Feasibility of a Telemedicine-Delivered Cognitive Behavioral Therapy for Insomnia in Rural Breast Cancer Survivors
Objectives: To evaluate a nurse-led, telemedicine-delivered cognitive behavioral therapy for insomnia (CBTI) in rural breast cancer survivors (BCSs).
Sample & Setting: 18 BCSs diagnosed with stage I–III breast cancer in the rural western United States.
Methods & Variables: In this prospective, pre-/post-test, quasiexperimental feasibility pilot trial, BCSs attended six weekly sessions of CBTI via Internet videoconference. Feasibility was assessed using recruitment and acceptability of the intervention. Primary outcomes were diary-based sleep efficiency (SE), sleep latency (SL), total sleep time, wake after sleep onset, and number of nightly awakenings; secondary outcomes included quality of life (QOL), mental health, and daily functioning.
Results: Following the intervention, participants reported improvements in sleep outcomes, including SE and SL. QOL and daily functioning improved, but anxiety and depression did not.
Implications for Nursing: Nurse-led, telemedicine-delivered CBTI for rural BCSs is feasible and may be effective in managing insomnia. Additional research is needed to determine widespread effectiveness and best practices for dissemination and implementation.
Jump to a section
Insomnia is a complex yet common condition that affects 42%–69% of women after primary treatment for breast cancer (Savard, Ivers, Villa, Caplette-Gingras, & Morin, 2011). Defined as having difficulty with initiating or maintaining sleep and experiencing associated daytime symptoms that can impair daily functioning (e.g., fatigue, sleepiness, cognitive disturbances) (American Psychiatric Association, 2013), insomnia can occur at the time of breast cancer diagnosis and throughout cancer treatment (Palesh et al., 2010). In addition, it often persists for years after the completion of cancer treatment (Fontes, Severo, Gonçalves, Pereira, & Lunet, 2017; Lowery-Allison et al., 2018; Savard et al., 2011). Previous research has shown that sleep disturbances in breast cancer survivors (BCSs) can decrease quality of life (QOL) (Lowery-Allison et al., 2018), depress the immune system, and increase mortality (Trudel-Fitzgerald et al., 2017).
Background
Cognitive behavioral therapy for insomnia (CBTI) is a multicomponent therapy aimed at eliminating a broad range of symptoms and perpetuating factors of insomnia. Components include empirically supported sleep restriction, stimulus control, sleep hygiene education, and cognitive therapy, with or without relaxation techniques. Trained therapists focus on addressing negative thoughts and feelings about sleep and changing behaviors that can perpetuate insomnia (Matthews, Carter, Page, Dean, & Berger, 2018). CBTI is an effective therapy that can lead to an improvement in sleep outcomes and a decrease in associated daytime symptoms among the general population (Aricò, Raggi, & Ferri, 2016; Trauer, Qian, Doyle, Rajaratnam, & Cunnington, 2015; van Straten et al., 2018) and in patients after cancer treatment (Fleming & MacMahon, 2015; Johnson et al., 2016). CBTI has been effectively delivered to patients in person (Matthews et al., 2014; Morin et al., 2009; van Straten et al., 2018), as well as via telephone (Arnedt et al., 2013; McCurry et al., 2016) and the Internet (Espie et al., 2012; Ritterband et al., 2017; Seyffert et al., 2016). It has proven to be a more effective long-term treatment than sleep medication alone (Beaulieu-Bonneau, Ivers, Guay, & Morin, 2017; Heckler et al., 2016; Morin et al., 2009). In-person individual and group CBTI is the recommended therapy for insomnia after breast cancer treatment (Aricò et al., 2016), although a video-based version of CBTI has shown promise (Savard, Ivers, Savard, & Morin, 2014, 2016). To date, there are no reports of testing a synchronous, Internet-based, telemedicine-delivered CBTI in rural BCSs.
Although CBTI is an established treatment for insomnia, it is unavailable to many people with cancer, particularly those in rural areas with limited healthcare services. Even in urban centers, CBTI providers are scarce. However, they are virtually nonexistent in the rural United States. Similarly, there are fewer mental health providers in rural areas (Beraldi et al., 2015), but the need for treatment may be greater. Many studies show that psychological distress after cancer treatment, including anxiety and depression, is prevalent in rural settings (Andrykowski, Steffens, Bush, & Tucker, 2014; Burris & Andrykowski, 2010; Weaver, Geiger, Lu, & Case, 2013).
Internet and mobile (i.e., cellphone application) technologies can provide new platforms for the delivery of telemedicine worldwide and may be helpful in addressing challenges related to the delivery of CBTI and mental health interventions (Seyffert et al., 2016). However, such technologies remain underused in clinical practice. With Internet and mobile technologies, providers can bridge geographic and transportation barriers and make home-based sleep promotion and mental health services available to rural residents. The American Academy of Sleep Medicine supports the use of telemedicine to deliver sleep interventions, provided that the intervention is consistent with what is offered in traditional in-person therapy (Singh et al., 2015).
An efficient and cost-effective way to increase access to CBTI is to train nurses as frontline providers (Fields, Schutte-Rodin, Perlis, & Myers, 2013; Manber et al., 2012). Multiple studies indicate that nurse-led CBTI is clinically effective (Espie et al., 2007; Karlin, Trockel, Taylor, Gimeno, & Manber, 2013; Matthews et al., 2014). The use of trained nurses to deliver CBTI could contribute to the creation of a larger skilled workforce to meet survivors’ needs. Given the prevalence of insomnia during and after cancer treatment, oncology nurses working with BCSs are ideally situated to provide this intervention, but little is known about the feasibility and outcomes of nurse-led, telemedicine-delivered CBTI in rural settings.
The theoretical framework for this study was the Spielman 3P model of insomnia (Spielman, Caruso, & Glovinsky, 1987). This model details the predisposing, precipitating, and perpetuating factors that contribute to insomnia. Predisposing factors are biopsychosocial determinants that contribute to an individual’s propensity for developing insomnia. Precipitating factors are events that directly trigger insomnia (e.g., a breast cancer diagnosis and the associated chemotherapy and radiation therapy). Perpetuating factors include cognitive and behavioral changes made by the individual that contribute to the continuation of insomnia and are specifically targeted in CBTI. Examples of these perpetuating factors include fatigue-driven adaptations that are common during cancer treatment, such as a decrease in exercise and/or an increase in napping.
