Symptom Occurrence, Frequency, and Severity During Acute Colorectal Cancer Survivorship
Objectives: To examine colorectal cancer (CRC) survivors’ symptom characteristics (occurrence, frequency, and severity) during acute cancer survivorship.
Participants & Setting: A cross-sectional study of 117 CRC survivors was conducted at a National Cancer Institute–designated cancer center in South Florida.
Methods & Variables: Symptom characteristics were assessed by the Therapy-Related Symptom Checklist. Participants completed a 25-item demographic questionnaire. Mann-Whitney U and Kruskal-Wallis H tests assessed between-group differences based on sex, age, education, and months since diagnosis. Exploratory factor analysis was performed to identify preliminary symptom clusters.
Results: 117 CRC survivors completed the study (age range = 21–88 years, 56% male, and 79% stage IV). Common symptoms included peripheral neuropathy, fatigue/feeling sluggish, and skin changes. Significance was found between months since diagnosis and number of symptoms (p = 0.03), suggesting that symptoms accumulate with time. Chemotherapy (85%) was the most common treatment type, and exploratory factor analysis identified two chemotherapy-related symptom clusters.
Implications for Nursing: Nurses are poised to identify, prevent, and promote self-management skills to reduce symptoms.
Jump to a section
Colorectal cancer (CRC) is a cancer of the digestive tract, colon, or rectum (American Cancer Society [ACS], 2021a). CRC is the fourth-leading cancer type and the second-leading cause of cancer-related deaths in the United States (ACS, 2021a; National Cancer Institute [NCI], 2021; Siegel et al., 2019). Because of advances in early detection (e.g., colonoscopy, genetic screening) and treatment (e.g., surgical interventions), mortality rates have decreased by 2% since 2012 (Howlader et al., 2019; Siegel et al., 2019). However, CRC survivors continue to manage the effects of a CRC diagnosis and its treatments. CRC survivors may develop numerous symptoms (e.g., depression, abdominal cramps and pain, weakness), physical or psychological subjective signs indicating an abnormal condition within the human body, typically caused by a disease or injury, which are measured by occurrence, frequency, and severity (Sreedhar, 2021; Stark et al., 2012).
Like other cancer survivors, CRC survivors develop a wide range of symptoms, including depression, body image disorder, sexual dysfunction, and neuropathy of the hands and feet (Röhrl et al., 2019; Wu et al., 2021). Symptoms can affect survivors’ functional status and quality of life throughout survivorship, the length of time a person has lived since their cancer diagnosis until the end of life, inclusive of medical and psychosocial care (Deshields et al., 2014; Miaskowski et al., 2017; Mosher & DuHamel, 2012; Xiao, 2010). Studies suggest that symptoms are reported more during acute cancer survivorship, the length of time from cancer diagnosis to the completion of treatment (Mullan, 1985). Because survivors begin medical treatments (e.g., surgical resection, radiation therapy, chemotherapy) that are dependent on cancer stage and location, they are prone to the development of symptoms from the CRC and its treatments (Albusoul et al., 2017; Röhrl et al., 2019; Sheikh-Wu et al., 2020, 2021; Tantoy et al., 2016). However, most research has been limited to symptoms among breast, prostate, and lung cancer survivors, with few studies examining CRC survivors, despite the increase in CRC diagnoses (Howlader et al., 2019; Siegel et al., 2019). As a result, there is a lack of knowledge about the CRC survivor population, particularly regarding their symptom characteristics (occurrence, frequency, and severity) during acute cancer survivorship.
In general, studies show that survivors often experience up to 13 symptoms during cancer survivorship; the physical and psychological symptoms vary and may include a range of symptoms such as body image disturbance, stress, anxiety, depression, pain, fatigue, sleep disturbances, and social isolation (Barsevick, 2016; Deshields et al., 2014; Mosher & DuHamel, 2012; Sheikh-Wu et al., 2020, 2021). Survivors may encounter a single symptom, but more frequently, two or more symptoms, also called a symptom cluster, coexist, (Albusoul et al., 2017; Miaskowski et al., 2017; Sheikh-Wu et al., 2020; Xiao, 2010). Approximately 20% of CRC survivors experience more than five symptoms, and 66% experienced at least two symptoms simultaneously while receiving treatment (curative, maintenance, or palliative) (O’Gorman et al., 2018; Röhrl et al., 2019; Røhrl et al., 2016). If left unmanaged, these symptoms may persist and become more severe during an after treatment, which may exacerbate other physical, psychological, and emotional symptoms that disrupt a patient’s overall quality of life (Juul et al., 2018; Miaskowski et al., 2017; Rasmussen et al., 2015; Schuh-Hofer et al., 2018; Sheikh-Wu et al., 2021; Siminoff et al., 2014; Tantoy et al., 2016).
