Auricular Point Acupressure to Manage Aromatase Inhibitor–Induced Arthralgia in Postmenopausal Breast Cancer Survivors: A Pilot Study
Purpose/Objectives: To assess the feasibility of auricular point acupressure to manage aromatase inhibitor–induced arthralgia.
Design: Wait list control design.
Setting: Outpatient clinics and oncology center.
Sample: 20 women with aromatase inhibitor–induced arthralgia.
Methods: After baseline data were collected, participants waited one month before they received acupressure once per week for four weeks at a convenient time. The baseline data served as the control comparison. Self-reported measures and blood samples were obtained at baseline, at preintervention, weekly during the intervention, and at post-intervention.
Main Research Variables: The primary outcomes included pain intensity, pain interference, stiffness, and physical function. Inflammatory cytokines and chemokines were tested.
Findings: After the four-week intervention, participants reported decreases in worst pain and pain interference, and improvements in physical function, cancer-related symptom severity, and interference. The proinflammatory cytokines and chemokines displayed a trend of a mean percentage reduction. The anti-inflammatory cytokine interleukin-13 increased from pre- to postintervention.
Conclusions: Auricular point acupressure is feasible and may be effective in managing arthralgia in breast cancer survivors.
Implications for Nursing: Nurses can administer acupressure in clinical settings, which could enhance the management of aromatase inhibitor–induced arthralgia and contribute to a shift from traditional disease-based biomedical models to a broader, integrative, medical paradigm for managing aromatase inhibitor–induced arthralgia.
Jump to a section
Aromatase inhibitor (AI) therapy has become an important standard of care for postmenopausal breast cancer survivors (PBCS) (Early Breast Cancer Trialists’ Collaborative Group, 2015). Adherence to this adjuvant endocrine therapy is an essential part of the multimodality treatment regimen of hormone-responsive breast cancer (Burstein et al., 2014; Hershman et al., 2010, 2011); however, adherence is challenging for patients because AI therapy requires daily use of an oral medication that must be continued for five years or longer (Hershman et al., 2010, 2011; Murphy, Bartholomew, Carpentier, Bluethmann, & Vernon, 2012). AI-induced arthralgia (AIA), particularly its high pain intensity, is a major challenge for optimal adherence to AI therapy (Hershman et al., 2010, 2011; Hershman, Loprinzi, & Schneider, 2015) and contributes to a 20%–50% rate of premature discontinuation (Henry et al., 2008, 2012; Howell et al., 2005; Mao et al., 2009; Presant et al., 2007). No effective treatment for AIA has been established (Hershman et al., 2015). The use of nonsteroidal anti-inflammatory drugs (NSAIDs), switching to an alternative AI, drug holidays, or exercise are common strategies to manage AIA (Hershman et al., 2015); however, NSAIDS are not particularly effective against AIA and have their own adverse effects (Benyamin et al., 2008; Glare et al., 2014). Exercise is too burdensome for many PBCS (Irwin et al., 2015), which limits its use.
Auricular point acupressure (APA), one type of auricular therapy, has a long history in traditional Chinese medicine and was first brought to the attention of Western medical providers by Paul Nogier, MD, in 1957 (Nogier, 1981, 1987, 2014). Nogier provided a new theoretical underpinning for traditional APA that is considered a specialized form of acupressure in traditional Chinese medicine. The ear is viewed as a microsystem of the body (Oleson, 2014), and specific acupoints on the ear are stimulated without using needles to achieve therapeutic effects (Yeh, Chien, & Suen, 2014). The therapeutic benefits of auricular therapy for pain have been recognized by the World Health Organization (1990). The underlying theory of auricular therapy posits that nerves in the outer ear correspond to specific areas of the brain, and these areas have a reflex connection with specific parts of the body (Huang, 2005; Oleson, 2014). This correlation of ear points and brain activity has been validated by functional magnetic resonance imaging (Alimi, Geissmann, & Gardeur, 2002; Romoli et al., 2014).
Although the exact etiology of AIA remains unclear, the precipitous decline in estrogen levels following initiation of AI therapy (Hannan, Felson, Anderson, Naimark, & Kannel, 1990; Hu, Shuang, Zou, & Yang, 2015; Mao et al., 2011; Nevitt et al., 1996; Prieto-Alhambra et al., 2015) and the consequent regulating of immune cells and cytokines related to joint pain (Burstein, 2007; Kramer, Kramer, & Guan, 2004) may be potential mechanisms. Estrogen has naturally antinociceptive properties that are believed to be mediated by opioid-containing neurons in the spinal cord that express estrogen receptors (Dawson-Basoa & Gintzler, 1998). Evidence suggests that the proinflammatory cytokines (interleukin [IL]-1, IL-6, and tumor necrosis factor-alpha) are spontaneously elevated in the first few years after menopause, a time when the natural incidence of joint symptoms is high (Din, Dodwell, Wakefield, & Coleman, 2010). Estrogen levels are also associated with levels of inflammatory cytokine production (Islander, Jochems, Lagerquist, Forsblad-d’Elia, & Carlsten, 2011).
Therefore, the purposes of this study were to examine (a) the feasibility of an easily administered APA intervention to manage AIA, (b) patient adherence to APA practice, and (c) initial effectiveness of the APA intervention for AIA.
Methods
A wait list control pilot study was conducted to examine the feasibility of a four-week APA intervention. A recruitment letter with study information was mailed to 73 women enrolled in a descriptive study of adjuvant treatment and cardiovascular risk in PBCS. After consent was obtained and baseline data were collected, participants waited a month and then completed the same data questionnaire before they started to receive a four-week APA intervention. Outcomes were assessed after completion of the four-week APA.
Participants
PBCS were eligible for the study if they met the following criteria: (a) were postmenopausal women with a history of breast cancer (non-metastatic); (b) were currently receiving AIs (anastrazole [Arimidex®], letrozole [Femara®], exemestane [Aromasin®]), according to chart documentation, for at least two months (This decreases the likelihood of the patient needing a drug holiday or switching to another AI treatment, which usually occurs within four to eight weeks of the initiation of AI therapy in the authors’ clinical settings, or concurrent treatment with other/newer/similar agents with different side effect profiles.); (c) were able to read and write English; (d) had joint pain attributable to AI, or had preexisting joint pain that worsened after the initiation of AIs, and had worst joint pain rated as 4 or more on a 0–10 numeric rating scale in the previous week; (e) were willing to commit to weekly study visits for four weeks during the intervention and follow-up visits; and (f) were able to apply pressure to the seeds taped to the ears.