Objectives
The purposes of this study are to (a) evaluate the feasibility of a nurse-led, telemedicine-delivered CBTI to improve insomnia and (b) conduct an initial assessment of the effectiveness of the intervention in rural BCSs. Primary outcomes are sleep parameters, insomnia severity, and knowledge/beliefs about sleep. Secondary outcomes are QOL, physical functioning, fatigue, anxiety, depression, and menopause symptoms.
Methods
Design and Participants
A pre-/post-test quasiexperimental design was chosen to evaluate the feasibility and preliminary effects of telemedicine-delivered CBTI. Study procedures were approved by the Colorado Multi-Institutional Review Board. BCSs from rural and frontier counties in Colorado were recruited through oncology providers, newspaper and radio advertisements, and flyers posted in the community. Participants were women who were diagnosed and treated with surgery, radiation therapy, or chemotherapy for nonmetastatic breast cancer at least one month but not more than five years (60 months) prior to study enrollment. Women currently receiving primary breast cancer treatment were not eligible because the acute treatment of cancer can increase systemic inflammatory responses, which can contribute to sleep disturbances (Liu et al., 2012; Wang et al., 2012). Participants were age restricted (aged 35–65 years) because breast cancer usually occurs after age 35 years and sleep patterns can change with older age (Yoon et al., 2003), which could confound the study outcomes.
Participants had to live in rural or frontier Colorado counties, as designated by the U.S. Health Resources and Services Administration. They also were required to have access to a computer with Internet access for videoconferences; this computer could be a personal computer, such as one in their home or workplace, or a public computer that could be accessed privately, such as one in a library or a healthcare provider’s office. Participants had to be on a stable dose of anti-estrogen agents and medications for hot flashes, meet the diagnostic criteria for subthreshold insomnia (Insomnia Severity Index [ISI] score of 8 or greater), speak and write English, and be on stable doses of psychotropic medications (excluding hypnotics), opioids, anti-endocrine medications, or high-dose steroids (less than 10% change in dosage per week).
Patients who met the following criteria were excluded from study participation:
• Presence of a serious unstable physical illness other than cancer
• Presence of dementia, major depression, psychosis, or other serious psychiatric disorder as determined by the Insomnia Interview Schedule (IIS), with questions adapted from the Structured Clinical Interview for DSM-IV
• Presence of a sleep disorder other than insomnia based on self-report in initial interview (IIS) when asked about specific sleep disorders
• Unstable doses of psychotropic medications (excluding hypnotics), opioids, anti-endocrine medications, or high-dose steroids
• Evening or night shift employment
• Residence in an urban county of Colorado
• No access to a private computer
Feasibility studies are designed to assess whether an intervention can be conducted and whether it warrants further testing (Bowen et al., 2009). Common feasibility metrics include recruitment, methodology, and outcomes (Orsmond & Cohn, 2015). In the current study, feasibility was assessed using recruitment, availability and acceptability of the technology necessary to provide the intervention, and initial outcomes of telemedicine-delivered CBTI.
Primary sleep-related outcomes were collected via daily sleep diaries and included the following parameters:
• Sleep efficiency (SE): ratio (%) of actual sleep time to time in bed multiplied by 100
• Sleep latency (SL): minutes to fall asleep after lights out
• Wake after sleep onset (WASO): sum of minutes awake after sleep onset
• Total sleep time (TST): sleep period minus SL and WASO
• Number of awakenings
Secondary variables, including QOL, mental health, and daily functioning, were collected in surveys prior to the CBTI intervention and at the end of the intervention.
Procedures
To assess eligibility, women engaged in a scripted telephone screening, including insomnia severity and ability to consent, using a brief mental status screening. Women were considered eligible for study participation if they had an ISI score of 8 or greater (Bastien, Vallières, & Morin, 2001; Savard, Simard, Ivers, & Morin, 2005). Eligible, interested participants received study information via telephone, and an informed consent form was emailed to participants. During initial videoconference sessions between the researcher and the participant, the consent form was reviewed, ample time was provided to answer questions, the participant’s Internet connectivity was tested, and each participant’s comfort with the Adobe Connect software, which facilitates virtual meetings, was strengthened. Participants mailed the signed informed consent form to the researcher. After the consent form was received, participants received a link to the baseline measures and study materials via email. Demographic and pre- and postintervention data were entered directly by participants into a secure web application (REDCap) (Harris et al., 2009). The surveys were configured to mandate forced completion to ensure that all questions were answered. Sleep-wake pattern data, which were reported by participants via weekly sleep diaries, were discussed during weekly meetings between the researcher and the participant. Participants received gift cards totaling $50 for completion of the study. Screening and enrollment details are outlined in Figure 1. 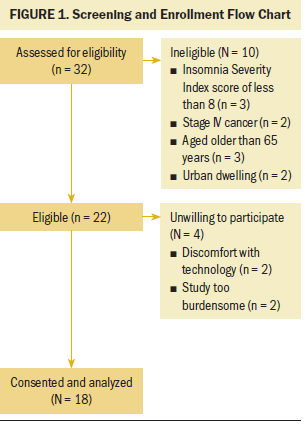
Intervention
The CBTI intervention consisted of six individual sessions, each lasting 30–60 minutes and taking place over a period of six weeks, which is a standard dose of CBTI (Perlis, Jungquist, Smith, & Posner, 2005). The first two sessions included education on insomnia and the main components of CBTI, which are as follows (Perlis et al., 2005):
• Sleep restriction: builds up sleep drive by restricting sleep and excessive amounts of time in bed to compensate for sleep loss; as sleep becomes consolidated, the sleep window is extended until a sleep schedule that optimizes daytime alertness is achieved.
• Stimulus control: re-establishes the discriminative properties of sleep and sleep-compatible stimuli with the act of sleeping; this is often achieved by avoiding sleep-incompatible behaviors in the bedroom, reinforcing a regular sleep-wake schedule, and strengthening the bed as a cue for sleep.