Symptoms can produce adverse side effects including but not limited to a disruption in functional ability and social relationships, which may exacerbate underlying illnesses (Miaskowski et al., 2017; Rasmussen et al., 2015; Schuh-Hofer et al., 2018; Sheikh-Wu et al., 2021). In addition, unmanaged symptoms put survivors at a higher risk of developing chronic conditions (e.g., depression, anxiety) that can negatively impact prognosis, recovery, and quality of life, ultimately leading to increased use of the healthcare system (Barsevick, 2016; Goldberg et al., 2019; Miaskowski et al., 2017). In addition, data reported among breast, prostate, or lung cancer survivors demonstrate more adverse outcomes when symptoms are not managed early in cancer survivorship, which may be true for CRC survivors, too. However, there is a dearth of knowledge about symptoms (occurrence, frequency, and severity) among CRC survivors during acute cancer survivorship. Therefore, it is crucial to understand the symptom occurrence, frequency, and severity among CRC survivors during the acute phase of survivorship, to inform strategies to improve health-related outcomes in the CRC population. The purpose of this study was to examine CRC survivors’ symptom occurrence, frequency, and severity during acute cancer survivorship.
Methods
Design and Sample
A total of 117 CRC survivors undergoing active treatment who met inclusion criteria were recruited from a NCI–designated cancer center in South Florida. A cross-sectional study was conducted from April 2021 to August 2021. The institutional review board at the University of Miami approved the study, and electronic informed consent and Health Insurance Portability and Accountability Act of 1996 (HIPAA) waivers were obtained prior to data collection. Study procedures were completed at the clinical setting or over a HIPAA-compliant virtual platform. Participants completed the electronic study surveys at their preferred location (e.g., clinical setting, at home) to ensure comfort and privacy.
Inclusion criteria were as follows: (a) aged at least 18 years, (b) able to comprehend English, (c) diagnosed with colon, rectal, or colorectal cancer, and (d) were undergoing active treatment (e.g., chemotherapy, radiation, surgical resection, adjuvant therapy). Exclusion criteria were as follows: (a) diagnosed with severe mental illness (e.g., active bipolar disorder, schizophrenia), (b) completed cancer treatment, documented as terminally ill, or initiated hospice, comfort care, or end-of-life measures, and (c) unable or unwilling to give informed consent.
Instruments
Instruments with established scoring procedures and evidence of validity among mixed cancer survivors were used in this study. All self-report measures were completed in REDCap, an electronic database (Harris et al., 2019). The demographic questionnaire, a 25-item self-report questionnaire used to assess participant demographic information, included age, marital status, income, education level, employment status, cancer treatment history, and diagnosis questions. A modified Therapy-Related Symptom Checklist (Williams et al., 2013, 2015) of 49 symptoms was used to assess the participants’ symptom occurrence, frequency (randomly [1–3 times], weekly [4–6 times], almost daily [7–9 times], and daily [10 or more times]), and severity (mild, moderate, severe, and very severe). The original checklist has shown good internal consistency with Cronbach’s alpha above 0.8 in samples of different types of cancer survivors (n = 110 and 385) (Mansouri et al., 2017; Sreedhar, 2021; Williams et al., 2013, 2015). The Therapy-Related Symptom Checklist was modified by the authors to include additional symptoms (n = 24) common among CRC survivors (e.g., mucus in the stool, feeling pressure in gastrointestinal [GI] tract, anal pain).
Statistical Analysis
GPower, version 3.1 (Faul et al., 2009), was used to estimate the post-hoc power and sample size for the research study. A power of 0.99 was achieved with 117 participants for a bivariate model with an effect size of 0.2 and α = 0.05. All statistical analyses were conducted using IBM SPSS Statistics, version 22.0. A p value less than 0.05 was considered statistically significant.
Measures of central tendencies and percentages were obtained to determine the study sample demographics and symptom characteristics. For between-group differences in symptom burden (number of symptoms) based on sex, the Mann-Whitney U test (Hart, 2001) was performed. For between-group differences in symptom frequency (average symptom frequency per survivor) and symptom severity (average symptom severity per survivor) based on age, education, and months since diagnosis, the Kruskal-Wallis H test (Ostertagová et al., 2014) was performed. Nonparametric statistic tests were used to test noncontinuous data.
A principal component analysis (PCA) with varimax rotation (Watkins, 2018) was performed to explore symptom clustering. All 49 symptoms based on occurrence were included in the PCA. Factor structure was guided by the scree plot and eigenvalues. Recommendations from Costello and Osborne were used for interpreting the scree plot, and eigenvalues greater than 1 were used for factor retention according to Kaiser criteria (Costello & Osborne, 2005; Watkins, 2018). Factor loadings were determined using a priori criteria; primary factor loadings on one factor and primary factor loadings greater than 0.4 (Cortina, 1993). Items not meeting the criteria were removed, and the factor was labeled according to items that met criteria. PCA has been used across cancer survivor populations and has been shown to identify specific symptom clusters based on the particular cancer type, location, and treatments (Dirksen et al., 2016; Lee et al., 2020; Sheikh-Wu et al., 2020).