Participants were excluded if they (a) had metastatic breast cancer; (b) had finished cytotoxic chemotherapy and/or radiation therapy fewer than four weeks prior to enrollment (because chemotherapy and radiation therapy can cause temporary exacerbation of joint symptoms that typically resolve spontaneously); (c) had bone fracture/surgery of an affiliated extremity during the preceding six months; (d) were using corticosteroids or narcotics; (e) had ear skin disease; (f) had an allergy to the tape used for the study; (g) had previous auricular therapy (because they would be unable to be blinded for the study); or (h) had been hospitalized for mental health reasons within the previous three months.
Auricular Point Acupressure Intervention Protocol
The APA protocol adhered to the International Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines (MacPherson et al., 2010). Auricular diagnosis, an objective and systematic procedure (Yeh & Huang, 2013), was used to locate ear points. With this diagnosis, the search for reactive ear points begins within an ear zone area that corresponds to body locations (Oleson, 2014). Specific points were then located by the Chinese Standard Ear-Acupoints Chart (Chinese Academy of Acupuncture-Moxibustion, 2008). The points for intervention comprised points corresponding to the body pain location (i.e., arm, knee, or foot, depending on patient’s pain location) and three points know for alleviating stress and pain (i.e., shenmen, sympathetic, and nervous subcortex). Bilateral auricular points were identified for treatment. Vaccaria seeds (natural, nontoxic botanical seeds of no medicinal value, roughly 2 mm in diameter) were placed on the ear points for stimulation, and small pieces of waterproof tape were used to secure the seeds onto the ears.
Measures
Primary outcomes: Primary outcomes included pain intensity, pain interference, stiffness, and physical function. The Brief Pain Inventory–Short Form (BPI-SF) (Cleeland & Ryan, 1994) was used to assess the severity of pain, the impact of pain on daily function (pain interference), the location of pain, any pain medications being used, and the amount of pain relief. The Quick Disabilities of the Arm, Shoulder, and Hand score (QuickDASH) (Beaton, Wright, & Katz, 2005) was used to assess the perceived level of disability in participants’ upper extremities. The stiffness and physical function subscales of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Bellamy, 2002) were used. Participants were asked to rate their conditions during the previous seven days, with high scores representing worse symptom severity.
Secondary outcomes: The two-item Pain Self-Efficacy Questionnaire (PSEQ-2) (Nicholas, McGuire, & Asghari, 2015) is a short version of the PSEQ (Nicholas, 2007) that assesses participants’ confidence in their ability to carry out activities, even when in pain. The questions are scored on a seven-point Likert-type scale ranging from 0 (not at all confident) to 6 (completely confident).
The Acupressure Expectancy Scale (AES) was adapted from the Acupuncture Expectancy Scale (Mao, Xie, & Bowman, 2010) to assess participants’ specific expectation about the outcomes of APA. AES is a four-item scale scored from 1 (not at all agree) to 5 (completely agree), with good reliability and known group validity (mean = 11.5 in previously used acupuncture; mean = 9.5 in acupuncture-naive patients; p = 0.002) (Mao et al., 2010). The internal consistency is good in the current study (Cronbach alpha = 0.86).
The MD Anderson Symptom Inventory (MDASI) (Cleeland et al., 2000) is a 13-item assessment of cancer-related symptoms and a 6-item assessment of symptom interference (13 items accounted for 64% of the variance in symptom distress).
The Patient-Reported Outcomes Measurement Information System (PROMIS)-29 assesses quality of life by addressing the following domains: physical function, anxiety, depression, fatigue, sleep disturbance, and social functioning (Craig et al., 2014; PROMIS Health Organization and PROMIS Cooperative Group, 2016). Known group validity for PROMIS was demonstrated with large effect sizes (pain intensity: 1.42; pain interference: 1.25; fatigue: 0.85) (Broderick et al., 2013).
The Medication Quantification Scale (MQS), version III, provides a single numeric value for a participant’s analgesic use profile in the previous 24 hours according to drug class, dosage, and detriment (risk). The data were collected using an e-diary.
Table 1 lists the study measures used in the study, including the number of items in each scale, score range, and internal consistency. Internal consistency (Cronbach alpha) for each measure was acceptable.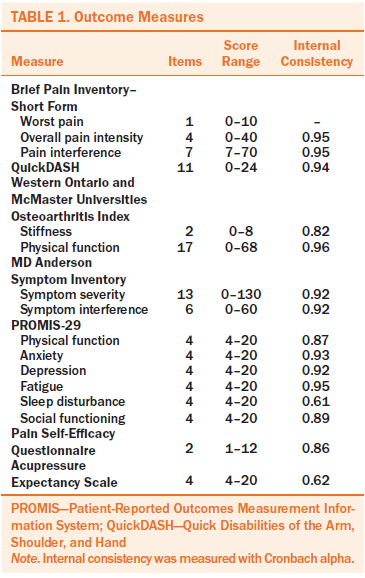
Demographic information: A demographic questionnaire was used to collect information on age, marital status, educational level, living arrangement, ethnicity, disease diagnosis, body mass index, past use of tamoxifen (Nolvadex®), and duration of AI therapy.
Daily diary: A daily diary was given to participants to record their APA self-treatment (including frequency and duration of pressing the seeds taped to their ears and any side effects), medication use (including supplements), and four symptoms (worst pain, fatigue, sleep disturbance, and depression) on a scale ranging from 0 (not present) to 10 (as bad as you can imagine).
Inflammatory Biomarker
Luminex cytokine analysis via xMAP® was used to measure IL-1a, IL-1b, IL-2, IL-6, IL-12, IL-4, IL-10, IL-13, eotaxin, and monocyte chemoattractant protein-1 (MCP-1). Serum was assayed in the Luminex Core Laboratory at the University of Pittsburgh Medical Center Hillman Cancer Center using a multiplex bead-based immunofluorescence assay performed by a blinded technician. A five-parameter regression formula was used to calculate the sample concentrations from the standard curves. The quantification of biomarkers was performed in duplicate to verify the results. These assays typically exhibit high precision and reproducibility (84.5% sensitivity; 98% specificity; 92% of the patients in the active disease group correctly classified from a cross-validation serum set) (Linkov et al., 2007).
