A Qualitative Study of the Everyday Impacts of Cognitive Difficulties After Stem Cell Transplantation
Purpose: To explore how cognitive difficulties affect the everyday lives of survivors of allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Participants & Setting: 20 survivors of allo-HSCT attending follow-up care at a tertiary cancer center in Toronto, Canada.
Methodologic Approach: This qualitative, descriptive study used semistructured interviews.
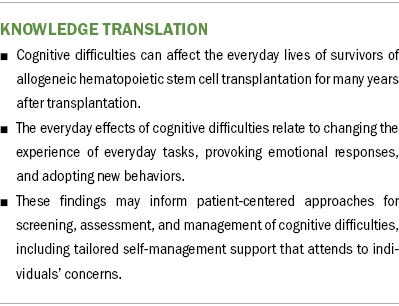
Findings: Cognitive symptoms affected the everyday lives of allo-HSCT survivors by changing the experience of everyday tasks, provoking emotional responses, and prompting adoption of mitigation strategies. Subthemes within each of these themes highlight the ways in which cognitive impairment shapes how allo-HSCT survivors feel about themselves, interact with others, and navigate coping challenges.
Implications for Nursing: These findings demonstrate the multidimensional experience of cognitive difficulties following allo-HSCT and may inform the development of patient-centered approaches to assessing and managing cognitive difficulties.
Jump to a section
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a treatment modality indicated for many hematologic cancers and diseases that involves high-dose chemotherapy and/or radiation therapy to destroy malignant or dysfunctional cells, followed by the infusion of healthy donor stem cells to restore immune function. Improvements in allo-HSCT techniques and supportive care have led to positive survival outcomes and greater recognition of the long-term sequelae experienced by survivors. Even after the risk of malignancy relapse has abated, individuals treated with allo-HSCT are at risk for physical, psychological, and functional impairments that may interfere with readjustment after treatment (Syrjala et al., 2012). With the number of recipients of allo-HSCT increasing and evolving, greater understanding of patient-oriented outcomes and the development of appropriate health services to support them are needed (Battiwalla et al., 2017; Bevans et al., 2017).
There is growing evidence that cognitive functioning, particularly in the domains of memory, concentration, information speed, and executive functioning, may be negatively affected among adult recipients of allo-HSCT (Harder et al., 2002; Harrison et al., 2021; Mayo, Wozniczka, et al., 2020; Syrjala et al., 2011). Although as many as one-third of recipients demonstrated deficits on objective neuropsychological tests of cognitive functioning prior to transplantation, there is evidence of further persistent declines in the months to years after allo-HSCT (Sharafeldin et al., 2018; Syrjala et al., 2004, 2011). After the first year following allo-HSCT, as many as 60% of survivors self-reported cognitive symptoms, with up to 40% demonstrating impairment on objective cognitive testing (Bevans et al., 2017). At five years after allo-HSCT, Syrjala et al. (2011) found that 41.5% of their cohort of 66 survivors demonstrated at least mild cognitive deficits on objective testing as compared to 19.5% of healthy controls. Those who experience greater severity of post-transplantation complications and systemic inflammation appear to be at greater risk for poorer cognitive outcomes (Hoogland et al., 2019; Jim et al., 2012; Mayo, Messner, et al., 2020), although explanatory mechanisms involving biological, psychological and social factors remain an active area of research (Harrison et al., 2021). Cognitive difficulties have been associated with poorer quality-of-life outcomes after allo-HSCT, particularly related to mood, social functioning, and employment status (Harder et al., 2002; Murdaugh et al., 2020). The need for large, multisite clinical research to characterize incidence, risk factors, and pathogenesis of cognitive impairment among the growing population of allo-HSCT survivors has been identified as a research priority (Bevans et al., 2017; Kelly et al., 2018).
An understanding of allo-HSCT survivors’ experiences of cognitive difficulties is also needed to inform the development of patient-centered supportive care approaches. Firsthand descriptive accounts from survivors may offer important information about the specific contexts in which cognitive difficulties affect their daily lives, but few qualitative studies have addressed this issue. In an early qualitative study of HSCT-focused support groups, Sherman et al. (2005) reported that memory and concentration issues were common and frustrating among participants. However, the specific effects on daily life were not described. Wu et al. (2019) conducted structured qualitative interviews with 69 autologous and allogeneic HSCT survivors and found that 71% of respondents experienced cognitive difficulties after transplantation, mostly related to memory and attention or concentration, and that these difficulties were associated with depressed mood, anxiety, and lower quality of life. In response to focused questions on the effect of cognitive difficulties on work and interacting with others, common themes reflected greater difficulty with productivity (e.g., taking longer to complete work-related tasks) and engaging effectively in social interactions (e.g., embarrassment, repeating stories). However, beyond these specific situations, the ways that survivors manage cognitive difficulties in daily life were not addressed. Descriptive accounts could contribute to a more fulsome understanding of the significance of cognitive difficulties in the lives of allo-HSCT survivors, which could inform the contexts in which supportive care interventions may be developed.
This article expands on the literature by exploring the everyday effects of cognitive difficulties among survivors of allo-HSCT and the ways that individuals cope. Semistructured interviews were conducted with allo-HSCT survivors as part of a longitudinal study of cognitive outcomes after allo-HSCT.