• Sleep hygiene education: psychosocial intervention not thought to be an effective monotherapy although it is generally considered to be an integral part of CTBI; poor sleep hygiene is rarely the primary cause of insomnia, but it increases the likelihood of insomnia perpetuation.
• Cognitive therapy: addresses dysfunctional beliefs held by most patients with insomnia (e.g., mild sleep deprivation is detrimental to functioning) or individualized concerns, unwanted intrusive ideation, or worry using a more traditional cognitive therapy model
Sleep diaries kept by participants were verbally reviewed at each session, and sleep schedules were adjusted according to standard practice. If the participant adhered to the prescribed wake time and continued to have difficulty falling asleep or was waking during the night, sleep was further restricted until the participant was able to maximize the percentage of time he or she was asleep during the night. The third, fourth, and fifth sessions, which each lasted 30 minutes, consisted of reviewing the participant’s sleep diary, adjusting sleep schedules, and reinforcing education from earlier sessions. The sixth session, which lasted 30–45 minutes, focused on relapse prevention.
CBTI sessions were completed using the Adobe Connect platform, which provided a videoconference link between the researcher and the participant. All sessions were conducted by the primary researcher, who is an advanced practice nurse with CBTI training, and were based on established standard practice (Perlis et al., 2005). Sessions were scheduled on a day and at a time that was convenient for each participant and repeated on that day and at that time for six weeks.
Measures
Feasibility of the intervention was determined using three metrics: recruitment, availability and acceptability of technology, and initial outcomes of treatment (Orsmond & Cohn, 2015). Participants reported demographic and medical information; primary outcome data regarding sleep parameters, insomnia severity, and knowledge of sleep; and secondary data regarding QOL, physical functioning, fatigue, anxiety, depression, and menopause symptoms. Demographic data included questions about age, cancer stage, marital status, education level, income, and cancer treatment.
The Consensus Sleep Diary (CSD) (Carney et al., 2012) is a subjective sleep assessment tool that standardizes instructions and core measures of sleep. It is a widely used clinical and research tool that monitors daily sleep characteristics and patterns (Carney et al., 2012). In this study, participants were instructed to fill out the sleep diary every morning when they woke up. Items recorded included the estimated time they went to bed, time of lights out, time they fell asleep, time of last awakening, number of nighttime awakenings, and WASO. These data were used to calculate sleep parameters, including TST, SE, and SL. Reliability of sleep diaries has been supported with as few as three nights of data (r = 0.8) (Thomas & Burr, 2009). Concurrent validity was previously supported in the comparison of duration of sleep between the CSD and an Actiwatch accelerometer (made by Philips Respironics) (r = 0.49) and the Pittsburgh Sleep Quality Index (PSQI) (r = 0.75) (Landry, Best, & Liu-Ambrose, 2015).
The ISI (Bastien et al., 2001) is a seven-item tool designed to measure the severity and impact of insomnia during the past two weeks. Item scores range from 0–4, with 0 being the least severe and 4 being the most severe; total scores range from 0–28. A total score of 0–7 represents no clinically significant insomnia, 8–14 is subthreshold insomnia, 15–21 is clinical insomnia (moderate severity), and 22–28 is clinical insomnia (severe). The ISI has been shown to be a reliable tool among BCSs over time, with a Cronbach alpha of 0.64–0.85 (Dirksen & Epstein, 2008). Concurrent validity was tested against the PSQI, and the total scores were significantly correlated (r = 0.8, p < 0.05) (Bastien et al., 2001; Morin, Belleville, Bélanger, & Ivers, 2011).
The Dysfunctional Beliefs and Attitudes about Sleep-16 (DBAS-16) measures knowledge and beliefs about sleep-related issues (Morin, Vallières, & Ivers, 2007) that can perpetuate misconceptions about sleep. It contains 16 questions, each of which is scored on a 0–10 Likert-type scale. An individual’s total DBAS-16 score is determined by adding up all item scores and dividing by 16. The lowest possible score is 0 and the highest possible score is 10. Higher scores indicate more dysfunctional knowledge and beliefs; if the mean score is 4 or greater, the individual may have dysfunctional beliefs about sleep, which should be addressed. The DBAS-16 has been shown to be reliable in clinical (Cronbach alpha of 0.77) and research (Cronbach alpha of 0.79–0.82) populations (Matthews et al., 2014; Morin et al., 2007), and concurrent validity has been established through correlations with the ISI (r = 0.45, p < 0.001) (Morin et al., 2007).
The European Organisation for Research and Treatment of Cancer QOL Questionnaire–Core 30 (EORTC QLQ-C30), version 3.0, is a 30-item self-report questionnaire designed to measure global health status/QOL, symptom burden, and daily functioning in populations of patients with cancer (Aaronson et al., 1993). It is comprised of functional and symptom scales and a global health status/QOL scale that is made up of two items: overall health status and QOL. Higher scores indicate better functioning and QOL and range from 0–100. For the current study, global health status/QOL and daily function measures were assessed. Internal reliability of the global EORTC QLQ-C30 was high when tested in BCSs (Cronbach alpha of 0.73–0.88) (Garabeli Cavalli Kluthcovsky et al., 2012; Matthews et al., 2014). Concurrent validity of the global health status/QOL scale is supported when the EORTC QLQ-C30 is compared to the Psychosocial Adjustment to Illness Scale (r = 0.63) and the Profile of Mood States (r = 0.56). The EORTC QLQ-C30 has been validated for use in multiple languages and is regularly used as a QOL measure in BCSs (Koch et al., 2013).
The revised Piper Fatigue Scale (PFS) is a 22-item questionnaire that reflects fatigue using four subscales. Questions are answered on a 0–10 scale, with higher scores reflecting greater fatigue. The total PFS score ranges from 0–10, in which 0 indicates no fatigue, 1–3 is mild fatigue, 4–6 is moderate fatigue, and 7–10 is severe fatigue. Subscale reliability has been confirmed: behavioral/severity (Cronbach alpha of 0.89), sensory (Cronbach alpha of 0.87), cognitive/mood (Cronbach alpha of 0.87), and affective meaning (Cronbach alpha of 0.87) (Piper et al., 1998; Reeve et al., 2012). Construct validity was supported through item-subscale correlations (r > 0.65), and concurrent validity was supported through a positive correlation with the fatigue subscale of the Profile of Mood States (r = 0.50–0.78) (Cantarero-Villanueva et al., 2014).