Results
Sample Characteristics
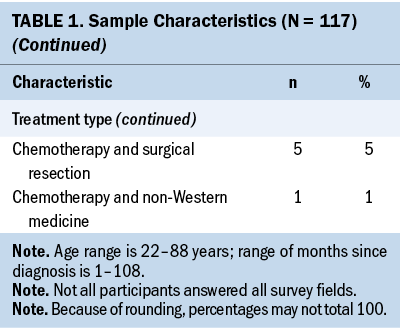
The study consisted of 117 survivors of colon (n = 77, 66%), rectal (n = 12, 10%), and colorectal (n = 28, 24%) cancers. The sample’s mean age was 55 years and was 44% women (n = 51) (see Table 1).
Symptom Characteristics
The Therapy-Related Symptom Checklist assessed 49 symptoms based on occurrence, frequency, and severity. Table 2 lists all symptoms and provides a rank order based on symptom occurrence; symptom frequency and severity are also reported. The most common symptoms, in order of highest occurrence rate, were as follows: (a) neuropathy of the hands/feet, (b) feeling sluggish/fatigue, (c) skin changes—sensitivity, discoloration, texture, and dryness, (d) sleep disturbances, and (e) generalized weakness.
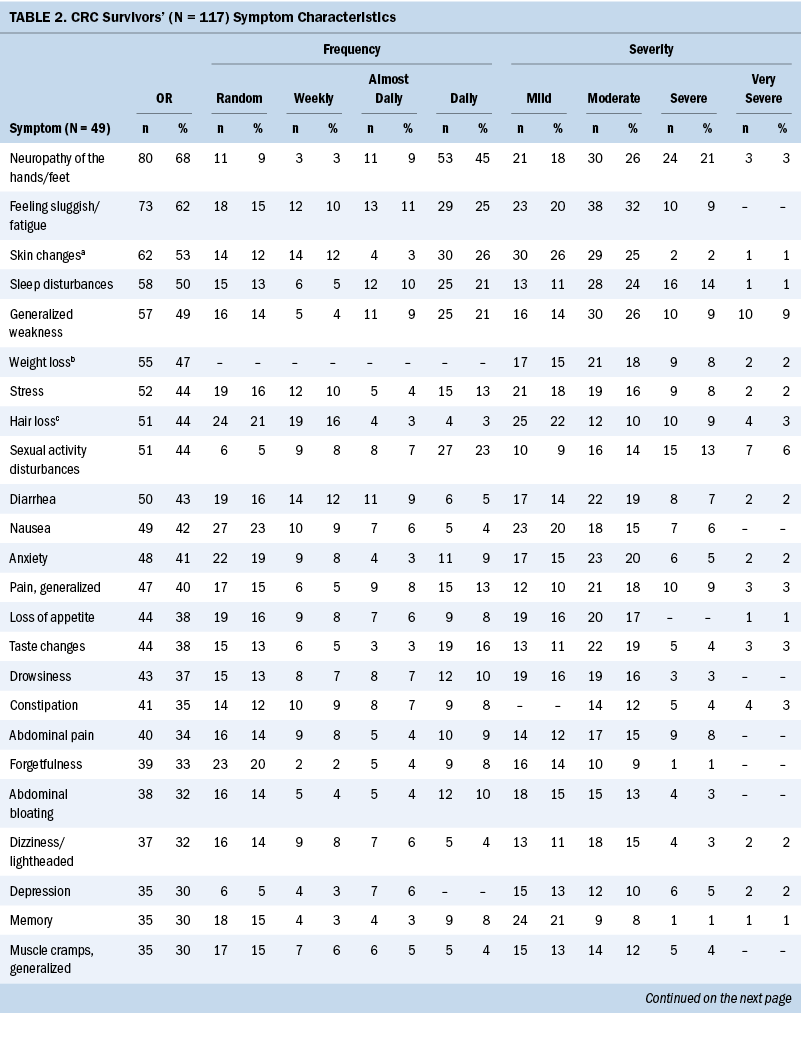
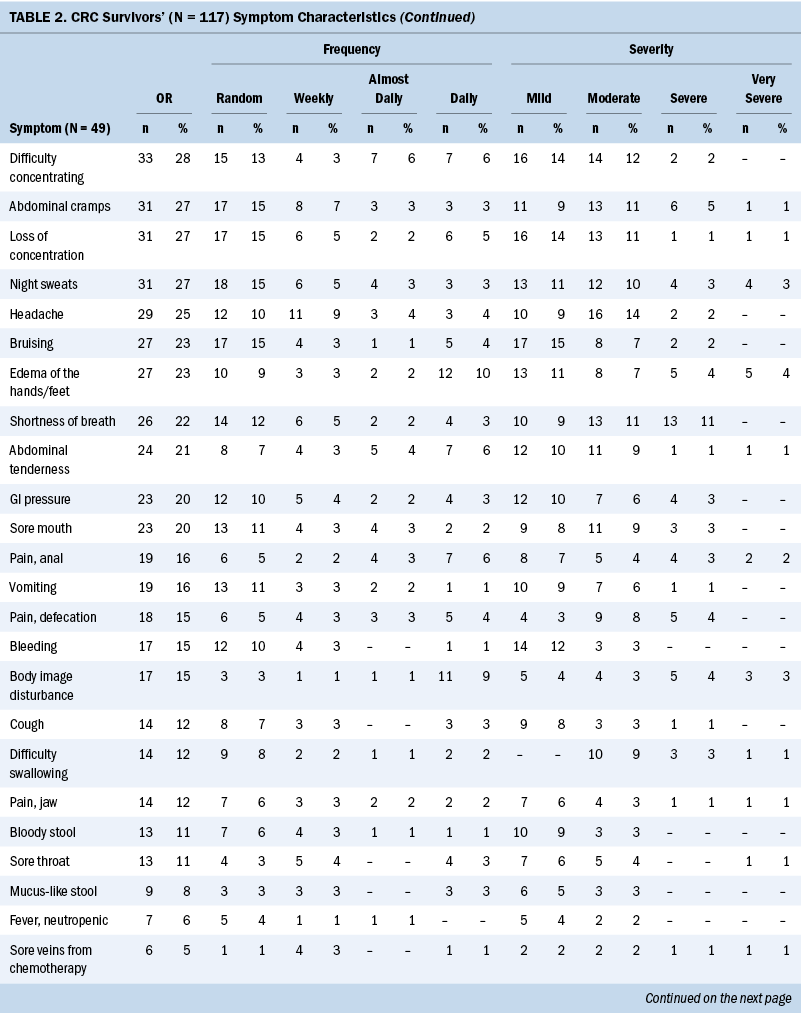
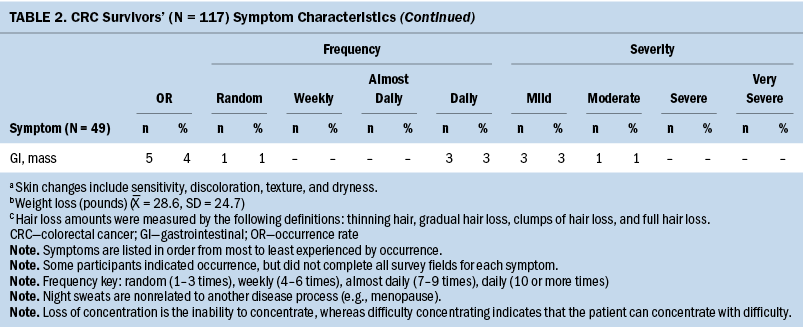
Most participants were undergoing chemotherapy (n = 87, 85%). The remainder of survivors were being treated by adjuvant therapy (n = 12, 12%) (e.g., chemotherapy paired with radiation therapy, surgical resection, or non-Western medicine), surgical resection (n = 2, 2%), and radiation therapy (n = 1, 1%). Common chemotherapy-related symptoms, in order of highest occurrence rate, were as follows: (a) neuropathy of the hands/feet, (b) feeling sluggish/fatigue, (c) skin changes—sensitivity, discoloration, texture, and dryness, (d) sleep disturbances, and (e) weight loss (see Supplemental Table 1).

The sample primarily consisted of colon cancer survivors (n = 77, 66%). The team assessed occurrence, frequency, and severity of symptoms reported among each cancer diagnosis (see Supplemental Table 2). Colon and CRC survivors’ common symptoms, in order of highest occurrence rate, were as follows: (a) neuropathy of the hands/feet, (b) feeling sluggish/fatigue, (c) sleep disturbances or sexual activity disturbances, and (d) weight loss. Rectal cancer survivors’ experienced symptoms, in order of highest occurrence rate, were as follows: (a) generalized weakness, (b) skin changes—sensitivity, discoloration, texture, and dryness, (c) neuropathy of the hands/feet, (d) feeling sluggish/fatigue, and (e) sexual activity disturbances.
Group Difference and Symptom Characteristics
The team assessed for between-group differences in symptom burden, frequency, and severity, based on sex, age, education, and months since diagnosis. The only significant between-group difference was observed between months since diagnosis and number of symptoms (p = 0.03). With regards to the number of symptoms and months since diagnosis, 14.27 symptoms were reported at 6 months, 11.21 symptoms at 7–12 months, 10.88 symptoms at 13–18 months, 14.68 symptoms at 19–24 months, and 16.58 symptoms at more than 25 months.
