Nicoll, J., Paquette, R., . . . Sawyers, C.L. (2006). Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. New England Journal of Medicine, 354, 2531–2541.
Thompson, H. (1877). Middlesex hospital: Case of leucocythaemia. Lancet, 109, 125–126.
Trask, P.C., Cella, D., Powell, C., Reisman, A., Whiteley, J., & Kelly, V. (2013). Health-related quality of life in chronic myeloid leukemia. Leukemia Research, 37, 9–13.
About the Author(s)
Hefner is a consultant of psychosomatic medicine and psychotherapy in the Department of Internal Medicine at the University of Wuerzburg in Germany; Csef is an assistant physician in the Department of Oncology, Gastroenterology, and Infectiology at St. Franziskus Hospital in Muenster, Germany; and Kunzmann is a professor of medical oncology in the Department of Internal Medicine at the University of Wuerzburg. No financial relationships to disclose. Mention of specific products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society. Hefner and Kunzmann contributed to the conceptualization and design. Csef completed the data collection. Hefner provided the statistical support and contributed to the analysis. All authors contributed to the manuscript preparation. Hefner can be reached at hefner_j@ukw.de, with copy to editor at ONFEditor@ons.org. Submitted November 2014. Accepted for publication June 28, 2015.
References
Apperley, J.F. (2015). Chronic myeloid leukaemia. Lancet, 385, 1447–1459.
Baccarani, M., Castagnetti, F., Gugliotta, G., Palandri, F., & Rosti, G. (2014). Treatment recommendations for chronic myeloid leukemia. Mediterranean Journal of Hematology and Infectious Diseases, 6, e2014005.
Baccarani, M., Rosti, G., de Vivo, A., Bonifazi, F., Russo, D., Martinelli, G., . . . Tura, S. (2002). A randomized study of interferon-alpha versus interferon-alpha and low-dose arabinosyl cytosine in chronic myeloid leukemia. Blood, 99, 1527–1535.
Bonifazi, F., de Vivo, A., Rosti, G., Guilhot, F., Guilhot, J., Trabacchi, E., . . . Baccarani, M. (2001). Chronic myeloid leukemia and interferon-alpha: A study of complete cytogenetic responders. Blood, 98, 3074–3081.
Brintzenhofe-Szoc, K.M., Levin, T.T., Li, Y., Kissane, D.W., & Zabora, J.R. (2009). Mixed anxiety/depression symptoms in a large cancer cohort: Prevalence by cancer type. Psychosomatics, 50, 383–391.
Burgess, C., Cornelius, V., Love, S., Graham, J., Richards, M., & Ramirez, A. (2005). Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ, 330, 702.
Deck, R., & Röckelein, E. (1999). [On the elicitation of socio-demographic and socio-medical Indicators in research associations of rehabilitation sciences]. DRV-Schriften, 16, 84–102.
D’Souza, V., Blouin, E., Zeitouni, A., Muller, K., & Allison, P.J. (2013). An investigation of the effect of tailored information on symptoms of anxiety and depression in head and neck cancer patients. Oral Oncology, 49, 431–437.
Efficace, F., Breccia, M., Saussele, S., Kossak-Roth, U., Cardoni, A., Caocci, G., . . . Sprangers, M. (2012). Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Annals of Hematology, 91, 1371–1381.
Gambacorti-Passerini, C., Antolini, L., Mahon, F.X., Guilhot, F., Deininger, M., Fava, C., . . . Kim, D.W. (2011). Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. Journal of the National Cancer Institute, 103, 553–561.
Garcia, S. (2014). The effects of education on anxiety levels in patients receiving chemotherapy for the first time: An integrative review. Clinical Journal of Oncology Nursing, 18, 516–521.
Gater, A., Heron, L., Abetz-Webb, L., Coombs, J., Simmons, J., Guilhot, F., & Rea, D. (2012). Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leukemia Research, 36, 817–825.
Guilhot, F., Chastang, C., Michallet, M., Guerci, A., Harousseau, J.L., Maloisel, F., . . . Tanzer, J. (1997). Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. New England Journal of Medicine, 337, 223–229.
Hefner, J., Kapp, M., Drebinger, K., Dannenmann, A., Einsele, H., Grigoleit, G.U., . . . Mielke, S. (2014). High prevalence of distress in patients after allogeneic hematopoietic SCT. Bone Marrow Transplant, 49, 581–584.
Henderson, V.P., Clemow, L., Massion, A.O., Hurley, T.G., Druker, S., & Hebert, J.R. (2012). The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: A randomized trial. Breast Cancer Research and Treatment, 131, 99–109.
Herschbach, P., Berg, P., Dankert, A., Duran, G., Engst-Hastreiter, U., Waadt, S., . . . Henrich, G. (2005). Fear of progression in chronic diseases: Psychometric properties of the Fear of Progression Questionnaire. Journal of Psychosomtic Research, 58, 505–511.
Herschbach, P., Book, K., Dinkel, A., Berg, P., Waadt, S., Duran, G., . . . Henrich, G. (2010). Evaluation of two group therapies to reduce fear of progression in cancer patients. Supportive Care in Cancer, 18, 471–479.
Herschbach, P., & Dinkel, A. (2014). Fear of progression. Recent Results in Cancer Research, 197, 11–29.
Hochhaus, A. (2011). Educational session: Managing chronic myeloid leukemia as a chronic disease. Hematology, 2011, 128–135.
Holland, J.C., Andersen, B., Breitbart, W.S., Buchmann, L.O., Compas, B., Deshields, T.L., . . . Freedman-Cass, D.A. (2013). Distress management. Journal of the National Comprehensive Cancer Network, 11, 190–209.