Methods
Design
This study used a qualitative, descriptive methodology (Sandelowski, 2000, 2010) to prioritize a comprehensive understanding of participant experiences while staying close to the data to best facilitate the development of interventions (Sullivan-Bolyai et al., 2005). Qualitative description provides a rich description of an experience in easily understood language. It enables a study to explore, describe, understand, and present participants’ experiences from their own unique, subjective perspectives with less interpretation than other qualitative methodologies (Sandelowski, 2000, 2010; Sullivan-Bolyai et al., 2005). Qualitative description is appropriate for research questions focused on discovering the who, what, and where of events or experiences and gaining insights from participants about poorly understood phenomena (Sandelowski, 2000, 2010). Although a variety of data collection methods can be employed in qualitative description studies, individual interviews facilitate deeper exploration of topics and provide the richness of data desired for this study (Sandelowski, 2000, 2010).
Participants and Setting
Semistructured interviews were conducted with 20 allo-HSCT survivors between November 2018 and December 2019 at Princess Margaret Cancer Centre in Toronto, Ontario, Canada. All study participants were enrolled in an initial longitudinal study of cognitive outcomes after allo-HSCT, with data collected during pretreatment, on day 100 post-transplantation, and at six months post-transplantation (Mayo, Messner, et al., 2020). Participants were enrolled in an additional follow-up study conducted six years after allo-HSCT (Mayo, Wozniczka, et al., 2020). Eligible participants for the initial longitudinal study were aged 18 years or older at the time of allo-HSCT and English-speaking. Participants were excluded from the six-year follow-up study if they had a new cancer diagnosis, a significant neurologic comorbidity (e.g., traumatic brain injury), or new diagnosis of psychotic disorder since the time of allo-HSCT; were currently receiving chemotherapy treatment; or reported significant substance use (defined as more than three alcoholic drinks per day or use of any illegal drugs during the past 30 days [Cysique et al., 2011]).
The initial longitudinal study had baseline enrollment of 59 participants, and 20 participants were enrolled in the six-year follow-up study, with attrition because of death or relapse (n = 28), inability to contact (n = 7) and decline of consent (n = 4) (Mayo, Wozniczka, et al., 2020). Data collection at the six-year follow-up included neuropsychological testing, measurement of cognitive difficulties using the standardized European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 Cognitive Functioning Subscale (Aaronson et al., 1993), and semistructured qualitative interviews. The results of neuropsychological testing and the standardized cognitive functioning questionnaire were published previously (Mayo, Wozniczka, et al., 2020). This article presents an analysis of the semistructured qualitative interviews completed during the six-year follow-up.
Written informed consent was obtained from all participants prior to participation in the follow-up study. Participation included a $50 honorarium. All study procedures were conducted with approval from the Research Ethics Boards of the University Health Network (#18-5151) and University of Toronto (#00036242).
Data Generation and Analysis
All interviews were conducted by one of two authors (S.J.M. and I.W.). Semistructured interviews were guided by open-ended questions designed to gather participants’ descriptions of their experience of post-transplantation cognitive difficulties and related effects on everyday functioning, with additional probes and questions evolving during the course of the study (see Figure 1). A total of 362 minutes of interview data were collected, with a median interview length of 15 minutes (range = 6–44). Demographic and clinical characteristics were extracted from baseline data and electronic health records.

Interviews were audio recorded and transcribed verbatim. NVivo, version 11, was used for data management and coding. Analysis occurred concurrently with data collection to allow emerging themes to be incorporated in subsequent interviews. Data were analyzed and themes were developed according to the tenets of thematic analysis (Braun & Clarke, 2006). Three investigators (B.E., I.W., and S.J.M.) independently listened to all audio recordings and conducted close reading of all interview transcripts. Inductive codes were developed and assigned to passages in the data by B.E. and refined with input from I.W. and S.J.M. Key patterns in the coded passages were explored among the three investigators and sorted into themes relevant to the study aims. Themes were reviewed related to the entire dataset for further modification, and illustrative participant quotes were extracted. Consultation with the full research team resulted in final agreement. Data saturation, determined by little to no change to the codes and code definitions based on the data collected from later interviews (Guest et al., 2006), was achieved within the 20 interviews.
The analytic approach represented an accurate and trustworthy representation of the experience of the study participants (Lincoln & Guba, 1985). Credibility was maintained by the prolonged engagement with participants leading up to the time of the interviews, triangulation of multiple investigators during data analysis, and involvement of researchers with clinical expertise in allo-HSCT. An audit trail of analytic material and illustrative passages from transcripts was maintained as supporting evidence of the themes to enhance confirmability.