Exploratory Factor Analysis
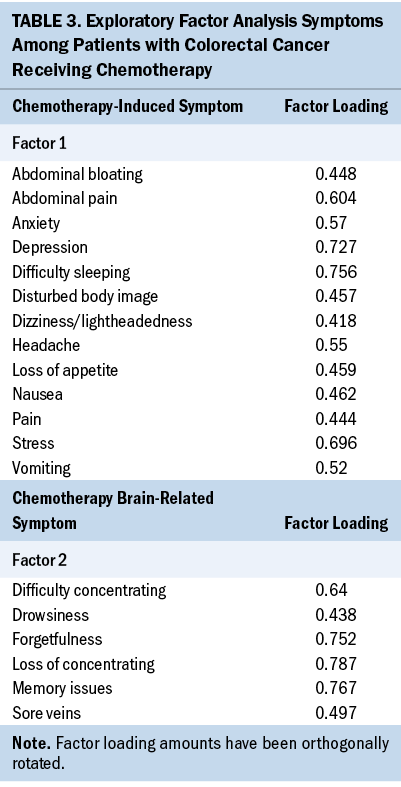
PCA was performed to explore factor loading of symptoms during acute cancer survivorship among CRC survivors. Two factors were identified based on the compositions of the symptoms, which were named to reflect the two symptom clusters. The two symptom clusters were as follows: (a) chemotherapy-induced symptoms (e.g., pain, loss of appetite, nausea, vomiting, difficulty sleeping and concentrating, depression, stress, anxiety, disturbed body image, abdominal pain and bloating, dizziness/lightheadedness, headache), and (b) chemotherapy brain-related symptoms (e.g., difficulty concentrating, loss of concentration, sore veins, forgetfulness, drowsiness, memory issues). Table 3 shows the factor loadings associated with each symptom within an identified symptom cluster.
Discussion
This is one of only a few studies to assess the symptom occurrence, frequency, and severity among CRC survivors during acute cancer survivorship. CRC survivors will likely undergo two or more types of treatments, depending on their cancer prognosis, and will encounter adverse symptoms at least once during acute cancer survivorship (Sreedhar, 2021). Therefore, a better understanding of CRC survivors’ symptom occurrence, frequency, and severity will directly help healthcare teams to systematically track and prioritize survivor-reported symptoms, which can improve survivors’ awareness of influencing factors that may worsen symptom burden, frequency, and severity throughout their treatment. Early identification of symptoms by survivors and healthcare teams may promote the survivors’ self-management skills, mitigating the adverse side effects of treatments during acute cancer survivorship. However, it is necessary for nurse researchers to examine CRC survivors’ symptoms at a larger scale. Research at multisite facilities that include diverse CRC populations should be considered a priority to determine symptom occurrence, frequency, severity, and potential outcome differences over longitudinal studies throughout acute cancer survivorship.
The demographics of the sample, particularly age, sex, cancer stage, and education levels, reflect the national CRC population statistics (ACS, 2021b; Siegel et al., 2020). This sample’s age range (21–88 years) reflected the national CRC population (ACS, 2019; NCI, 2021). There has been a rise in new CRC survivors aged younger than 55 years, and research indicates that young CRC survivors are diagnosed with more advanced stages (III and IV) when compared to survivors aged older than 55 years (ACS, 2020a; Howlader et al., 2019; Siegel et al., 2019). In this study, the mean age was 55 years, indicating that half of the sample was aged younger than 55 years, and the majority of those participants had stage IV CRC. This is consistent with the national statistics. In addition, CRC incidence rates are higher in men, which was true for this sample (n = 66, 56% men) (ACS, 2021a). The sample reflected the education levels seen in the national CRC data, with the majority of survivors having a high school diploma or having an associate or bachelor’s degree (ACS, 2021b; Siegel et al., 2020).
The sample’s cancer stage (stage IV: n = 93, 79%) and treatment type (chemotherapy: n = 87, 85%) coincided with the national CRC data. In the United States, more than 60% of people with CRC are diagnosed with stage II, III, or IV (NCI, 2021), and more than 65% of CRC survivors undergo chemotherapy treatment (solo or adjuvant) (ACS, 2021a). Depending on cancer type and location, CRC survivors may continue maintenance or oral chemotherapy treatment until disease progression, and survivors with a higher stage (III or IV) are more likely to undergo multiple treatments (ACS, 2020b). For example, colon, CRC, and rectal cancer survivors with stage IV may undergo surgical resection in conjunction with chemotherapy and radiation because of metastases (e.g., liver, lung) (ACS, 2020b). For these reasons, the percentage of survivors undergoing chemotherapy is reflective of late-stage CRC and the CRC population in the United States. In addition, these data suggest that during acute cancer survivorship, the type of treatment contributes the most to symptoms rather than cancer diagnosis.
This sample is reflective of CRC survivors and the treatments used in the United States. Therefore, the data related to symptom occurrence, frequency, and severity may be applicable across settings where protocol-driven chemotherapy decisions are used. Chemotherapy is a significant contributor to CRC survivors’ symptoms (e.g., peripheral neuropathy, fatigue/feeling sluggish, skin changes), and data from multiple studies converged to show that common symptoms occur during chemotherapy independent of cancer diagnosis (Röhrl et al., 2019; Sheikh-Wu et al., 2021; Sreedhar, 2021; Wu et al., 2021). In this study, the exploratory factor analysis identified two factors associated with chemotherapy. Although not fully powered for a robust PCA, the preliminary findings are consistent with other reports of symptoms related to chemotherapy (Albusoul et al., 2017; Lee et al., 2020; Miaskowski et al., 2017). This has implications for nursing. In particular, nurses should develop symptom-management strategies focused on common symptoms during chemotherapy that could be employed across cancer diagnoses, evaluate these strategies not only for efficacy but for tailoring these nurse-driven interventions, and evaluate the effects of symptoms and management strategies on short, intermediate, and long-term outcomes.