Jabbour, E., & Kantarjian, H. (2014). Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management. American Journal of Hematology, 89, 547–556.
Jabbour, E.J., Bixby, D., & Akard, L.P. (2012). Clinical roundtable monograph: Unmet needs in the management of chronic myelogenous leukemia. Clinical Advances in Hematology Oncology, 10(12, Suppl. 22), 1–16.
Jabbour, E.J., Kantarjian, H., Eliasson, L., Cornelison, A.M., & Marin, D. (2012). Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. American Journal of Hematology, 87, 687–691.
Jayakar, V. (2012). Chronic myeloid leukemia: In pursuit of perfection. South Asian Journal of Cancer, 1, 16–24.
Kantarjian, H., Giles, F., Wunderle, L., Bhalla, K., O’Brien, S., Wassmann, B., . . . Ottmann, O.G. (2006). Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. New England Journal of Medicine, 354, 2542–2551.
Kantarjian, H., Shah, N.P., Hochhaus, A., Cortes, J., Shah, S., Ayala, M., . . . Baccarani, M. (2010). Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. New England Journal of Medicine, 362, 2260–2270.
Kantarjian, H.M., Shah, N.P., Cortes, J.E., Baccarani, M., Agarwal, M.B., Undurraga, M.S., . . . Hochhaus, A. (2012). Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Two-year follow-up from a randomized phase 3 trial (DASISION). Blood, 119, 1123–1129.
Koch, L., Jansen, L., Brenner, H., & Arndt, V. (2013). Fear of recurrence and disease progression in long-term (>/= 5 years) cancer survivors. Psycho-Oncology, 22, 1–11.
Kris, M.G., Benowitz, S.I., Adams, S., Diller, L., Ganz, P., Kahlenberg, M.S., . . . Petrelli, N.J. (2010). Clinical cancer advances 2010: Annual report on progress against cancer from the American Society of Clinical Oncology. Journal of Clinical Oncology, 28, 5327–5347.
Ludman, E.J., McCorkle, R., Bowles, E.A., Rutter, C.M., Chubak, J., Tuzzio, L., . . . Wagner, E.H. (2015). Do depressed newly diagnosed cancer patients differentially benefit from nurse navigation? General Hospital Psychiatry, 37, 236–239.
Mann, K.S. (2011). Education and health promotion for new patients with cancer. Clinical Journal of Oncology Nursing, 15, 55–61.
McClellan, W., Klemp, J.R., Krebill, H., Ryan, R., Nelson, E.L., Panicker, J., . . . Stegenga, K. (2013). Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncology Nursing Forum, 40, 254–262.
McPhillips, D., Evans, R., Ryan, D., Daneshvar, C., Sarkar, S.A., & Breen, D. (2015). The role of a nurse specialist in a modern lung-cancer service. British Journal of Nursing, 24(Suppl. 4), S21–S27.
Mehnert, A., Herschbach, P., Berg, P., Henrich, G., & Koch, U. (2006). [Fear of progression in breast cancer patients—Validation of the short form of the Fear of Progression Questionnaire]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 52, 274–288.
Mehnert, A., Koch, U., Sundermann, C., & Dinkel, A. (2013). Predictors of fear of recurrence in patients one year after cancer rehabilitation: A prospective study. Acta Oncologica, 52, 1102–1109.
Mo, X.D., Jiang, Q., Xu, L.P., Liu, D.H., Liu, K.Y., Jiang, B., . . . Huang, X.J. (2014). Health-related quality of life of patients with newly diagnosed chronic myeloid leukemia treated with allogeneic hematopoietic SCT versus imatinib. Bone Marrow Transplant, 49, 576–580.
Noens, L., van Lierde, M.A., De Bock, R., Verhoef, G., Zachee, P., Berneman, Z., . . . Abraham, I. (2009). Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia. Blood, 113, 5401–5411.
Phillips, K.M., Pinilla-Ibarz, J., Sotomayor, E., Lee, M.R., Jim, H.S., Small, B.J., . . . Jacobsen, P.B. (2013). Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A controlled comparison. Supportive Care in Cancer, 21, 1097–1103.
Polat, U., Arpaci, A., Demir, S., Erdal, S., & Yalcin, S. (2014). Evaluation of quality of life and anxiety and depression levels in patients receiving chemotherapy for colorectal cancer: Impact of patient education before treatment initiation. Journal of Gastrointestinal Oncology, 5, 270–275.
Shields, C.G., Ziner, K.W., Bourff, S.A., Schilling, K., Zhao, Q., Monahan, P., . . . Champion, V. (2010). An intervention to improve communication between breast cancer survivors and their physicians. Journal of Psychosocial Oncology, 28, 610–629.
Siekkinen, M., Pyrhonen, S., Ryhanen, A., Vahlberg, T., & Leino-Kilpi, H. (2015). Psychosocial outcomes of e-feedback of radiotherapy for breast cancer patients: A randomized controlled trial. Psycho-Oncology, 24, 515–522.
Swanson, J., & Koch, L. (2010). The role of the oncology nurse navigator in distress management of adult inpatients with cancer: A retrospective study. Oncology Nursing Forum, 37, 69–76.
Talpaz, M., Shah, N.P., Kantarjian, H., Donato, N., Nicoll, J., Paquette, R., . . . Sawyers, C.L. (2006). Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. New England Journal of Medicine, 354, 2531–2541.
Thompson, H. (1877). Middlesex hospital: Case of leucocythaemia. Lancet, 109, 125–126.
Trask, P.C., Cella, D., Powell, C., Reisman, A., Whiteley, J., & Kelly, V. (2013). Health-related quality of life in chronic myeloid leukemia. Leukemia Research, 37, 9–13.