Findings
Sample Characteristics
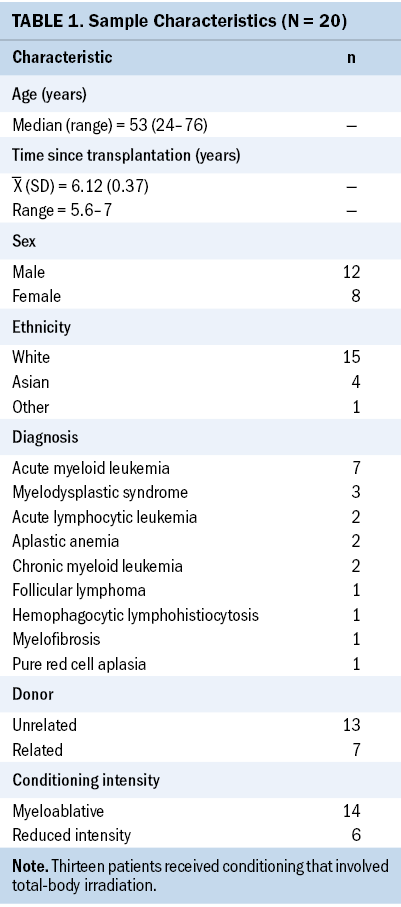
Participant characteristics are provided in Table 1. Interviews occurred an average of 6.12 years (SD = 0.37, range = 5.6–7) after allo-HSCT, most frequently for acute leukemia or myelodysplastic syndrome (n = 12). Most participants were White (n = 15) and more than half were male (n = 12). The median age of participants was 53 years (range = 24–76). At the time of the interview, average scores on the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 Cognitive Functioning Subscale met the criteria for clinical importance, and eight participants met the International Cognition and Cancer Task Force criteria for cancer-related cognitive impairment (Mayo, Wozniczka, et al., 2020). All participants self-reported experiencing some degree of cognitive difficulties following transplantation.
Cognitive difficulties affected the everyday lives of participants by changing the experience of everyday tasks, provoking emotional responses, and prompting adoption of mitigation strategies. Figure 2 presents the themes and subthemes that emerged during this study.

Changing the Experience of Everyday Tasks
Participants described the tangible ways that cognitive difficulties affected their functional ability. Rudimentary tasks, such as meal planning or selecting a parking spot, were experienced as more cognitively demanding. Participants attributed these challenges to feeling as though it required more effort to recall information quickly and accurately, particularly when under pressure. Participants described being less able to stay on task, feeling easily distracted, and having difficulty finding words. These cognitive difficulties affected participants’ ability to complete tasks in the home, at work, and in social environments. They described how accessing and applying their knowledge and skills seemed to require more cognitive effort than it had previously, although they could still achieve their goals. One participant described,
The way I best described it was it’s like a pencil. You can still write with a pencil, but it just wasn’t quite as sharp. So, I was able to get the things done, but just not with the precision that I was used to. (participant 3)
Routines: Cognitive difficulties frequently became more apparent on returning to routines or sequences of actions that demanded focus. A common example provided by participants was the routine of grocery shopping. It became challenging to move through the grocery store without a list and to make meal planning decisions. Processes seemed to require more thought or an explicit effort to break tasks down into steps. For one participant, this also included preparing for the grocery trip itself:
It doesn’t seem like much to get your coat on, boots on, and get out the door to go grocery shopping, but I remember that was such a big task because it involved making a list. Then I had to get dressed. And then I had to get ready to go outside. And then I had to grab the bags. It seems silly now talking about it, but at the time I felt like it was a million things to do before I could get out the door. (participant 3)
Decision-making: Participants also described difficulties managing different inputs. This was evident when they were attempting to focus on one specific task or when they were required to multitask. It was also an issue when participants faced decisions; sustained effort to weigh relative advantages and disadvantages could be overwhelming, leading to frustration or abandonment of the decision altogether. As one participant stated, “I can’t make decisions. Making decisions, that’s a big deal. I can’t even decide between two cans of food. ‘Oh, should I get this one? Should I get that one?’ I don’t know. ‘Oh no, I just won’t get anything’” (participant 20).
Social interactions: Participants also described how cognitive difficulties affected the ways they interacted with others, including forgetting names, misusing words when speaking or writing, or feeling overwhelmed when trying to follow conversations:
People that don’t know me, when you’re talking to them . . . when the words don’t come out and you’re just like there, when you can’t think of the words and you go into states like this—you’re just kind of, “Um, uh, um,” they just look at you and smile. “There’s something wrong with her. Oh, gosh.” (participant 20)
Provoking Emotional Responses
Changes in the experience of everyday tasks provoked a variety of emotional responses among participants. These ranged from negative emotions, such as frustration, feeling overwhelmed, and isolation, to feelings of acceptance.
Frustration and feeling overwhelmed: Participants described a complex process by which the difficulty in completing a specific task often could not be separated from the meaning attributed to this difficulty. For example, initial frustration with a particular task, if not managed, could quickly lead to negative emotions, such as inadequacy about oneself and hopelessness about one’s situation. These emotions heightened the experience of feeling overwhelmed, which further exacerbated the initial cognitive difficulty associated with the task at hand. This process is illustrated by a quote from one participant who stated, “Because it’d be like, ‘Oh, I forget this.’ And I get frustrated. And then I get sad and then it’s just like a downhill spiral” (participant 12). The emotions are further explained by another participant who described, “Well, you kind of lose your confidence. . . . It’s like you start questioning, ‘Is this something—am I going to be able to do some of the things I’m going to be able to do again?’ And you don’t really know” (participant 3). For some, the emotional impacts were a consequence of the cumulative symptoms and health issues experienced in the post-treatment phase, as described by another participant:
So, you have a lot of things that you need to deal with mentally, emotionally in terms of health. It can be an extra layer of stress on top of everything else. Yeah, some days are good, but occasionally it can trigger the feeling of depression or sad[ness], and it does that kind of thing. It does happen. (participant 6)
Preventing feeling overwhelmed required recognizing and managing the initial frustration to limit further progression: “If I’m getting frustrated then, typically, my pulse is up, my breathing’s up, my blood pressure’s probably up. And it just takes a moment to just collect myself again and approach it again” (participant 2).