The Hospital Depression and Anxiety Scale (HADS) is a 14-item self-assessment tool designed to measure anxiety and depression in the past week. It has two seven-item subscales (anxiety and depression) that are scored 0–3 for a total possible score of 0–21. Higher scores indicate greater anxiety and depression (Zigmond & Snaith, 1983). A subscale score of 0–7 is normal, 8–10 is borderline normal, and 11–21 is abnormal. Internal reliability of both subscales has been established in cancer survivors: The Cronbach alpha for the anxiety subscale was 0.85, and the Cronbach alpha for the depression subscale was 0.81 (Matthews et al., 2014). Concurrent validity was established by correlating scores from Beck’s Depression Inventory against the HADS depression subscale and scores from the Spielberger State-Trait Anxiety Inventory against the HADS anxiety subscale (Bjelland, Dahl, Haug, & Neckelmann, 2002).
The Menopause Rating Scale (MRS) (Heinemann et al., 2004) is an 11-item self-assessment questionnaire in which respondents score the severity of menopausal symptoms from 0 (none) to 4 (very severe); higher scores (range = 0–44) indicate greater symptom severity. Three subscales comprise the MRS: psychological (irritability, anxiety; range = 0–16); somatic (physical and sleep complaints; range = 0–16); and urogenital (sexual and urinary complaints; range = 0–12). External reliability (r = 0.8–0.96) and high internal reliability (Cronbach alpha of 0.6–0.9) were established with test-retest stability in a population of women aged 40–70 years (r = 0.8–0.96) (Heinemann et al., 2004). Concurrent validity was established when the MRS was compared to the clinically used Kupperman Index (r = 0.91, 95% confidence interval [0.89, 0.93] (Heinemann et al., 2004).
Data Analysis
An a priori power analysis was performed based on a previous study that evaluated a CBTI intervention in BCSs (Savard et al., 2005). To reach an alpha level of 0.01 and a power of 0.9, a sample size of 16 women was required to detect an effect size of 1. Dependent t tests were run to determine differences between pre- and postintervention sleep and other measures. All data were analyzed using IBM SPSS Statistics, version 23.0.
Results
Eighteen women enrolled in and completed the study. The average age of the participants was 57.72 years (SD = 6.49), and most women had been diagnosed with either stage I (n = 9) or stage II (n = 7) breast cancer (see Table 1). Most had undergone radiation therapy (n = 16). All participants had undergone surgery, which ranged from a lumpectomy to a double mastectomy with delayed reconstruction. Primary breast cancer treatment was conducted at an urban academic medical center for 7 participants, whereas 11 women sought treatment at hospitals in their rural communities. No participants had undergone insomnia treatment prior to this study, and all participants stated that their insomnia symptoms either started or worsened with breast cancer diagnosis or treatment. The majority of participants were college educated or higher (n = 13), and the median annual household income was $60,000–$79,999. These demographics are consistent with an in-person study of urban BCSs also conducted in Colorado (Matthews et al., 2014). 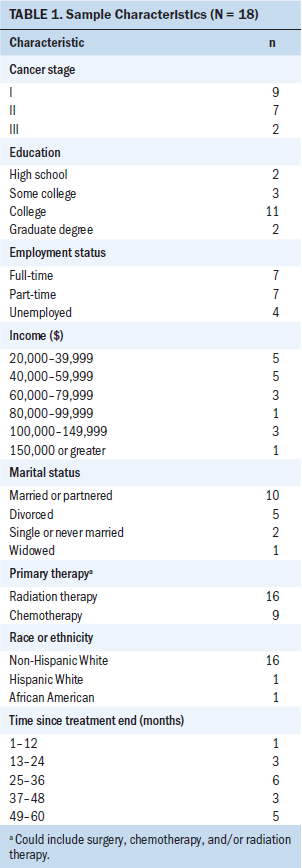
The three metrics used to assess feasibility were recruitment, availability and acceptability of technology, and initial outcomes of treatment (Orsmond & Cohn, 2015). Thirty-two women were assessed for eligibility. Of 22 eligible women, 18 were recruited into the study. All rural participants had private computers and Internet access, which functioned at an acceptable speed, according to participant self-report. No sessions were canceled because of problems with technology, but three sessions were each delayed by 10 minutes. Outcomes of treatment were consistent with those of in-person CBTI (Matthews et al., 2014).
Primary Outcomes
A significant increase in diary-based SE scores was noted postintervention, with a mean SE score increase from 77.79% preintervention to 88.05% postintervention (p < 0.001) (see Table 2). From pre- to postintervention, the mean SL decreased from 36.81 minutes to 16.02 minutes; WASO decreased from 44.42 minutes to 25.27 minutes; and the number of awakenings decreased from 2.37 to 0.94 (p < 0.001). ISI scores decreased an average of seven points after the intervention (p < 0.001). Dysfunctional beliefs about sleep decreased after the six weeks of CBTI, as evidenced by a mean DBAS-16 score decrease of 1.52 points (p < 0.001). 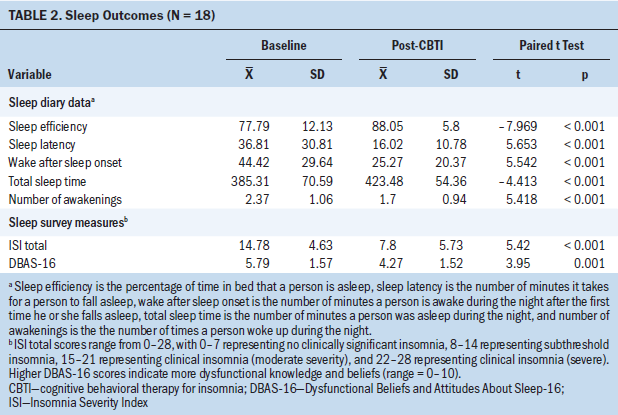
Secondary Outcomes
Global health status/QOL improved after CBTI, which was reflected in a 21.29-point increase in the mean score of the global health status/QOL subscale of the EORTC QLQ-C30 (p < 0.001). The emotional (mean = 13.42, p < 0.001) and cognitive (mean = 14.81, p = 0.001) function subscales improved, whereas the physical, role, and social subscales did not change. Fatigue, as measured by the PFS, decreased after the intervention (mean = 2.1, p = 0.000).