The researchers added additional items related to GI symptoms (e.g., diarrhea, disturbed body image, abdominal bloating, pain, cramps, pressure, mass/lump, tenderness, bloody stool, anal pain, mucus in stool, painful defecation) to the Therapy-Related Symptom Checklist because of the nature of CRC. One-third of the participants reported GI symptoms of constipation, abdominal pain, bloating, cramping, tenderness, pressure, and anal pain during defecation. This study suggests that GI symptoms were predominantly seen early during radiation therapy among rectal cancer survivors. However, GI symptoms may have been caused by the treatment type, cancer diagnosis, or from the time since diagnosis during survivors’ acute cancer survivorship. Because one-third of the sample reported these symptoms and it is unclear if any treatment exacerbated them, it is important to include these items when assessing symptoms among CRC survivors. The addition of these items to the questionnaire enhanced the understanding of symptoms during acute cancer survivorship in this population. Had this not been done, potentially important symptoms affecting patients in acute survivorship would not be known.
In addition, the number of symptoms varied according to length of time across acute cancer survivorship. There was an increase in symptoms corresponding with a greater length of time in acute survivorship, suggesting that symptom burden accumulates during treatment. Observations about symptom burden throughout acute cancer survivorship are not well known in the CRC literature. Studies suggest that symptoms worsen among all cancer types, particularly toward the end of life (increase in symptom occurrence, frequency, and severity) (Goldberg et al., 2019; Seow et al., 2020). However, longitudinal studies are needed to expand the understanding of CRC survivors’ symptom burden in acute survivorship. Understanding the changes within symptom burden throughout treatment among CRC survivors would allow researchers to develop preventive care strategies to identify and treat the most bothersome symptoms, and could be tailored to other cancer diagnoses.
Limitations
This study had several limitations. Because this was a cross-sectional study, data were obtained at a single point in time, making it difficult to assess symptom trajectories. However, this concern was minimized by sampling CRC survivors across the acute cancer survivorship continuum. Future work should include prospective, repeated measures to best identify symptom trajectories among CRC survivors based on treatment type, cancer stage, and cancer type. This sample under-represented non-Hispanic Black patients, which may limit generalizability. However, the sample was reflective of the national CRC data related to age, sex, cancer stage, and education levels. In addition, this sample is reflective of the south Florida population with a higher number of Hispanic CRC survivors when compared to the national statistics.
Implications for Nursing
Oncology nurses are well poised to identify, prevent, and promote self-management skills to reduce adverse symptoms among CRC survivors. Early identification and prioritization of survivor-reported symptoms may improve survivors’ awareness of factors that may worsen symptoms throughout acute cancer survivorship. In addition, early recognition provides the opportunity for nurses to promote evidence-based self-management skills to mitigate symptoms during acute cancer survivorship. In addition, GI-specific and treatment-related symptoms should be assessed in patients with CRC during acute cancer survivorship, as these could be missed in some standardized symptom surveys. Finally, longitudinal studies are needed to evaluate symptoms and symptom trajectories among patients with CRC during and after acute cancer survivorship to obtain a better understanding of the symptoms and their effects on survivorship.
Conclusion
This study evaluated 49 symptoms to better understand CRC survivors’ symptom characteristics by occurrence, frequency, and severity during acute cancer survivorship. This study is one of the first to gather new insight regarding CRC survivors’ symptom characteristics throughout acute cancer survivorship. Additional understanding of CRC survivors’ symptom occurrence, frequency, and severity throughout treatment is needed and will help nurses to systematically track and prioritize symptoms. Regardless of cancer type, recognizing symptoms that cluster together may improve survivors’ awareness of influencing factors that may worsen symptom burden over time. Additional studies are needed to identify key places for prevention and self-management strategies to reduce symptom burden among CRC survivors.
About the Authors
Sameena F. Sheikh-Wu, PhD, BA, BSN, RN-BC, is a postdoctoral student, Debbie Anglade, PhD, RN, MSN, CPHQ, CCM, is an associate professor of clinical nursing, and Karina Gattamorta, PhD, is a research associate professor, all in the School of Nursing and Health Studies at the University of Miami in Florida; Canhua Xiao, PhD, RN, FAAN, is an associate professor in the Nell Hodgson Woodruff School of Nursing at Emory University in Atlanta, GA; and Charles A. Downs, PhD, ACNP-BC, FAAN, is an associate professor in the School of Nursing and Health Studies at the University of Miami. No financial relationships to disclose. Sheikh-Wu, Anglade, Xiao, and Downs contributed to the conceptualization and design. Sheikh-Wu completed the data collection. Sheikh-Wu and Gattamorta provided statistical support and analysis. All authors contributed to the manuscript preparation. Sheikh-Wu can be reached at sfs23@miami.edu, with copy to ONFEditor@ons.org. (Submitted October 2021. Accepted February 8, 2022.)