Isolation: Participants also experienced social isolation linked to their cognitive difficulties, in that they felt “different” from their peers. For those who had been physically isolated because of their disease and allo-HSCT treatment, cognitive difficulties contributed to further feelings of isolation. As one participant stated,
So, I had a lot of issues socializing, and that’s something that I really wasn’t prepared for. . . . I mean, there’s already issues of isolation going on with going through illness and going through treatment, and then the fact that you have difficulty socializing or being in a social environment with lots of noise or lots of people, no one kind of prepares you for that. So, it almost feels like, even though your treatment is done, that you still can’t necessarily—you’re still not a part of the world in a very, very concrete way. You still feel like there’s something unusual about you. So, it’s very isolating. (participant 5)
Acceptance: Participants also described times when they responded to their cognitive difficulties with neutral feelings or acceptance. This was particularly the case when survivors attributed their cognitive difficulties to aging or the physical symptoms they were experiencing at the time. For symptoms like fatigue, acceptance was linked to expectations that overall symptom burden would eventually improve.
I just took the attitude to listen to my body so when that happened, I would just say to myself, “I’m going to go to bed. I’m going to lie down. I’m going to sleep because my body’s telling me that’s what I have to. So, what is the win of pushing yourself when you don’t feel that you can do it?” . . . But again, it was patience. This isn’t going to be forever. This is just today. (participant 10)
Another participant described how mitigating the impacts of cognitive difficulties helped them move toward acceptance in the context of their treatment journey:
Well, if it’s really, really important, I write it down. Do this, and do this, and do this. It’s like when you go shopping, you make your grocery list, and you do whatever. Otherwise, life goes on. Thank God that I’m alive. It was a long ride. Very, very long. (participant 4)
Prompting Adoption of Mitigation Strategies
The experience of cognitive difficulties necessitated finding a way to cope. However, participants rarely sought specific healthcare solutions to address these needs. Instead, participants developed their own strategies over time to mitigate disruption to everyday tasks and emotional health.
Positive mindset and self-talk: Participants expressed that coping with cognitive difficulties depended on their mindset or general approach to appraising their situation. An approach that was more positive and flexible appeared to allow individuals to steadily meet their challenges without getting overwhelmed by frustration. This approach was bolstered by using phrases such as “being patient with yourself,” “listen to your body,” or “take it one day at a time” when describing how they coped in the early stages post-treatment or the advice they would give to a peer experiencing cognitive difficulties.
So, I don’t remember ever being frustrated by not being able to do something mostly because, every day, it was kind of, “OK. I made it this far. This is really good,” and I guess it’s a positive outlook and it’s, “What do I need to do next?” (participant 13)
However, it was also noted that there were often challenges regarding negative self-talk over time. Feelings of resignation showed up in thoughts that needed to be countered with thoughts of patience and encouragement.
I just kind of rolled with the punches. I’m a very positive person. So, I would never get upset and I would never get frustrated or angry. I just kind of, “Oh, my gosh. Isn’t this hysterical? I can’t even remember my own—I can’t remember where my socks are.” Something silly like that and just kind of roll with the punches. But that’s just my nature. . . . So, that’s how I handled it. Yeah. Never beat myself up. (participant 10)
Finding strategies that work through trial and error: Participants described learning how to apply strategies for mitigating disruptions related to cognitive difficulties. Even after receiving tips and tactics from others, processes of trial and error were necessary to establish what would work best in the context of their specific work, home, and social demands. Commonly described actions and strategies included having routines, planning ahead, making lists, breaking up tasks into smaller pieces, trying new hobbies, or changing one’s routines to continue to challenge oneself mentally. However, participants acknowledged that strategies would be different for everyone based on personal interests and standard ways of coping. The development of individualized strategies is highlighted in one participant’s description of the method he developed to keep apprised of his schedule:
What I do now is, I have a dry erase board. So, I’m putting it right there, so it’s—every morning I wake up, it’s in my room, I have to look at it. So, you kind of find ways of making yourself looking at what you write down. . . . And you don’t have to spend time memorizing because then that’s a stress by itself. . . . So, I find when you write it down and you have a place where you have to or you must pass every day . . . and I have a little red kind of magnet that I put on the change on the date, so I just look for the red button, and . . . that’s today. And if there’s anything written there, then I know I have to read it. And if there’s nothing there, then I know it’s just a normal day. (participant 11)
Leaning on others for support: Participants acknowledged the role of their support network for being able to tolerate shifts in existing relationships. For example, survivors needed to rely more heavily on their partners, children, parents, coworkers, or friends for help in ways that were not previously needed. Support included encouragement, understanding and acceptance, humor, but also practical support, such as managing schedules, assisting with medications, and planning events. Sometimes this required specific efforts to ask for help:
[My daughters] weren’t really getting the fact that I was having a hard time trying to make [dinner] happen. So, I finally said, . . . “I’m having a really hard time making this happen, and how you guys are involved in this isn’t making me feel any better. It’s actually making me feel worse. So, I need you guys to know this is what I need you to do so that I can get through this.” . . . They couldn’t put themselves in my shoes, and I was so consumed with myself I couldn’t put myself in theirs. (participant 3)