Study Procedures
After University of Pittsburgh Medical Center Institutional Review Board approval, potential participants were approached by a member of the research team who provided introductory information about the study and determined the potential participants’ willingness to participate. After signing consent, a questionnaire was mailed to the participants to collect baseline data to serve as within-subject control data. After a one-month waiting period, participants completed the same set of questionnaires; the data served as the preintervention. Participants then received one APA treatment each week for four weeks by a trained therapist. Each weekly cycle included one office visit, five days of wearing the tape and seeds on both ears, and two days without, minimizing the risk of allergic reactions to the tape and allowing the points on the ear to recover and restore sensitivity prior to the next treatment. After seed placement by the therapist, participants were instructed to apply pressure to the seeds on all ear points with the thumb and index finger three times per day (morning, noon, and evening) for three minutes each time to manage AIA. During the office visit for seeds placement, participants also filled out the outcome assessments (weekly data). Blood (10 ml) was collected by a trained nurse from participants in a red-top vacutainer, using standard phlebotomy procedures. Tubes containing blood samples were labeled with the participant’s ID number and time of collection, placed on a level rack at room temperature, and left undisturbed for 1.5 hours. After the tubes were centrifuged at 1,500 rpm for 10 minutes, the serum was transferred into 0.5 ml polypropylene microcentrifuge tubes and stored at –80°C until assayed. Because of budget limitations, data were only analyzed for the pre-APA (baseline) and post-APA treatment (after the four-week APA treatment).
Data Analysis
Intent-to-treat analysis was used to include the data of all participants. Missing values of the outcome variables were replaced by “last value carried forward” for intent-to-treat analysis. Descriptive statistics were used to present demographic characteristics and study measures. The equality of the mean change score from control to pre- and postintervention was examined. The percentage change score was also calculated by dividing the raw change score by the baseline score and multiplying by 100. A cut point of 30% improvement for primary outcomes was used to determine if the mean score changes reached clinical significance (Dworkin et al., 2008). In addition to the descriptive statistics used, medians at the 25th to 75th percentiles were included to present cytokine measures because of skewness of data distribution. One-way analysis of variance was used to examine the outcome changes across different time points. The adherence rate of APA was defined by the number of participants who completed at least two-thirds of the suggested pressing times (i.e., at least two times per day, two minutes per time) to determine the feasibility of participants’ practicing APA at home.
To evaluate the trajectory of daily symptom change (pain, fatigue, sleep disturbance, and depression), the joinpoint regression modeling approach was used to estimate the linear trend of symptom improvement in percentages of symptom scores over time (National Cancer Institute [NCI], 2013). The model was constructed by fitting a linear regression to the improved percentage of each symptom using ordinal day (i.e., beginning the day after receiving the APA intervention) as a regression predictor. This approach can analyze trends with different lines connected at certain joinpoints; each joinpoint represents a significant change in the slope of the trend. A permutation test determined the best number of joinpoints in the final model of each measurement in each group (Kim, Fay, Feuer, & Midthune, 2000). The authors used the calculated daily percentage change (DPC) to characterize the trend of improved percentages from baseline (day 0) to the completion of the four-week APA intervention (day 28). Data analyses were performed using SPSS®, version 22. The trend analysis was performed with the Joinpoint Regression Program, version 4.0.4 (NCI, 2013). Spearman’s rho correlation coefficient was used to examine the linear association of the changes score of cytokines and clinical outcomes from pre- to post-treatment. Statistical significance was defined as a p value less than 0.05.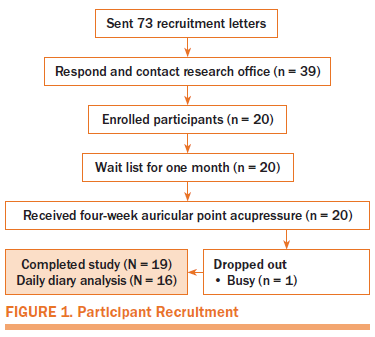
Results
The authors received 39 self-referrals within three weeks in response to the recruitment letter (see Figure 1). The response rate was 53%. Nineteen participants were excluded because they were unable to keep a study appointment, 20 enrolled, 1 dropped out from being too busy after the first APA intervention, and 19 participants completed the study (95% retention rate). Table 2 presents the demographic characteristics of participants.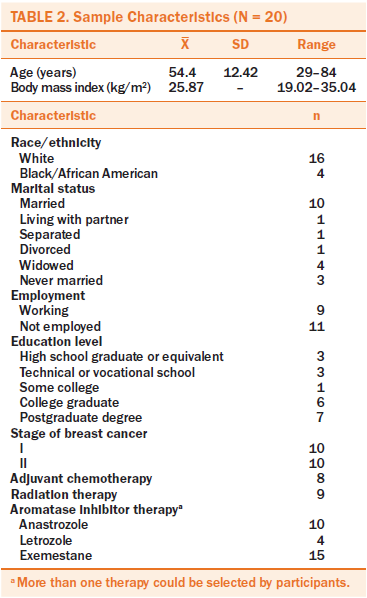
Feasibility for Participants
The results indicate that participants were able to perform APA during the four-week intervention at suggested stimulation dosage (three times per day three minutes per time) for at least a rate of 96% or higher throughout the four-week APA. The average pressing time was 11.49 minutes per day. Patients reported that the adverse effects of APA on their ears were minimal and bearable. Any discomfort usually appeared on day 1 or 2 and then gradually diminished.