No significant changes were noted in the scores for either the HADS anxiety subscale (mean = –0.55, p = 0.417) or the HADS depression subscale (mean = –0.83, p = 0.16). Total menopausal symptoms, as measured by the MRS, decreased (mean = 6.22, p < 0.001). Scores on the MRS’s psychological, somatic, and urogenital subscales decreased after the CBTI intervention (p = 0.001) (see Table 3). 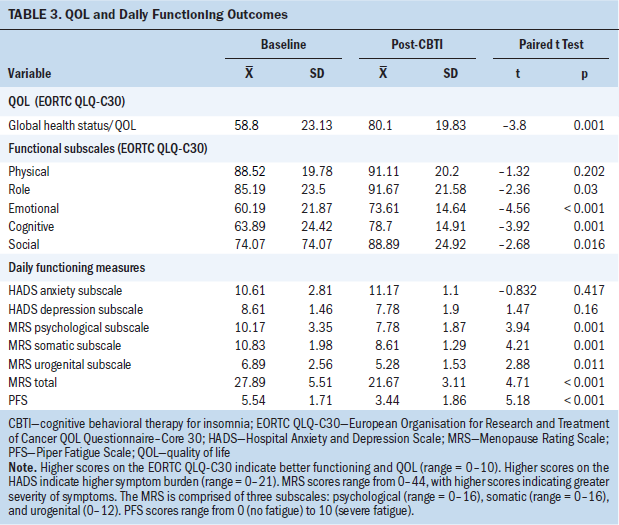
Discussion
This study is the first to pilot test the feasibility of a telemedicine-delivered CBTI in rural BCSs. CBTI is an established intervention to treat insomnia in BCSs, and it is most often provided as an in-person individual or group therapy. Participants were successfully recruited to the intervention and found the intervention to be acceptable; all were able to complete six sessions of CBTI without a loss of Internet connection or failure of computer hardware.
Consistent with in-person CBTI findings, there was significant improvement in sleep outcomes immediately after CBTI intervention. All diary-based outcomes (SE, SL, WASO, TST, number of awakenings) showed significant improvement after the six-week intervention. The most important clinical sleep outcome may be SE, which is the primary outcome that is targeted in CBTI. An SE score of 85% is the clinical threshold for distinguishing a good sleeper from a bad one (Perlis et al., 2005). The improvement in SE from 78%–88% from pre- to postintervention indicates a clinically important step from inefficient to efficient sleep. Spending excessive time in bed while awake increases SE and is counterproductive to healthy sleep; this is an example of a common perpetuating factor for chronic insomnia in the Spielman 3P model of insomnia (Spielman et al., 1987). Sleep restriction and stimulus control are key components of CBTI that address this perpetuating factor. SL and WASO represent minutes spent awake in bed that can be harmful to forming good sleep habits. In this study, both measures improved significantly, with a clinically meaningful gain of almost 40 minutes of sleep per night.
ISI scores improved, which suggests that the participants perceived CBTI to be effective in reducing insomnia severity and impact. ISI scores were reduced by nearly half (14.78 to 7.8), representing a drop from moderate to subthreshold insomnia. This change shows a moderately improved insomnia score (Morin et al., 2011). Consistent with findings about in-person CBTI in BCSs (Matthews et al., 2014), the current study showed an improvement in knowledge and beliefs regarding sleep-related issues as assessed by the DBAS-16.
In this study, participants reported a significant increase in QOL, as measured by the global health status/QOL subscale of the EORTC QLQ-C30. Additional examination of the EORTC QLQ-C30 subscales revealed an interesting pattern related to functional improvement. Following the intervention, scores for functional subscales that reflect psychosocial dimensions (role, emotional, cognitive, social) showed significant improvement, whereas scores for physical symptom subscales did not reflect any significant improvement. Pain subscale scores showed significant improvement postintervention, which may be related to the well-established interaction between sleep and perception of pain (Matre et al., 2015).
Participants’ fatigue also significantly improved after the intervention, as measured by the PFS and the fatigue subscale of the EORTC QLQ-C30. About one in four BCSs experience severe fatigue, an independent construct that often co-occurs with insomnia. CBTI has been shown to reduce cancer-related fatigue (Fleming, Dixon, Frampton, & Merry, 2012; Heckler et al., 2016); however, whether this is independent of or related to the associated decrease in sleep disturbance is unknown. In the current study, the decrease in fatigue was correlated with the reported improvement in sleep.
Rural areas throughout Colorado vary greatly in access to community services and in perceived isolation (Pedro & Schmiege, 2014), which can influence depression and hopelessness (Koopman et al., 2001). The mean scores for the HADS anxiety and depression subscales in this study did not change significantly from pre- to postintervention, but both changes approached clinical significance. This is consistent with findings from a study by Fleming, Randell, Harvey, and Espie (2014) showing that although fatigue and insomnia decrease after CBTI, improvements in anxiety and depression may take longer to manifest.
In a study of 116 cancer survivors, Burris and Andrykowski (2010) examined the differences in mental health functioning between rural and nonrural cancer survivors one to five years after cancer treatment. They found that rural cancer survivors scored higher on the HADS anxiety (effect size [ES] = 0.7) and HADS depression (ES = 0.47) subscales than nonrural survivors (Burris & Andrykowski, 2010). It is possible that the HADS is not a sensitive tool in the rural cancer survivor population or that telemedicine-delivered CBTI focused on sleep improvements was not an effective tool for treating underlying anxiety or depression. Improving sleep habits may affect anxiety and depression in the long term, but this would require a longer-term study with more outcome points.