Participants also noted an important role for healthcare providers and cancer programs to link them with information, as well as peers who had also undergone allo-HSCT and experienced similar cognitive difficulties. The need to hear from others who had been through similar experiences, such as in a peer support group, were described:
[When talking to others in a support group] . . . You have this problem. OK. “How did you do it?” That might even be better because I don’t know if you [the interviewer] would be able to tell me how to make my memory better, but this person experienced the same thing. Tried this. And they got it better. So, I was like, “OK. Well, yeah. Maybe that.” (participant 12)
Discussion
This qualitative study contributed novel insights to the impact of cognitive difficulties on the day-to-day lives of long-term allo-HSCT survivors. This study expanded on the existing literature regarding the consequences of cognitive difficulties on quality of life by highlighting participants’ experiences and outcomes. Participants in this study described challenges related to their ability to efficiently and meaningfully engage in instrumental activities of daily living, which triggered feelings of frustration, loss of confidence, and social isolation. Over time, participants developed their own ways to cope with these challenges, typically by reducing negative feelings through reframing expectations and finding ways to accommodate cognitive changes into their everyday lives.
Cognitive difficulties were found to affect the experience of everyday tasks by increasing the level of required effort compared to before treatment. Changes in tasks requiring decision-making were seen as particularly challenging, which is congruent with previous evidence that poorer performance on objective cognitive tests after allo-HSCT is associated with reduced understanding of risks and benefits, as well as poorer medication management ability (Mayo et al., 2016; Zaubler et al., 2010). These findings may help to explain the ways that cognitive difficulties influence quality-of-life outcomes, such as employment and relationships after allo-HSCT. They may also serve to inform dialogue with individuals experiencing cognitive difficulties after allo-HSCT and identify targets for developing patient-oriented interventions. Patient counseling and self-management support interventions that explicitly address everyday difficulties, such as bolstering patients’ ability to follow conversations or manage decision-making, may be of particular benefit.
The emotional experience of cognitive difficulties was significant, consistent with research conducted in other cancer populations (Selamat et al., 2014). Emotions related to withdrawal and isolation are particularly salient in the context of allo-HSCT because individuals may experience significant isolation during and after treatment that separate them from their peers. Individuals treated with allo-HSCT often experience physical isolation as part of the protective environment during hospital admission and post-treatment because of their recovering immune function, during which loneliness and psychological distress may be experienced (Biagioli et al., 2017). The findings of the current study suggest that cognitive difficulties can further exacerbate isolation in this population. Among individuals treated with allo-HSCT, discordance between recovery expectations and post-transplantation functioning have been associated with psychological distress (Andrykowski et al., 1995).
Participants described the range of ways they found to cope with the cognitive difficulties they were experiencing. These included focusing on organizational strategies, modifying the physical and social environment, reducing stress, and stimulating the mind. Similar findings have been reported among breast cancer survivors (Selamat et al., 2014; Von Ah et al., 2013). A novel finding from this study is evidence of survivors’ persistence at applying different strategies until they discovered what worked and under what circumstances. For these survivors, who were six years post-treatment on average, persistence was required as they experienced multiple setbacks and frustrations. This is reflective of the hard work of cancer self-management (Haase et al., 2020). Skills training interventions are effective in other populations (Fernandes et al., 2019; Richard et al., 2019), and future research could explore appropriate tailoring of these interventions for allo-HSCT survivors.
Relationships with others, particularly family caregivers, significantly shaped participants’ experience of cognitive difficulties. Processes of engaging with others, such as through conversations or fulfilling commitments, frequently exposed limitations and created stress. At the same time, supportive relationships with trusted peers or family members bolstered participants’ ability to cope with these difficulties. Although some survivors’ experiences may not be fully evident to those around them, family members observe behavioral changes, such as greater difficulty following conversations and need for constant reminders, that may signal cognitive difficulties (Fitch, 2021). Interventions that facilitate nonjudgmental dialogue between the patient and their support network may reduce the stress associated with this experience.
These findings contribute to efforts to theorize the patient experience of cancer-related cognitive impairment. In a meta-ethnography of qualitative studies describing the experience of “chemobrain” among people with breast cancer, Selamat et al. (2014) characterized the experience by the struggle for validation of cognitive difficulties, impacts on activities across life domains, struggles to adjust and self-manage, effects on relationships and work or school, and being thankful for life, yet fearful of the future. Findings from this study support the transferability of this experience to the context of patients treated with allo-HSCT. This study advances insight into the everyday processes that contribute to these broader experiences. It also contributes to the body of qualitative research in hematologic cancer survivorship, which has lagged behind other cancer groups (Laidsaar-Powell et al., 2019).