Outcomes of the Intervention
The outcome change between control and pre-APA were minimal (see Table 3). After the four-week APA intervention, participants had a clinically significant (i.e., improvement of 30% or greater) decrease in worst pain (50%), pain interference (42%), and improvement of physical function (31%). Stiffness improved by a moderate level (28%). Cancer-related symptom severity and interference improved (48% for each). The changes between the baseline assessment and pre-APA intervention were less than 9% in all primary outcomes. Participants’ pain self-efficacy and expectancy also improved (25% for pain self-efficacy and 33% for expectancy).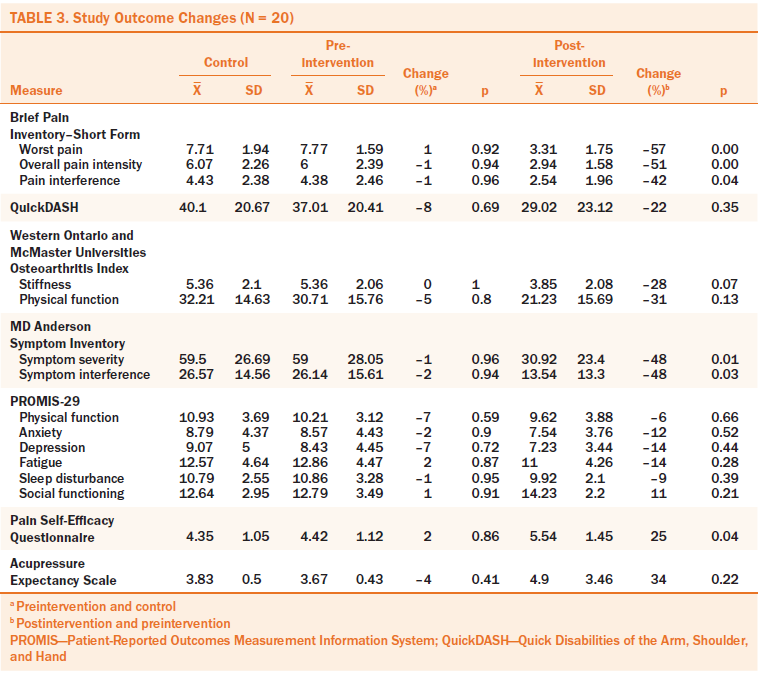
Weekly Change During the Intervention
Percentage changes in outcomes from pre-APA to post-APA were calculated to standardize the improvement outcomes. All outcomes (worst pain, stiffness, physical function, disability, symptom severity, and symptom interferences) decreased during the four-week APA intervention (see Figure 2).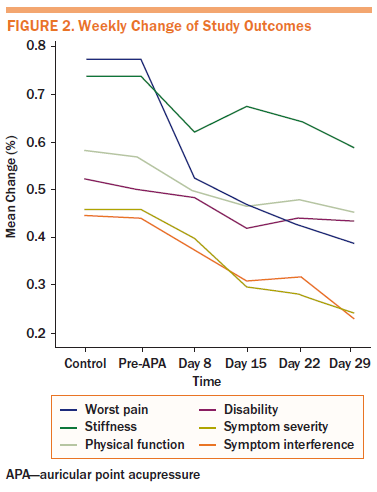
Daily Change of Daily Symptoms
Because of incomplete daily diary data, only 16 participants were included for analysis. Figures 3–6 shows four linear trends of the change in worst pain intensity, fatigue, sleep disturbance, and depression from the pre-APA (day 0) to the post-APA assessment (day 28). Trends in percentage improvement of each symptom are presented in Table 4. The observed worst pain mean score decreased 27% after the first day of the APA intervention and reached the largest decrease (63%) at day 28. Two significant joinpoints were found at day 2 and day 24, suggesting that three significant changes occurred in the improvement pattern of worst pain. The greatest improvement shown, as indicated by the DPC, was 19% (95% CI [–33.87, 0.23], p = 0.05) per day from day 0 to day 2. This improvement steadily continued from day 2 to day 24 (DPC = 1.54%, 95% CI [–2.06, –1.01], p < 0.0001), which suggests continuous improvement of worst pain for 22 of 28 days after beginning the APA intervention.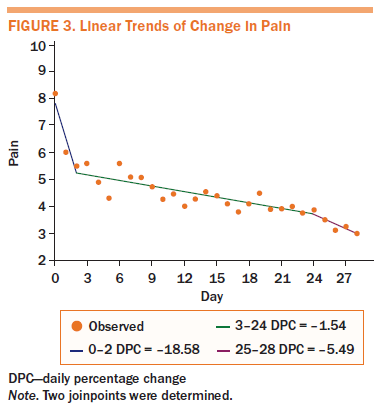
Fatigue and sleep disturbance displayed similar improvement patterns. On the first day of treatment, the mean score for fatigue decreased by 34%, and the mean score for sleep disturbance decreased by 34%. A steady improvement was noted throughout the four-week APA intervention, with a statistically significant DPC of 3% (95% CI [–4.11, –2.38], p < 0.0001) for fatigue and 4% (95% CI [–5.21, –3.11], p < 0.0001) for sleep disturbance until the end of the treatment. The mean score for depression decreased by 48% after the first day of treatment.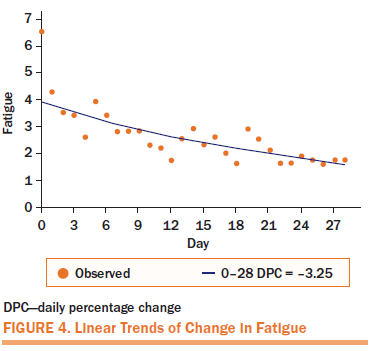
Characteristics of Biomarkers
Table 5 presents the descriptive characteristics of inflammatory cytokines, chemokines, and clinical outcome (worst pain, symptom severity, interference, disability, and stiffness) at pre- and post-APA treatment. The median is also presented because the biomarker data were skewed. After the four-week APA intervention, a trend of mean percentage reduction was found in the proinflammatory cytokines (–22% in IL-1a, –19% in IL-12) and chemokines (–9% in eotaxin). The anti-inflammatory cytokine IL-13 increased from pre- to post-APA treatment (29%); however, no significant changes were noted from pre- to post-APA treatment for all biomarkers.