Limitations
The current study had several strengths, such as inclusion of a rural population and the use of telemedicine, but limitations should be acknowledged. The study was designed to evaluate a telemedicine-delivered CBTI intervention in rural BCSs. Rural populations may have inherent differences based on their geographic and demographic characteristics (Pedro & Schmiege, 2014), and no attempt was made to factor this into the analysis. More detailed information and stratification of the participants by rural areas would have allowed for a richer analysis. In addition, the technology requirements for participation in this study may have limited and skewed the demographics of the sample. It is possible that the rural BCSs who chose to participate may not be representative of the larger rural BCS population. Finally, the small study population and the use of self-report measures limit generalizability of results.
Implications for Nursing Practice
A nurse-administered, telemedicine-delivered CBTI is a feasible intervention to treat rural BCSs who experience insomnia. Although Internet and cellphone access in rural areas can be challenging, in this study, sufficient access to high-speed Internet in rural counties existed to support a six-week telemedicine intervention. Rural women benefited from the CBTI intervention, as evidenced by the self-reported decrease in insomnia and increase in QOL and daily functioning.
Oncology nurses are increasingly engaged in the care of individuals with cancer throughout the trajectory of cancer treatment and survivorship. Nurse navigators help individuals and families negotiate the healthcare system to optimize treatment and symptom management. Insomnia is a common but undertreated condition that follows breast cancer treatment, which can negatively affect health and QOL. Nurses trained in CBTI would be well positioned to provide this evidence-based treatment to improve sleep in rural residents, particularly those with cancer.
Future research should expand telemedicine-delivered CBTI to other populations. For example, patients with lung or prostate cancer have high rates of insomnia and may benefit from telemedicine-delivered CBTI. The convenience of receiving insomnia therapy in the comfort of their own home may appeal to patients in urban as well as rural areas. In addition, additional research should evaluate a telemedicine-delivered CBTI intervention using smartphones. This could expand the reach of the intervention because patients would not be reliant on a broadband network for Internet connectivity. 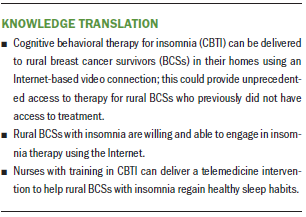
Conclusion
The results of this pilot feasibility study support a nurse-led telemedicine intervention to decrease insomnia in rural BCSs. Participants showed improvements in sleep, fatigue, QOL, and cognitive and emotional functioning. The intervention proved to be feasible in a rural population in the western United States. This study contributes to the growing body of literature that supports telemedicine interventions, but more research is needed to determine the effectiveness and sustainability of the intervention.
About the Author(s)
Michaela S. McCarthy, PhD, RN, is an advanced health services research fellow for the VA Eastern Colorado Health Care System and an adjunct faculty member in the College of Nursing at the University of Colorado, both in Denver; Ellyn E. Matthews, PhD, RN, AOCNS®, CBSM, FAAN, is an associate professor and endowed chair of oncology in the College of Nursing at the University of Arkansas for Medical Sciences in Little Rock; Catherine Battaglia, PhD, RN, is a nurse scientist in the Department of Veterans Affairs at the VA Eastern Colorado Health Care System and an associate professor in the Colorado School of Public Health at the University of Colorado; and Paula M. Meek, PhD, RN, FAAN, is a professor in the College of Nursing at the University of Colorado. This research was funded by a grant from the National Institutes of Health National Institute of Nursing Research (NRSA 1F31NR012097-01A1). Battaglia consults for and has ownership in the Joffit Group, Inc. McCarthy and Matthews contributed to the conceptualization and design and completed the data collection. McCarthy and Meek provided statistical support. McCarthy, Matthews, and Meek provided the analysis. McCarthy, Matthews, and Battaglia contributed to the manuscript preparation. McCarthy can be reached at michaela.mccarthy@ucdenver.edu, with copy to ONFEditor@ons.org. (Submitted January 2018. Accepted March 15, 2018.)
References
Aaronson, N.K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N.J., . . . de Haes, J.C. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Andrykowski, M.A., Steffens, R.F., Bush, H.M., & Tucker, T.C. (2014). Disparities in mental health outcomes among lung cancer survivors associated with ruralness of residence. Psycho-Oncology, 23, 428–436. https://doi.org/10.1002/pon.3440
Aricò, D., Raggi, A., & Ferri, R. (2016). Cognitive behavioral therapy for insomnia in breast cancer survivors: A review of the literature. Frontiers in Psychology, 7, 1162.
Arnedt, J.T., Cuddihy, L., Swanson, L.M., Pickett, S., Aikens, J., & Chervin, R.D. (2013). Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep, 36, 353–362. https://doi.org/10.5665/sleep.2448
Bastien, C.H., Vallières, A., & Morin, C.M. (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307.
Beaulieu-Bonneau, S., Ivers, H., Guay, B., & Morin, C.M. (2017). Long-term maintenance of therapeutic gains associated with cognitive-behavioral therapy for insomnia delivered alone or combined with zolpidem. Sleep, 40, zsx002. https://doi.org/10.1093/sleep/zsx002
Beraldi, A., Kukk, E., Nest, A., Schubert-Fritschle, G., Engel, J., Heußner, P., & Herschbach, P. (2015). Use of cancer-specific mental health resources—Is there an urban-rural divide? Supportive Care in Cancer, 23, 1285–1294. https://doi.org/10.1007/s00520-014-2467-x
Bjelland, I., Dahl, A.A., Haug, T.T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52, 69–77.
Bowen, D.J., Kreuter, M., Spring, B., Cofta-Woerpel, L., Linnan, L., Weiner, D., . . . Fernandez, M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36, 452–457.