References
Albusoul, R.M., Berger, A.M., Gay, C.L., Janson, S.L., & Lee, K.A. (2017). Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. Journal of Pain and Symptom Management, 53(5), 880–886. https://doi.org/10.1016/j.jpainsymman.2016.12.339
American Cancer Society. (2019). Cancer facts and figures, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
American Cancer Society. (2020a). Colorectal cancer rates rise in younger adults. https://www.cancer.org/latest-news/colorectal-cancer-rates-rise-in-youn…
American Cancer Society. (2020b). Treatment of colon cancer, by stage. https://www.cancer.org/cancer/colon-rectal-cancer/treating/by-stage-col…
American Cancer Society. (2021a). Cancer facts and figures, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
American Cancer Society. (2021b). Survival rates for colorectal cancer. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-s…
Barsevick, A. (2016). Defining the symptom cluster: How far have we come? Seminars in Oncology Nursing, 32(4), 334–350. https://doi.org/10.1016/j.soncn.2016.08.001
Cortina, J.M. (1993). What is coefficient alpha? An examination of theory and applications. Journal of Applied Psychology, 87(1), 98–104. https://doi.org/10.1037/0021-9010.78.1.98
Costello, A.B., & Osborne, J.W. (2005). Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment Research and Evaluation, 10(10), 1–7. https://doi.org/10.7275/jyj1-4868
Deshields, T.L., Potter, P., Olsen, S., & Liu, J. (2014). The persistence of symptom burden: Symptom experience and quality of life of cancer patients across one year. Supportive Care in Cancer, 22(4), 1089–1096. https://doi.org/10.1007/s00520-013-2049-3
Dirksen, S.R., Belyea, M.J., Wong, W., & Epstein, D.R. (2016). Transitions in symptom cluster subgroups among men undergoing prostate cancer radiation therapy. Cancer Nursing, 39, 3–11. https://doi.org/10.1016/j.jpainsymman.2015.02.016
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. https://doi.org/10.3758%2Fbrm.41.4.1149
Goldberg, S.L., Paramanathan, D., Khoury, R., Patel, S., Jagun, D., Arunajadai, S., . . . Pecora, A.L. (2019). A patient-reported outcome instrument to assess symptom burden and predict survival in patients with advanced cancer: Flipping the paradigm to improve timing of palliative and end-of-life discussions and reduce unwanted health care costs. Oncologist, 24(1), 76–85. https://doi.org/10.1634/theoncologist.2018-0238
Harris, P.A., Taylor, R., Minor, B.L., Elliott, V., Fernandez, M., O’Neal, L., . . . Duda, S.N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. https://doi.org/10.1016/j.jbi.2019.103208
Hart, A. (2001). Mann-Whitney test is not just a test of medians: Differences in spread can be important. British Medical Journal, 323(7309), 391. https://doi.org/10.1136%2Fbmj.323.7309.391
Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Brest, A., Yu, M., . . . Cronin, K.A. (2019). SEER cancer statistics review (CSR) 1975–2016. National Cancer Institute. https://seer.cancer.gov/archive/csr/1975_2016/
Juul, J.S., Hornung, N., Andersen, B., Laurberg, S., Olesen, F., & Vedsted, P. (2018). The value of using the faecal immunochemical test in general practice on patients presenting with non-alarm symptoms of colorectal cancer. British Journal of Cancer, 119(4), 471–479. https://doi.org/10.1038/s41416-018-0178-7
Lee, L.J., Ross, A., Griffith, K., Jensen, R.E., & Wallen, G.R. (2020). Symptom clusters in breast cancer survivors: A latent class profile analysis. Oncology Nursing Forum, 47(1), 89–100. https://doi.org/10.1188/20.ONF.89-100
Mansouri, A., Motaghedi, R., Rashidian, A., Ashouri, A., Kagrar, M., Hajibabaei, . . . Ansari, S. (2017). Validity and reliability assessment of the Persian version of Therapy-Related Symptom Checklist. Iranian Journal of Medical Sciences, 42(3), 292–300.