Discussion
This study examines the feasibility and initial treatment effects of a four-week APA protocol to manage AIA in PBCS and to evaluate the association of APA intervention with biomarkers and patient-reported symptom change in patients with AIA. The study findings indicate that APA is feasible for managing AIA in PBCS in terms of recruitment, retention, and adherence. The authors recruited 39 potential participants who responded to the 73 recruitment letters. This indicates that PBCS with AIA were receptive to the APA intervention. The retention rate was 95% (one participant dropped out). For adherence to APA practice, participants exhibited a 96% or greater adherence rate throughout the four-week APA. The use of APA to manage AIA symptoms is promising. After the four-week APA, patients with AIA showed improved clinical outcomes (reductions of pain intensity, pain interference, symptom severity, and symptom interferences). After the four-week APA, the authors observed a small decrease in pro-inflammatory cytokines (such as IL-1a and IL-12) and an increase in an anti-inflammatory cytokine (IL-13). Although the findings are promising, the interpretation and extrapolation of the study findings are limited by several factors, including small sample size because of the pilot nature of the study and lack of a sham therapy group to control not only for APA placebo effects, but also for nonspecific psychological placebo effects. These shortcomings should be addressed in a future, larger-scale study.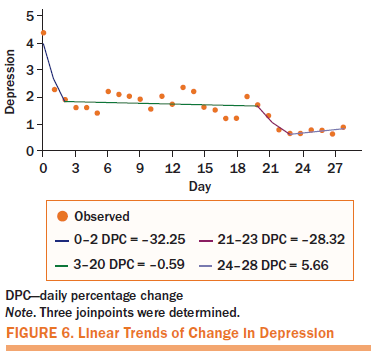
In terms of APA efficacy for pain management, point specificity is an important factor to affect APA outcomes on arthralgia (Asher et al., 2011; Yeh, Chiang, et al., 2014). To date, few studies have addressed the point specificity of auricular therapy (Zhang, Yang, Zhang, May, & Xue, 2014). Different from body acupoints, ear acupoints exhibit changes after the development of physical disease. For example, normal ear points are flat and of normal skin color; however, in the presence of disease or symptoms, the ear points may show discoloration, deformity, papules, or angiosclerosis, in addition to a decrease in auricular cutaneous electrical resistance and pain threshold (Huang, 2005, 2006; Yeh & Huang, 2013). Such changes are not usually observed in body acupoints. Experimental and clinical studies have reported evidence of changes not only in ear skin, but also in electrodermal skin resistance for a number of physical ailments, such as stomach and duodenal ulcer disease (Szopinski et al., 2003), acute and viral hepatitis (Szopinski et al., 2006), musculoskeletal pain (Oleson, Kroening, & Bresler, 1980), and heart disorders (Saku, Mukaino, Ying, & Arakawa, 1993). The results of two meta-analyses examining the efficacy of using auricular therapy to manage pain demonstrate that auricular therapy has better treatment outcomes than sham/control treatments (Asher et al., 2011; Yeh, Chiang, et al., 2014); however, the studies included in these meta-analyses were limited by their small sample size and lack of controls for placebo effects. Whether or not ear acupoints are point-specific warrants additional study.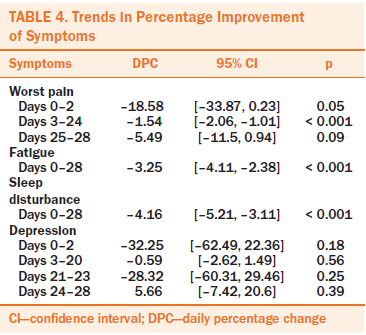
The presence of nonspecific placebo effects is another important confounding factor involved with APA outcomes. The current study has shown that pressure on the selected ear points can reduce AIA symptoms; however, the authors were not able to determine in this pilot study whether the positive outcomes were from the APA intervention itself or possible placebo effects, such as the patient’s treatment beliefs (Furlan et al., 2012; Lundeberg & Lund, 2008; Lundeberg, Lund, & Naslund, 2007; Vickers et al., 2010), the patient’s expectations (Hsu et al., 2014; Schafer et al., 2012; Schnur et al., 2007), or the patient–provider relationship (Lind et al., 2005; Shelton et al., 2013), which could play a role in treatment outcomes. Therefore, future studies of APA on managing pain or cancer symptoms need to address these nonspecific psychological placebo effects.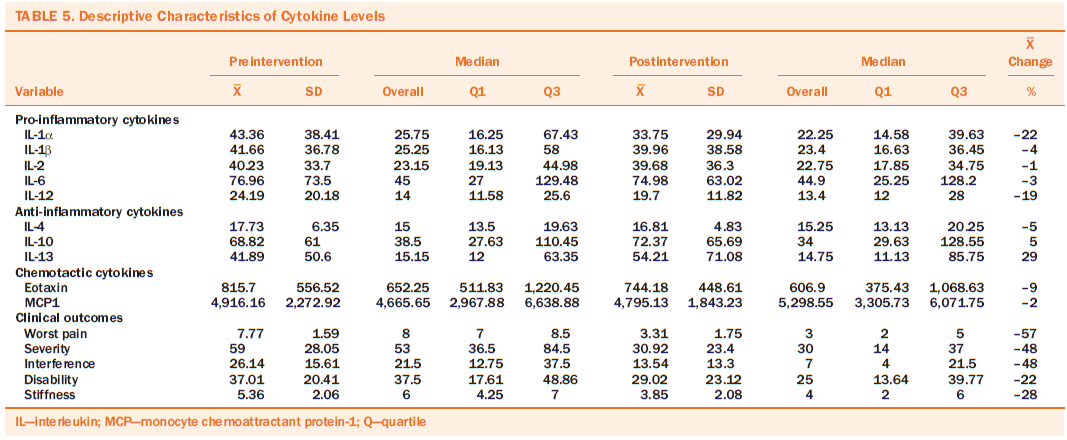
The authors’ findings suggest the potential inflammatory reactions of APA effects on AIA symptom relief. The authors speculate that the effects of APA on AIA may be through the modulation of type-I helper T (Th1) cells and macrophages. For example, the Th1 cells host cellular immune system and activated macrophages by releasing interferon-alpha and IL-2 and, as positive feedback, macrophages release IL-12 to trigger differentiation of Th1 cells (Kaiko, Horvat, Beagley, & Hansbro, 2008). Both Th1 cells and macrophages are inhibited by IL-10 (Zhu & Paul, 2008). Macrophages secrete proinflammatory cytokines when they are exposed to inflammatory stimuli, such as rheumatoid arthritis (Kinne, Bräuer, Stuhlmüller, Palombo-Kinne, & Burmester, 2000). The authors’ findings of APA on symptoms relief may be through decreasing IL-2 and IL-12 and increases in IL-10 observed in the serum.
The current study demonstrates the promise of an APA intervention to manage AIA for PBCS. The authors plan to conduct a large-scale randomized, controlled trial to determine the efficacy of APA intervention for AIA that addresses potential confounding variables such as the effect(s) of treatment variables (i.e., point specificity and stimulation), placebo effects, and patient expectations of treatment outcomes.