Limitations
The transferability of these findings is likely limited by the nature of the sample, which was predominantly White and receiving follow-up care at a Canadian tertiary cancer center for transplantations received in 2012–2013. Variability in clinical conditioning regimens and management of complications, such as graft-versus-host disease, as well as availability of supportive care services, may influence transferability of these findings to other allo-HSCT settings. The sample also varied regarding various clinical (e.g., disease, transplantation conditioning regimen, receiving total-body irradiation) and demographic (e.g., sex, age) characteristics that may have influenced the severity and experience of cognitive difficulties, although the specific biologic mechanisms underlying these difficulties among allo-HSCT survivors remain unclear and warrant further research. In addition, study respondents were six years post–allo-HSCT, and many found it difficult to link their experiences to specific time periods relative to transplantation or articulate how their experience fluctuated over time. Although all respondents self-reported cognitive difficulties at some point following transplantation, including at the time of the interview, individual descriptions of their experiences may have been limited by recall bias. Overall, despite these limitations, this study contributes novel insights into common experiences across this heterogenous sample of allo-HSCT survivors, which can be extended with focused qualitative studies on the experiences within specific clinical circumstances that consider how supportive care interventions may need to be tailored based on underlying pathophysiology.
Implications for Nursing
Despite the high prevalence of cognitive effects in the early phase after allo-HSCT that can interfere with self-care and other daily life activities (Bevans et al., 2008; Mayo et al., 2016), recommendations specific to the routine monitoring and management of cognitive outcomes among survivors are limited (Majhail et al., 2012; Mayo, Lustberg, et al., 2020; National Comprehensive Cancer Network, 2022; Von Ah et al., 2014). These findings provide insights into how cognitive difficulties can shape allo-HSCT survivors’ feelings about themselves, their interactions with others, and the ways in which they cope. For nurses working with individuals experiencing cognitive difficulties after allo-HSCT, these findings may inform the development of patient-centered approaches for screening, assessment, and management of cognitive difficulties, including tailored self-management support that attends to individual concerns.
Conclusion
Long-term survivors of allo-HSCT experience a range of everyday impacts related to cognitive difficulties. These include changing the experience of everyday tasks, provoking emotional responses, and adopting new behaviors. Overall, this study demonstrates the multidimensional experience of cognitive difficulties, and its findings can guide the development of patient-centered interventions to manage cognitive difficulties and bolster survivors’ quality of life.
The authors gratefully acknowledge the valuable contribution of the late Hans Messner, MD, PhD, who was instrumental in the conceptualization and design of this study.
About the Authors
Samantha J. Mayo, RN, PhD, is an assistant professor in the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto and the RBC Financial Group Chair in Oncology Nursing Research in the Princess Margaret Cancer Centre at the University Health Network; Isabel Wozniczka, RN, BScN, MSc, is a former research assistant in the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto and currently a clinical research coordinator in the Princess Margaret Cancer Centre at the University Health Network; Beth Edwards, MPH, PhD, is a scientific associate at the Cancer Rehabilitation and Survivorship program at the University Health Network; Sean B. Rourke, PhD, FCAHS, is a scientist in the MAP Centre for Urban Health Solutions at St. Michael’s Hospital and a professor in the Department of Psychiatry at the University of Toronto; Doris Howell, RN, PhD, is an emeritus scientist in the Princess Margaret Cancer Centre at the University Health Network; Kelly A. Metcalfe, RN, PhD, is a professor in the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto and a senior scientist at the Women’s College Hospital Research Institute; Arta Taghavi Haghayegh is a research assistant in the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto; and Jeffrey H. Lipton, MD, PhD, is a staff physician in the Princess Margaret Cancer Centre at the University Health Network and a professor in the Department of Medicine at the University of Toronto, all in Toronto, Ontario, Canada. This study was funded by a grant from the Oncology Nursing Foundation (principal investigator: Mayo). Mayo, Rourke, Howell, Metcalfe, and Lipton contributed to the conceptualization and design. Mayo and Wozniczka completed the data collection. Mayo provided statistical support. Mayo, Edwards, Howell, Haghayegh, Lipton, and Wozniczka provided the analysis. Mayo, Wozniczka, Edwards, Rourke, Howell, Metcalfe, Haghayegh, and Lipton contributed to the manuscript preparation. Mayo can be reached at samantha.mayo@utoronto,ca, with copy to ONFEditor@ons.org. (Submitted August 2021. Accepted December 15, 2021.)
References
Aaronson, N.K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N.J., . . . Takeda, F. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(5), 365–376. https://doi.org/10.1093/jnci/85.5.365
Andrykowski, M.A., Brady, M.J., Greiner, C.B., Altmaier, E.M., Burish, T.G., Antin, J.H., . . . Henslee-Downey, P.J. (1995). ‘Returning to normal’ following bone marrow transplantation: Outcomes, expectations and informed consent. Bone Marrow Transplantation, 15(4), 573–581.