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P.A., Jacobsen, P., . . . Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of National Cancer Institute, 109(4), djw253. https://doi.org/10.1093/jnci/djw253
Mosher, C.E., & DuHamel, K.N. (2012). An examination of distress, sleep, and fatigue in metastatic breast cancer patients. Psycho-Oncology, 21(1), 100–107. https://doi.org/10.1002/pon.1873
Mullan, F. (1985). Seasons of survival: Reflections of a physician with cancer. New England Journal of Medicine, 313(4), 270–273. https://doi.org/10.1056/NEJM198507253130421
National Cancer Institute. (2021). Cancer stat facts: Colorectal cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/colorect.html
O’Gorman, C., Stack, J., O’Ceilleachair, A., Denieffe, S., Gooney, M., McKnight, M., & Sharp, L. (2018). Colorectal cancer survivors: An investigation of symptom burden and influencing factors. BMC Cancer, 18(1). https://doi.org/10.1186/s12885-018-4923-3
Ostertagová, E., Ostertag , O., & Kováč, J. (2014). Methodology and application of the Kruskal-Wallis Test. Applied Mechanics and Materials, 611, 115–120. https://doi.org/10.4028%2Fwww.scientific.net%2Famm.611.115
Rasmussen, S., Larsen, P.V., Søndergaard, J., Elnegaard, S., Svendsen, R.P., & Jarbøl, D.E. (2015). Specific and non-specific symptoms of colorectal cancer and contact to general practice. Family Practice, 32(4), 387–394. https://doi.org/10.1093/fampra/cmv032
Röhrl, K., Guren, M.G., Miaskowski, C., Cooper, B.A., Diep, L.M., & Rustøen, T. (2016). No differences in symptom burden between colorectal cancer patients receiving curative versus palliative chemotherapy. Journal of Pain Symptom Management, 52(4), 539–547. https://doi.org/10.1016/j.jpainsymman.2016.04.008
Röhrl, K., Guren, M.G., Småstuen, M.C., & Rustøen, T. (2019). Symptoms during chemotherapy in colorectal cancer patients. Supportive Care in Cancer, 27(8), 3007–3017. https://doi.org/10.1007/s00520-018-4598-y
Schuh-Hofer, S., Eichhorn, N., Grinevich, V., & Treede, R.-D. (2018). Sleep deprivation related changes of plasma oxytocin in males and female contraceptive users depend on sex and correlate differentially with anxiety and pain hypersensitivity. Frontiers in Behavioral Neuroscience, 12. https://doi.org/10.3389/fnbeh.2018.00161
Seow, H., Stevens, T., Barbera, L.C., Burge, F., McGrail, K., Chan, K.K.W., . . . Guthrie, D.M. (2020). Trajectory of psychosocial symptoms among home care patients with cancer at end-of-life. Psycho-Oncology, 30(1), 103–110. https://doi.org/10.1002/pon.5559
Sheikh-Wu, S.F., Downs, C.A., & Anglade, D. (2020). Interventions for managing a symptom cluster of pain, fatigue, and sleep disturbances during cancer survivorship: A systematic review. Oncology Nursing Forum, 47(4), E107–E119. https://doi.org/10.1188%2F20.onf.e107-e119
Sheikh-Wu, S.F., Kauffman, M.A., Anglade, D., Shamsaldeen, F., Ahn, S., & Downs, C.A. (2021). Effectiveness of different music interventions on managing symptoms in cancer survivors: A meta-analysis. European Journal of Oncology Nursing, 52, 101968.
Siegel, R.L., Miller, K.D., Goding Sauer, A., Fedewa, S.A., Butterly, L.F., Anderson, J.C., . . . Jemal, A. (2020). Colorectal cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(3), 145–164. https://doi.org/10.3322/caac.21601
Siegel, R.L., Miller, K.D., & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34.
Siminoff, L., Thomson, M., & Dumenci, L. (2014). Factors associated with delayed patient appraisal of colorectal cancer symptoms. Psycho-Oncology, 23(9), 981–988. https://doi.org/10.1002/pon.3506
Sreedhar, J.S. (2021). Symptom occurence, severity, and self-care methods by ethnicity and age group among adults with cancer. Oncology Nursing Forum, 48(5), 522–534.
Stark, L.L., Tofthagen, C., Visovsky, C., & McMillan, S.C. (2012). The symptom experience of patients with cancer. Journal of Hospice nd and Palliative Nursing, 14(1), 61–70.
Tantoy, I.Y., Cataldo, J.K., Aouizerat, B.E., Dhruva, A., & Miaskowski, C. (2016). A review of the literature on multiple co-occurring symptoms in patients with colorectal cancer who received chemotherapy alone or chemotherapy with targeted therapies. Cancer Nursing, 39(6), 437–445.
Watkins, M.W. (2018). Exploratory factor analysis: A guide to best practice. Journal of Black Psychology, 44(3), 219–246. https://doi.org/10.1177/0095798418771807
Williams, A.R., Williams, D.D., Williams, P.D., Alemi, F., Hesham, H., Donley, B., & Kheirbek, R.E. (2015). The development and application of an oncology Therapy-Related Symptom Checklist for Adults (TRSC) and Children (TRSC-C) and e-health applications. BioMedical Engineering OnLine, 14(Suppl. 2), S1.
Williams, P.D., Graham, K.M., Storlie, D.L., Pedace, T.M., Haeflinger, K.V., Williams, D.D., . . . Williams, A.R. (2013). Therapy-Related Symptom Checklist use during treatments at a cancer center. Cancer Nursing, 36(3), 245–254.
Wu, C.-J., Huang, K.-J., Tsai, Y.-C., Yeh, T.-P., Hsieh, C.-F., & Wang, Y.-J. (2021). Peripheral neuropathy: Comparison of symptoms and severity between colorectal cancer survivors and patients with diabetes. Clinical Journal of Oncology Nursing, 25(4), 395–403. https://doi.org/10.1188/21.CJON.395-403
Xiao, C. (2010). The state of science in the study of cancer symptom clusters. European Journal of Oncology Nursing, 14(5), 417–434. https://doi.org/10.1016/j.ejon.2010.05.011