Implications for Research
This preliminary research has demonstrated that APA results in promising AIA symptom relief and has the potential to alter inflammatory cytokines. Randomized, controlled trials are needed to determine the efficacy of APA in relieving AIA and to examine the underpinning mechanisms associated with APA-evoked AIA relief. In addition, future studies need to control possible placebo effects of APA effects, including treatment factor (i.e., ear point specificity) and nonspecific psychological factors (i.e., treatment beliefs and expectations).
Implications for Nursing Practice and Conclusion
APA is noninvasive, easily administered, self-managed, and nonpharmacologic, and can be used as an adjunct therapy for AIA. Nurses without training in acupuncture and traditional Chinese medicine can learn APA in brief seminars. The integration of APA into clinical practice could broaden the options of pain management and provide an option for patients who cannot afford acupuncture or other treatments. More importantly, once learned, patients can manage their AIA at home by engaging in the therapy daily anywhere and anytime as a practical tool for pain control. The availability of APA as an adjunct to standard care offers the potential to improve patients’ quality of life in a cost-effective manner.
About the Author(s)
Yeh is an associate professor in the School of Nursing at Johns Hopkins University in Baltimore, MD; Lin is a project director in the School of Nursing at the University of Pittsburgh in Pennsylvania; Suen is an associate professor in the School of Nursing at Hong Kong Polytechnic University; Park is an assistant professor in the School of Nursing at the University of Pittsburgh; Wood is the Amelia Peabody professor of nursing research at the MGH Institute of Health Professions in Charlestown, MA; van Londen is an assistant professor in the School of Medicine at the University of Pittsburgh; and Bovbjerg is director of the Biobehavioral Oncology Program at the University of Pittsburgh Cancer Institute and Hillman Cancer Center. This research was funded by a grant to Yeh from the American Cancer Society (124638-PEP-13-237-01-PCSM). Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Yeh, Suen, van Londen, and Bovbjerg contributed to the conceptualization and design. Yeh, Lin, and Park completed the data collection. Yeh and Lin provided statistical support. Yeh, Lin, van Londen, and Bovbjerg provided the analysis. All authors contributed to the manuscript preparation. Yeh can be reached at cyeh13@jhu.edu, with copy to editor at ONFEditor@ons.org. Submitted July 2016. Accepted for publication November 10, 2016.
References
Alimi, D., Geissmann, A., & Gardeur, D. (2002). Auricular acupuncture stimulation measured on functional magnetic resonance imaging. Medical Acupuncture, 13(2), 18–21.
Asher, G.N., Jonas, D.E., Coeytaux, R.R., Reilly, A.C., Loh, Y.L., Motsinger-Reif, A.A., & Winham, S.J. (2011). Auriculotherapy for pain management: A systematic review and meta-analysis of randomized controlled trials. Journal of Alternative and Complementary Medicine, 16, 1097–1108. doi:10.1089/acm.2009.0451
Beaton, D.E., Wright, J.G., & Katz, J.N. (2005). Development of the QuickDASH: Comparison of three item-reduction approaches. Journal of Bone and Joint Surgery, 87, 1038–1046. doi:10.2106/jbjs.d.02060
Bellamy, N. (2002). WOMAC® osteoarthritis index: User guide V. Queensland, Australia: WOMAC.
Benyamin, R., Trescot, A.M., Datta, S., Buenaventura, R., Adlaka, R., Sehgal, N., . . . Vallejo, R. (2008). Opioid complications and side effects. Pain Physician, 11(Suppl. 2), S105–S120.
Broderick, J.E., Schneider, S., Junghaenel, D.U., Schwartz, J.E., & Stone, A.A. (2013). Validity and reliability of patient-reported outcomes measurement information system instruments in osteoarthritis. Arthritis Care and Research, 65, 1625–1633.
Burstein, H.J. (2007). Aromatase inhibitor-associated arthralgia syndrome. Breast, 16, 223–234. doi:10.1016/j.breast.2007.01.011
Burstein, H.J., Temin, S., Anderson, H., Buchholz, T.A., Davidson, N.E., Gelmon, K.E., . . . Griggs, J.J. (2014). Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. Journal of Clinical Oncology, 32, 2255–2269. doi:10.1200/jco.2013.54.2258
Chinese Association of Acupuncture-Moxibustion. (2008). Nomenclature and location of auricular acupuncture points (GB/T13734-2008) of the National Standard of the People’s Republic of China. Beijing, China: China Standard Press.
Cleeland, C.S., Mendoza, T.R., Wang, X.S., Chou, C., Harle, M.T., Morrissey, M., & Engstrom, M.C. (2000). Assessing symptom distress in cancer patients: The MD Anderson Symptom Inventory. Cancer, 89, 1634–1646.
Cleeland, C.S., & Ryan, K.M. (1994). Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore, 23, 129–138.
Craig, B.M., Reeve, B.B., Brown, P.M., Cella, D., Hays, R.D., Lipscomb, J., . . . Revicki, D.A. (2014). US valuation of health outcomes measured using the PROMIS-29. Value in Health, 17, 846–853. doi:10.1016/j.jval.2014.09.005
Dawson-Basoa, M., & Gintzler, A.R. (1998). Gestational and ovarian sex steroid antinociception: Synergy between spinal kappa and delta opioid systems. Brain Research, 794, 61–67.
Din, O.S., Dodwell, D., Wakefield, R.J., & Coleman, R.E. (2010). Aromatase inhibitor-induced arthralgia in early breast cancer: What do we know and how can we find out more? Breast Cancer Research and Treatment, 120, 525–538. doi:10.1007/s10549-010-0757-7
Dworkin, R.H., Turk, D.C., Wyrwich, K.W., Beaton, D., Cleeland, C.S., Farrar, J.T., . . . Zavisic, S. (2008). Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain, 9, 105–121. doi:10.1016/j.jpain.2007.09.005
Early Breast Cancer Trialists’ Collaborative Group. (2015). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet, 386, 1341–1352. doi:10.1016/s0140-6736(15)61074-1
Furlan, A.D., Yazdi, F., Tsertsvadze, A., Gross, A., Van Tulder, M., Santaguida, L., . . . Tsouros, S. (2012). A systematic review and meta-analysis of efficacy, cost-effectiveness, and safety of selected complementary and alternative medicine for neck and low-back pain. Evidence-Based Complementary and Alternative Medicine, 2012, 953139. doi:10.1155/2012/953139
Glare, P.A., Davies, P.S., Finlay, E., Gulati, A., Lemanne, D., Moryl, N., . . . Syrjala, K.L. (2014). Pain in cancer survivors. Journal of Clinical Oncology, 32, 1739–1747. doi:10.1200/jco.2013.52.4629
Hannan, M.T., Felson, D.T., Anderson, J.J., Naimark, A., & Kannel, W.B. (1990). Estrogen use and radiographic osteoarthritis of the knee in women. The Framingham Osteoarthritis Study. Arthritis and Rheumatism, 33, 525–532.