Battiwalla, M., Tichelli, A., & Majhail, N.S. (2017). Long-term survivorship after hematopoietic cell transplantation: Roadmap for research and care. Biology of Blood and Marrow Transplantation, 23(2), 184–192. https://doi.org/10.1016/j.bbmt.2016.11.004
Bevans, M., El-Jawahri, A., Tierney, D.K., Wiener, L., Wood, W.A., Hoodin, F., . . . Syrjala, K.L. (2017). National Institutes of Health hematopoietic cell transplantation late effects initiative: The Patient-Centered Outcomes Working Group report. Biology of Blood and Marrow Transplantation, 23(4), 538–551. https://doi.org/10.1016/j.bbmt.2016.09.011
Bevans, M.F., Mitchell, S.A., & Marden, S. (2008). The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Supportive Care in Cancer, 16(11), 1243–1254. https://doi.org/10.1007/s00520-008-0420-6
Biagioli, V., Piredda, M., Alvaro, R., & de Marinis, M.G. (2017). The experiences of protective isolation in patients undergoing bone marrow or haematopoietic stem cell transplantation: Systematic review and metasynthesis. European Journal of Cancer Care, 26(5), e12461. https://doi.org/10.1111/ecc.12461
Braun, V., & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. https://doi.org/10.1191/1478088706qp063oa
Cysique, L.A., Franklin Jr., D., Abramson, I., Ellis, R.J., Letendre, S., Collier, A., . . . Heaton, R.K. (2011). Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. Journal of Clinical and Experimental Neuropsychology, 33(5), 505–522. https://doi.org/10.1080/13803395.2010.535504
Fernandes, H.A., Richard, N.M., & Edelstein, K. (2019). Cognitive rehabilitation for cancer-related cognitive dysfunction: A systematic review. Supportive Care in Cancer, 27(9), 3253–3279. https://doi.org/10.1007/s00520-019-04866-2
Fitch, M.I. (2021). Contrasting patient and family member perspectives about cognitive changes following cancer therapy. Canadian Oncology Nursing Journal, 31(2), 242–247.
Guest, G., Bunce, A., & Johnson, L. (2006). How many interviews are enough? An experiment with data saturation and variability. Field Methods, 18(1), 59–82. https://doi.org/10.1177/1525822X05279903
Haase, K.R., Avery, J., Bryant-Lukosius, D., Kryzanowska, M., Kukretti, V., Liu, G., . . . Howell, D. (2020). Patient and clinician perspectives of desired features for a web-based self-management program (icanmanage.ca): Exposing patients “hard work” of managing acute cancer. Supportive Care in Cancer, 29(4), 1989–1998. https://doi.org/10.1007/s00520-020-05683-8
Harder, H., Cornelissen, J.J., Van Gool, A.R., Duivenvoorden, H. J., Eijkenboom, W.M., & van den Bent, M.J. (2002). Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer, 95(1), 183–192. https://doi.org/10.1002/cncr.10627
Harrison, R.A., Sharafeldin, N., Rexer, J.L., Streck, B., Petersen, M., Henneghan, A.M., & Kesler, S.R. (2021). Neurocognitive impairment after hematopoietic stem cell transplant for hematologic malignancies: Phenotype and mechanisms. Oncologist, 26(11), e2021–e2033. https://doi.org/10.1002/onco.13867
Hoogland, A.I., Nelson, A.M., Gonzalez, B.D., Small, B.J., Breen, E.C., Sutton, S.K., . . . Jim, H.S.L. (2019). Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain, Behavior, and Immunity, 80, 308–314. https://doi.org/10.1016/j.bbi.2019.04.008
Jim, H.S.L., Small, B., Hartman, S., Franzen, J., Millay, S., Phillips, K., . . . Pidala, J. (2012). Clinical predictors of cognitive function in adults treated with hematopoietic cell transplantation. Cancer, 118(13), 3407–3416. https://doi.org/10.1002/cncr.26645
Kelly, D.L., Buchbinder, D., Duarte, R.F., Auletta, J.J., Bhatt, N., Byrne, M., . . . Shaw, B.E. (2018). Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation, 24(2), 228–241. https://doi.org/10.1016/j.bbmt.2017.09.004
Laidsaar-Powell, R., Konings, S., Rankin, N., Koczwara, B., Kemp, E., Mazariego, C., & Butow, P. (2019). A meta-review of qualitative research on adult cancer survivors: Current strengths and evidence gaps. Journal of Cancer Survivorship, 13(6), 852–889. https://doi.org/10.1007/s11764-019-00803-8
Lincoln, Y.S., & Guba, E.G. (1985). Naturalistic inquiry. Sage.