Burris, J.L., & Andrykowski, M. (2010). Disparities in mental health between rural and nonrural cancer survivors: A preliminary study. Psycho-Oncology, 19, 637–645. https://doi.org/10.1002/pon.1600
Cantarero-Villanueva, I., Fernández-Lao, C., Diaz-Rodríguez, L., Cuesta-Vargas, A.I., Fernández-de-las-Peñas, C., Piper, B.F., & Arroyo-Morales, M. (2014). The Piper Fatigue Scale–Revised: Translation and psychometric evaluation in Spanish-speaking breast cancer survivors. Quality of Life Research, 23, 271–276. https://doi.org/10.1007/s11136-013-0434-5
Carney, C.E., Buysse, D.J., Ancoli-Israel, S., Edinger, J.D., Krystal, A.D., Lichstein, K.L., & Morin, C.M. (2012). The Consensus Sleep Diary: Standardizing prospective sleep self-monitoring. Sleep, 35, 287–302. https://doi.org/10.5665/sleep.1642
Dirksen, S.R., & Epstein, D.R. (2008). Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. Journal of Advanced Nursing, 61, 664–675. https://doi.org/10.1111/j.1365-2648.2007.04560.x
Espie, C.A., Kyle, S.D., Williams, C., Ong, J.C., Douglas, N.J., Hames, P., & Brown, J.S. (2012). A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep, 35, 769–781. https://doi.org/10.5665/sleep.1872
Espie, C.A., MacMahon, K.M., Kelly, H.L., Broomfield, N.M., Douglas, N.J., Engleman, H.M., . . . Wilson, P. (2007). Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep, 30, 574–584.
Fields, B.G., Schutte-Rodin, S., Perlis, M.L., & Myers, M. (2013). Master’s-level practitioners as cognitive behavioral therapy for insomnia providers: An underutilized resource. Journal of Clinical Sleep Medicine, 9, 1093–1096.
Fleming, L., & MacMahon, K. (2015). CBT-I in cancer: We know it works, so why are we waiting? Current Sleep Medicine Reports, 1, 177–183. https://doi.org/10.1007/s40675-015-0021-0
Fleming, L., Randell, K., Harvey, C.-J., & Espie, C.A. (2014). Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psycho-Oncology, 23, 679–684. https://doi.org/10.1002/pon.3468
Fleming, T., Dixon, R., Frampton, C., & Merry, S. (2012). A pragmatic randomized controlled trial of computerized CBT (SPARX) for symptoms of depression among adolescents excluded from mainstream education. Behavioural and Cognitive Psychotherapy, 40, 529–541.
Fontes, F., Severo, M., Gonçalves, M., Pereira, S., & Lunet, N. (2017). Trajectories of sleep quality during the first three years after breast cancer diagnosis. Sleep Medicine, 34, 193–199. https://doi.org/10.1016/j.sleep.2017.03.022
Garabeli Cavalli Kluthcovsky, A.C., Urbanetz, A.A., de Carvalho, D.S., Pereira Maluf, E.M., Schlickmann Sylvestre, G.C., & Bonatto Hatschbach, S.B. (2012). Fatigue after treatment in breast cancer survivors: Prevalence, determinants and impact on health-related quality of life. Supportive Care in Cancer, 20, 1901–1909. https://doi.org/10.1007/s00520-011-1293-7
Harris, P.A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J.G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381.
Heckler, C.E., Garland, S.N., Peoples, A.R., Perlis, M.L., Shayne, M., Morrow, G.R., . . . Roscoe, J.A. (2016). Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: A randomized placebo-controlled trial. Supportive Care in Cancer, 24, 2059–2066. https://doi.org/10.1007/s00520-015-2996-y
Heinemann, K., Ruebig, A., Potthoff, P., Schneider, H.P., Strelow, F., Heinemann, L.A., & Thai, D.M. (2004). The Menopause Rating Scale (MRS) scale: A methodological review. Health and Quality of Life Outcomes, 2004, 45. https://doi.org/10.1186/1477-7525-2-45
Johnson, J.A., Rash, J.A., Campbell, T.S., Savard, J., Gehrman, P.R., Perlis, M., . . . Garland, S.N. (2016). A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Medicine Reviews, 27, 20–28. https://doi.org/10.1016/j.smrv.2015.07.001
Karlin, B.E., Trockel, M., Taylor, C.B., Gimeno, J., & Manber, R. (2013). National dissemination of cognitive behavioral therapy for insomnia in veterans: Therapist- and patient-level outcomes. Journal of Consulting and Clinical Psychology, 81, 912–917. https://doi.org/10.1037/a0032554
Koch, L., Jansen, L., Herrmann, A., Stegmaier, C., Holleczek, B., Singer, S., . . . Arndt, V. (2013). Quality of life in long-term breast cancer survivors—A 10-year longitudinal population-based study. Acta Oncologica, 52, 1119–1128. https://doi.org/10.3109/0284186x.2013.774461
Koopman, C., Angell, K., Turner-Cobb, J.M., Kreshka, M.A., Donnelly, P., McCoy, R., . . . Spiegel, D. (2001). Distress, coping, and social support among rural women recently diagnosed with primary breast cancer. Breast Journal, 7, 25–33.
Landry, G.J., Best, J.R., & Liu-Ambrose, T. (2015). Measuring sleep quality in older adults: A comparison using subjective and objective methods. Frontiers in Aging Neuroscience, 7, 166. https://10.3389/fnagi.2015.00166
Liu, L., Mills, P.J., Rissling, M., Fiorentino, L., Natarajan, L., Dimsdale, J.E., . . . Ancoli-Israel, S. (2012). Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, Behavior, and Immunity, 26, 706–713. https://doi.org/10.1016/j.bbi.2012.02.001
Lowery-Allison, A.E., Passik, S.D., Cribbet, M.R., Reinsel, R.A., O’Sullivan, B., Norton, L., . . . Kavey, N.B. (2018). Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliative and Supportive Care, 16, 325–334. https://doi.org/10.1017/s1478951517000311
Manber, R., Carney, C., Edinger, J., Epstein, D., Friedman, L., Haynes, P.L., . . . Trockel, M. (2012). Dissemination of CBTI to the non-sleep specialist: Protocol development and training issues. Journal of Clinical Sleep Medicine, 8, 209–218. https://doi.org/10.5664/jcsm.1786
Matre, D., Hu, L., Viken, L.A., Hjelle, I.B., Wigemyr, M., Knardahl, S., . . . Nilsen, K.B. (2015). Experimental sleep restriction facilitates pain and electrically induced cortical responses. Sleep, 38, 1607–1617. https://doi.org/10.5665/sleep.5058
Matthews, E., Carter, P., Page, M., Dean, G., & Berger, A. (2018). Sleep-wake distrubance: A systematic review of evidence-based interventions for management in patients with cancer. Clinical Journal of Oncology Nursing, 22, 37–52. https://doi.org/10.1188/18.CJON.37-52
Matthews, E.E., Berger, A.M., Schmiege, S.J., Cook, P.F., McCarthy, M.S., Moore, C.M., & Aloia, M.S. (2014). Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: A randomized, controlled trial. Oncology Nursing Forum, 41, 241–253. https://doi.org/10.1188/14.ONF.41-03AP
McCurry, S.M., Guthrie, K.A., Morin, C.M., Woods, N.F., Landis, C.A., Ensrud, K.E., . . . LaCroix, A.Z. (2016). Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: A MsFLASH randomized clinical trial. JAMA Internal Medicine, 176, 913–920. https://doi.org/10.1001/jamainternmed.2016.1795
Morin, C.M., Belleville, G., Bélanger, L., & Ivers, H. (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608.