Henry, N.L., Azzouz, F., Desta, Z., Li, L., Nguyen, A.T., Lemler, S., . . . Storniolo, A.M. (2012). Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of Clinical Oncology, 30, 936–942. doi:10.1200/jco.2011.38.0261
Henry, N.L., Giles, J.T., Ang, D., Mohan, M., Dadabhoy, D., Robarge, J., . . . Clauw, D.J. (2008). Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Research and Treatment, 111, 365–372. doi:10.1007/s10549-007-9774-6
Hershman, D.L., Kushi, L.H., Shao, T., Buono, D., Kershenbaum, A., Tsai, W.Y., . . . Neugut, A.I. (2010). Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. Journal of Clinical Oncology, 28, 4120–4128. doi:10.1200/jco.2009.25.9655
Hershman, D.L., Loprinzi, C., & Schneider, B.P. (2015). Symptoms: Aromatase inhibitor induced arthralgias. Advances in Experimental Medicine and Biology, 862, 89–100. doi:10.1007/978-3-319-16366-6_7
Hershman, D.L., Shao, T., Kushi, L.H., Buono, D., Tsai, W.Y., Fehrenbacher, L., . . . Neugut, A.I. (2011). Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Research and Treatment, 126, 529–537. doi:10.1007/s10549-010-1132-4
Howell, A., Cuzick, J., Baum, M., Buzdar, A., Dowsett, M., Forbes, J.F., . . . Tobias, J.S. (2005). Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet, 365, 60–62. doi:10.1016/s0140-6736(04)17666-6
Hsu, C., Sherman, K.J., Eaves, E.R., Turner, J.A., Cherkin, D.C., Cromp, D., . . . Ritenbaugh, C. (2014). New perspectives on patient expectations of treatment outcomes: Results from qualitative interviews with patients seeking complementary and alternative medicine treatments for chronic low back pain. BMC Complementary and Alternative Medicine, 14, 276. doi:10.1186/1472-6882-14-276
Hu, W., Shuang, F., Zou, H.X., & Yang, H.H. (2015). Association between estrogen receptor-alpha gene PvuII and XbaI polymorphisms and osteoarthritis risk: A meta-analysis. International Journal of Clinical and Experimental Medicine, 8, 1956–1965.
Huang, L.C. (2005). Auricular medicine: A complete manual of auricular diagnosis and treatment. Orlando FL: Auricular International Research and Training.
Huang, L.C. (2006). Auricular diagnosis with color photos. Fern Park, FL: Auricular Medicine International Research and Training Center.
Irwin, M.L., Cartmel, B., Gross, C.P., Ercolano, E., Li, F., Yao, X., . . . Ligibel, J. (2015). Randomized exercise trial of aromatase inhibitor–induced arthralgia in breast cancer survivors. Journal of Clinical Oncology, 33, 1104–1111. doi:10.1200/jco.2014.57.1547
Islander, U., Jochems, C., Lagerquist, M.K., Forsblad-d’Elia, H., & Carlsten, H. (2011). Estrogens in rheumatoid arthritis; the immune system and bone. Molecular and Cellular Endocrinology, 335, 14–29. doi:10.1016/j.mce.2010.05.018
Kaiko, G.E., Horvat, J.C., Beagley, K.W., & Hansbro, P.M. (2008). Immunological decision‐making: How does the immune system decide to mount a helper T‐cell response? Immunology, 123, 326–338. doi:10.1111/j.1365-2567.2007.02719.x
Kim, H.J., Fay, M.P., Feuer, E.J., & Midthune, D.N. (2000). Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine, 19, 335–351.
Kinne, R.W., Bräuer, R., Stuhlmüller, B., Palombo-Kinne, E., & Burmester, G.R. (2000). Macrophages in rheumatoid arthritis. Arthritis Research and Therapy, 2, 189–202.
Kramer, P.R., Kramer, S.F., & Guan, G. (2004). 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis and Rheumatism, 50, 1967–1975. doi:10.1002/art.20309
Lind, B.K., Lafferty, W.E., Tyree, P.T., Sherman, K.J., Deyo, R.A., & Cherkin, D.C. (2005). The role of alternative medical providers for the outpatient treatment of insured patients with back pain. Spine, 30, 1454–1459.
Linkov, F., Lisovich, A., Yurkovetsky, Z., Marrangoni, A., Velikokhatnaya, L., Nolen, B., . . . Ferris, R.L. (2007). Early detection of head and neck cancer: Development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiology, Biomarkers and Prevention, 16, 102–107. doi:10.1158/1055-9965.EPI-06-0602
Lundeberg, T., & Lund, I. (2008). Acupuncture for preconditioning of expectancy and/or Pavlovian extinction. Acupuncture Medicine, 26, 234–238.
Lundeberg, T., Lund, I., & Naslund, J. (2007). Acupuncture--Self-appraisal and the reward system. Acupuncture Medicine, 25, 87–99.
MacPherson, H., Altman, D.G., Hammerschlag, R., Youping, L., Taixiang, W., White, A., & Moher, D. (2010). Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT statement. PLOS ONE, 7(6), e1000261. doi:10.1371/journal.pmed.1000261
Mao, J.J., Stricker, C., Bruner, D., Xie, S., Bowman, M.A., Farrar, J.T., . . . DeMichele, A. (2009). Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer, 115, 3631–3639. doi:10.1002/cncr.24419
Mao, J.J., Su, H.I., Feng, R., Donelson, M.L., Aplenc, R., Rebbeck, T.R., . . . DeMichele, A. (2011). Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Research, 13(1), R8. doi:10.1186/bcr2813
Mao, J.J.., Xie, S.X., & Bowman, M.A. (2010). Uncovering the expectancy effect: The validation of the acupuncture expectancy scale. Alternative Therapies in Health and Medicine, 16(6), 22–27.
Murphy, C.C., Bartholomew, L.K., Carpentier, M.Y., Bluethmann, S.M., & Vernon, S.W. (2012). Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Research and Treatment, 134, 459–478.