Majhail, N.S., Rizzo, J.D., Lee, S.J., Aljurf, M., Atsuta, Y., Bonfim, C., . . . Tichelli, A. (2012). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplantation, 47(3), 337–341. https://doi.org/10.1038/bmt.2012.5
Mayo, S., Messner, H.A., Rourke, S.B., Howell, D., Victor, J.C., Kuruvilla, J., . . . Metcalfe, K. (2016). Relationship between neurocognitive functioning and medication management ability over the first 6 months following allogeneic stem cell transplantation. Bone Marrow Transplantation, 51(6), 841–847. https://doi.org/10.1038/bmt.2016.2
Mayo, S.J., Lustberg, M., Dhillon, H,M., Nakamura, Z.M., Allen, D.H., Von Ah, D., . . . Peters, K.B. (2020). Cancer-related cognitive impairment in patients with non-central nervous system malignancies: An overview for oncology providers from the MASCC Neurological Complications Study Group. Supportive Care in Cancer, 29(6), 2821–2840. https://doi.org/10.1007/s00520-020-05860-9
Mayo, S.J., Messner, H.A., Rourke, S.B., Howell, D., Victor, J.C., Lipton, J.H., . . . Metcalfe, K. (2020). Predictors of the trajectory of cognitive functioning in the first 6 months after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation, 55(5), 918–928. https://doi.org/10.1038/s41409-019-0746-3
Mayo, S.J., Wozniczka, I., Brennenstuhl, S., Rourke, S.B., Howell, D., Metcalfe, K.A., & Lipton, J.H. (2020). Late cognitive outcomes among allogeneic stem cell transplant survivors: Follow-up data from a 6-year longitudinal study. Supportive Care in Cancer, 29(5), 2621–2630. https://doi.org/10.1007/s00520-020-05761-x
Murdaugh, D.L., Bosworth, A., Patel, S.K., Sharafeldin, N., Chen, Y., Francisco, L., . . . Bhatia, S. (2020). Self-endorsed cognitive problems versus objectively assessed cognitive impairment in blood or bone marrow transplantation recipients: A longitudinal study. Cancer, 126(10), 2174–2182. https://doi.org/10.1002/cncr.32773
National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Survivorship [v. 1.2022]. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf
Richard, N.M., Bernstein, L.J., Mason, W.P., Laperriere, N., Maurice, C., Millar, B.-A., . . . Edelstein, K. (2019). Cognitive rehabilitation for executive dysfunction in brain tumor patients: A pilot randomized controlled trial. Journal of Neuro-Oncology, 142(3), 565–575. https://doi.org/10.1007/s11060-019-03130-1
Sandelowski, M. (2000). Whatever happened to qualitative description? Research in Nursing and Health, 23(4), 334–340. https://doi.org/10.1002/1098-240x(200008)23:4%3C334::aid-nur9%3E3.0.co;…
Sandelowski, M. (2010). What’s in a name? Qualitative description revisited. Research in Nursing and Health, 33(1), 77–84. https://doi.org/10.1002/nur.20362
Selamat, M.H., Loh, S.Y., Mackenzie, L., & Vardy, J. (2014). Chemobrain experienced by breast cancer survivors: A meta-ethnography study investigating research and care implications. PLOS ONE, 9(9), e108002. https://doi.org/10.1371/journal.pone.0108002
Sharafeldin, N., Bosworth, A., Patel, S.K., Chen, Y., Morse, E., Mather, M., . . . Bhatia, S. (2018). Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: Results from a prospective longitudinal study. Journal of Clinical Oncology, 36(5), 463–475. https://doi.org/10.1200/jco.2017.74.2270
Sherman, R.S., Cooke, E., & Grant, M. (2005). Dialogue among survivors of hematopoietic cell transplantation: Support-group themes. Journal of Psychosocial Oncology, 23(1), 1–24. https://doi.org/10.1300/J077v23n01_01
Sullivan-Bolyai, S., Bova, C., & Harper, D. (2005). Developing and refining interventions in persons with health disparities: The use of qualitative description. Nursing Outlook, 53(3), 127–133. https://doi.org/10.1016/j.outlook.2005.03.005
Syrjala, K.L., Artherholt, S.B., Kurland, B.F., Langer, S.L., Roth-Roemer, S., Elrod, J.B., & Dikmen, S. (2011). Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. Journal of Clinical Oncology, 29(17), 2397–2404. https://doi.org/10.1200/jco.2010.33.9119
Syrjala, K.L., Dikmen, S., Langer, S.L., Roth-Roemer, S., & Abrams, J.R. (2004). Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood, 104(10), 3386–3392. https://doi.org/10.1182/blood-2004-03-1155
Syrjala, K.L., Martin, P.J., & Lee, S.J. (2012). Delivering care to long-term adult survivors of hematopoietic cell transplantation. Journal of Clinical Oncology, 30(30), 3746–3751. https://doi.org/10.1200/jco.2012.42.3038
Von Ah, D., Jansen, C.E., & Allen, D.H. (2014). Evidence-based interventions for cancer- and treatment-related cognitive impairment. Clinical Journal of Oncology Nursing, 18(6, Suppl.), 17–25. https://doi.org/10.1188/14.cjon.s3.17-25
Von Ah, D., Storey, S., Jansen, C.E., & Allen, D.H. (2013). Coping strategies and interventions for cognitive changes in patients with cancer. Seminars in Oncology Nursing, 29(4), 288–299. https://doi.org/10.1016/j.soncn.2013.08.009
Wu, L.M., Kuprian, N., Herbert, K., Amidi, A., Austin, J., Valdimarsdottir, H., & Rini, C. (2019). A mixed methods analysis of perceived cognitive impairment in hematopoietic stem cell transplant survivors. Palliative Supportive Care, 17(4), 396–402. https://doi.org/10.1017/s1478951518000664
Zaubler, T., Fann, J.R., Roth-Roemer, S., Katon, W.J., Bustami, R., & Syrjala, K.L. (2010). Impact of delirium on decision-making capacity after hematopoietic stem-cell transplantation. Psychosomatics, 51(4), 320–329. https://doi.org/10.1176/appi.psy.51.4.320