Morin, C.M., Vallières, A., Guay, B., Ivers, H., Savard, J., Mérette, C., . . . Baillargeon, L. (2009). Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA, 301, 2005–2015.
Morin, C.M., Vallières, A., & Ivers, H. (2007). Dysfunctional Beliefs and Attitudes about Sleep (DBAS): Validation of a brief version (DBAS-16). Sleep, 30, 1547–1554.
Orsmond, G.I., & Cohn, E.S. (2015). The distinctive features of a feasibility study: Objectives and guiding questions. OTJR, 35, 169–177. https://doi.org/10.1177/1539449215578649
Palesh, O.G., Roscoe, J.A., Mustian, K.M., Roth, T., Savard, J., Ancoli-Israel, S., . . . Morrow, G.R. (2010). Prevalence, emographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center–Community Clinical Oncology Program. Journal of Clinical Oncology, 28, 292–298. https://doi.org/10.1200/JCO.2009.22.5011
Pedro, L.W., & Schmiege, S.J. (2014). Rural living as context: A study of disparities in long-term cancer survivors [Online exclusive]. Oncology Nursing Forum, 41, E211–E219. https://doi.org/10.1188/14.ONF.E211-E219
Perlis, M.L., Jungquist, C., Smith, M.T., & Posner, D. (2005). Cognitive behavioral treatment of insomnia: A session-by-session guide. New York, NY: Springer.
Piper, B.F., Dibble, S.L., Dodd, M.J., Weiss, M.C., Slaughter, R.E., & Paul, S.M. (1998). The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncology Nursing Forum, 25, 677–684.
Reeve, B.B., Stover, A.M., Alfano, C.M., Smith, A.W., Ballard-Barbash, R., Bernstein, L., . . . Piper, B.F. (2012). The Piper Fatigue Scale-12 (PFS-12): Psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Research and Treatment, 136, 9–20. https://doi.org/10.1007/s10549-012-2212-4
Ritterband, L.M., Thorndike, F.P., Ingersoll, K.S., Lord, H.R., Gonder-Frederick, L., Frederick, C., . . . Morin, C.M. (2017). Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: A randomized clinical trial. JAMA Psychiatry, 74, 68–75.
Savard, J., Ivers, H., Savard, M.H., & Morin, C.M. (2014). Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep, 37, 1305–1314. https://doi.org/10.5665/sleep.3918
Savard, J., Ivers, H., Savard, M.H., & Morin, C.M. (2016). Long-term effects of two formats of cognitive behavioral therapy for insomnia comorbid with breast cancer. Sleep, 39, 813–823. https://doi.org/10.5665/sleep.5634
Savard, J., Ivers, H., Villa, J., Caplette-Gingras, A., & Morin, C.M. (2011). Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. Journal of Clinical Oncology, 29, 3580–3586. https://doi.org/10.1200/JCO.2010.33.2247
Savard, J., Simard, S., Ivers, H., & Morin, C.M. (2005). Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. Journal of Clinical Oncology, 23, 6097–6106.
Seyffert, M., Lagisetty, P., Landgraf, J., Chopra, V., Pfeiffer, P.N., Conte, M.L., & Rogers, M.A. (2016). Internet-delivered cognitive behavioral therapy to treat insomnia: A systematic review and meta-analysis. PLOS ONE, 11, e0149139.
Singh, J., Badr, M.S., Diebert, W., Epstein, L., Hwang, D., Karres, V., . . . McCann, K. (2015). American Academy of Sleep Medicine (AASM) position paper for the use of telemedicine for the diagnosis and treatment of sleep disorders. Journal of Clinical Sleep Medicine, 11, 1187–1198. https://doi.org/10.5664/jcsm.5098
Spielman, A.J., Caruso, L.S., & Glovinsky, P.B. (1987). A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America, 10, 541–553.
Thomas, K.A., & Burr, R.L. (2009). Accurate assessment of mother and infant sleep: How many diary days are required? American Journal of Maternal/Child Nursing, 34, 256–260.
Trauer, J.M., Qian, M.Y., Doyle, J.S., Rajaratnam, S.M., & Cunnington, D. (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163, 191–204.
Trudel-Fitzgerald, C., Zhou, E.S., Poole, E.M., Zhang, X., Michels, K.B., Eliassen, A.H., . . . Schernhammer, E.S. (2017). Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. British Journal of Cancer, 116, 1239–1246. https://doi.org/10.1038/bjc.2017.85
van Straten, A., van der Zweerde, T., Kleiboer, A., Cuijpers, P., Morin, C.M., & Lancee, J. (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. https://doi.org/10.1016/j.smrv.2017.02.001
Wang, X.S., Williams, L.A., Krishnan, S., Liao, Z., Liu, P., Mao, L., . . . Cleeland, C.S. (2012). Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, Behavior, and Immunity, 26, 699–705. https://doi.org/10.1016/j.bbi.2011.12.007
Weaver, K.E., Geiger, A.M., Lu, L., & Case, L.D. (2013). Rural-urban disparities in health status among US cancer survivors. Cancer, 119, 1050–1057. https://doi.org/10.1002/cncr.27840
Yoon, I.Y., Kripke, D.F., Elliott, J.A., Youngstedt, S.D., Rex, K.M., & Hauger, R.L. (2003). Age-related changes of circadian rhythms and sleep-wake cycles. Journal of the American Geriatrics Society, 51, 1085–1091.
Zigmond, A.S., & Snaith, R.P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67, 361–370.