National Cancer Institute. (2013). Joinpoint Regression Program [v.4.4.0]. Rockville, MD: Author.
Nevitt, M.C., Cummings, S.R., Lane, N.E., Hochberg, M.C., Scott, J.C., Pressman, A.R., . . . Cauley, J.A. (1996). Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Archives of Internal Medicine, 156, 2073–2080.
Nicholas, M.K. (2007). The pain self-efficacy questionnaire: Taking pain into account. European Journal of Pain, 11, 153–163. doi:10.1016/j.ejpain.2005.12.008
Nicholas, M.K., McGuire, B.E., & Asghari, A. (2015). A 2-item short form of the Pain Self-Efficacy Questionnaire: Development and psychometric evaluation of PSEQ-2. Journal of Pain, 16, 153–163. doi:10.1016/j.jpain.2014.11.002
Nogier, P. (1981). Handbook to auriculotherapy. Moulins Les-Metz, France: Maisonneuve SA.
Nogier, P. (1987). Points reflexes auricularis. Moulin Les-Metz, France: Maisonneuve SA.
Nogier, R. (2014). How did Paul Nogier establish the map of the ear? Medical Acupuncture, 26(2), 76–83.
Oleson, T. (2014). Auriculotherapy manual: Chinese and Western systems of ear acupuncture (4th ed.). Edinburgh, Scotland: Churchill Livingstone.
Oleson, T.D., Kroening, R.J., & Bresler, D.E. (1980). An experimental evaluation of auricular diagnosis: The somatotopic mapping or musculoskeletal pain at ear acupuncture points. Pain, 8, 217–229.
Presant, C.A., Bosserman, L., Young, T., Vakil, M., Horns, R., Upadhyaya, G., . . . Howard, F. (2007). Aromatase inhibitor-associated arthralgia and/or bone pain: Frequency and characterization in non-clinical trial patients. Clinical Breast Cancer, 7, 775–778. doi:10.3816/CBC.2007.n.038
Prieto-Alhambra, D., Javaid, M.K., Judge, A., Maskell, J., Cooper, C., & Arden, N.K. (2015). Hormone replacement therapy and mid-term implant survival following knee or hip arthroplasty for osteoarthritis: A population-based cohort study. Annals of the Rheumatic Diseases, 74, 557–563. doi:10.1136/annrheumdis-2013-204043
PROMIS Health Organization and PROMIS Cooperative Group (2016). PROMIS-29 profile v2.0. Retrieved from http://www.healthmeasures.net/administrator/components/com_instruments/…
Romoli, M., Allais, G., Airola, G., Benedetto, C., Mana, O., Giacobbe, M., . . . Fornari, E. (2014). Ear acupuncture and fMRI: A pilot study for assessing the specificity of auricular points. Neurological Sciences, 35(Suppl.), 189–193. doi:10.1007/s10072-014-1768-7
Saku, K., Mukaino, Y., Ying, H., & Arakawa, K. (1993). Characteristics of reactive electropermeable points on the auricles of coronary heart disease patients. Clinical Cardiology, 16, 415–419.
Schafer, L.M., Hsu, C., Eaves, E.R., Ritenbaugh, C., Turner, J., Cherkin, D.C., . . . Sherman, K.J. (2012). Complementary and alternative medicine (CAM) providers’ views of chronic low back pain patients’ expectations of CAM therapies: A qualitative study. BMC Complementary and Alternative Medicine, 12, 234. doi:10.1186/1472-6882-12-234
Schnur, J.B., Hallquist, M.N., Bovbjerg, D.H., Silverstein, J.H., Stojceska, A., & Montgomery, G.H. (2007). Predictors of expectancies for post-surgical pain and fatigue in breast cancer surgical patients. Personality and Individual Differences, 42, 419–429. doi:10.1016/j.paid.2006.07.009
Shelton, R.C., Clarke Hillyer, G., Hershman, D.L., Leoce, N., Bovbjerg, D.H., Mandelblatt, J.S., . . . Neugut, A.I. (2013). Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: An examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Research and Treatment, 137, 817–828.
Szopinski, J.Z., Lochner, G.P., Macura, T., Karcz-Socha, I., Kasprzyk-Minkner, A., Kielan, K., . . . Teng, X.H. (2006). Localization of auricular projection area of the liver and its use in the monitoring of viral hepatitis. Journal of Traditional Chinese Medicine, 26, 260–265.
Szopinski, J., Lochner, G., Szkliniarz, J., Karcz-Socha, I., Kasprzyk-Minkner, A., . . . Warakomski, P. (2003). Localization of auricular projection areas of the stomach and duodenum and their use in the monitoring of ulcer disease. Medical Acupuncture, 15, 31–34.
Vickers, A.J., Cronin, A.M., Maschino, A.C., Lewith, G., Macpherson, H., Victor, N., . . . Linde, K. (2010). Individual patient data meta-analysis of acupuncture for chronic pain: Protocol of the Acupuncture Trialists’ Collaboration. Trials, 11, 90. doi:10.1186/1745-6215-11-90
World Health Organization. (1990). Report of the working group on auricular acupuncture nomenclature. Retrieved from http://apps.who.int/medicinedocs/documents/s7144e/s7144e.pdf
Yeh, C.H., Chiang, Y.C., Hoffman, S.L., Liang, Z., Klem, M.L., Tam, W.W., . . . Suen, L.K. (2014). Efficacy of auricular therapy for pain management: A systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine, 2014, 934670. doi:10.1155/2014/934670
Yeh, C.H., Chien, L.C., & Suen, L.K.P. (2014). Application of auricular therapy for cancer-related pain for nursing care. Journal of Pain and Relief, 3, 139, doi:10.4172/2167-0846.1000139
Yeh, C.H., & Huang, L.C. (2013). Comprehensive and systematic auricular diagnosis protocol. Medical Acupuncture, 25, 423–436.
Zhang, C.S., Yang, A.W., Zhang, A.L., May, B.H., & Xue, C.C. (2014). Sham control methods used in ear-acupuncture/ear-acupressure randomized controlled trials: A systematic review. Journal of Alternative and Complementary Medicine, 20, 147–161.
Zhu, J., & Paul, W.E. (2008). CD4 T cells: Fates, functions, and faults. Blood, 112, 1557–1569. doi:10.1182/blood-2008-05-078154

