Precision Medicine Testing and Disparities in Health Care for Individuals With Non-Small Cell Lung Cancer: A Narrative Review
Problem Identification: Precision medicine initiatives provide opportunities for optimal targeted therapy in individuals with non-small cell lung cancer. However, there are barriers to these initiatives that reflect social determinants of health.
Literature Search: MEDLINE®, CINAHL®, PsycINFO®, Embase®, and Google ScholarTM databases were searched for articles published in English in the United States from 2016 to 2020.
Data Evaluation: Data that were collected included individual demographic information, specific diagnosis, status of targeted genomic testing, and receipt of targeted therapy. All studies were retrospective and involved database review of insurance claims or medical records.
Synthesis: Individuals with non-small cell lung cancer received less genetic testing and targeted therapy if they were of a lower socioeconomic status, had public health insurance or no health insurance, were Black, or lived in rural communities.
Implications for Nursing: Social determinants of health affect health equity, including in precision medicine initiatives for individuals with lung cancer. Gaining an understanding of this impact is the first step in mitigating inequities.
Jump to a section
Individuals who have a diagnosis of lung cancer with actionable genetic subtypes are poised to greatly benefit from advances in precision medicine. Lung cancer is consistently the cause of more than 100,000 deaths per year in the United States and is the number one cause of death among all cancer types (Krist et al., 2021). In 2003, the human genome was sequenced after a massive global effort (Connors & Schorn, 2018), and researchers began to understand the mechanisms that cause cancer to grow (Krzyszczyk et al., 2018). Ultimately, this led to the development of precision medicine and pharmacogenetics techniques, allowing healthcare providers to treat some cancers in a very specific way, including some cases of lung cancer. Advances in this realm have led to the development of drugs that can specifically target the action of the mutated proteins to inhibit tumor growth. Pharmaceutical companies have pushed the availability of these targeted therapies into the market (Knutsen, 2016).
Eighty-five percent of lung cancer cases are characterized histologically as non-small cell lung cancer (NSCLC). In 57% of cases, lung cancer is diagnosed after it has metastasized, and the five-year survival rate for these individuals is less than 6% (Goebel et al., 2019). In the past decade, individuals with NSCLC have been shown to carry an identifiable genetic variant in their tumor cells in more than 53% of cases. Providers use genetic findings to determine eligibility for individuals with lung cancer for targeted therapy, which has been shown to prolong survival and is often considered a first-line treatment (Rajurkar et al., 2020). Targeted therapies are not only associated with longer survival, but also fewer side effects than traditional forms of cancer treatment, such as chemotherapy and radiation therapy (Ginsburg & Phillips, 2018). Kehl et al. (2019) reported that individuals with stage IV NSCLC have a survival rate of 5.2 months with no systemic treatment, 9.8 months with chemotherapy, and 18.8 months with targeted therapy. Another study reported that median survival for those who received molecular testing and targeted therapies ranged from 14.9 months to 34.2 months (Al-Ahmadi et al., 2020). When compared to the traditional methods of cancer treatment, the development of targeted therapies is a welcomed option.
Although advances in precision medicine have led to the development of important protocols for individuals with NSCLC, many do not undergo the associated care measures, such as molecular testing and targeted therapy, or may not benefit from these therapies. Previous research reveals some social factors, including socioeconomic status (SES), health insurance status, race, and regional area, are related to testing frequency and/or targeted therapy; however, a structured approach to summarizing findings is warranted. The American Medical Association describes six domains that contribute to social determinants of health (SDOH): economic stability, neighborhood and physical environment, education, food, community and social context, and healthcare system (Bennett et al., 2018). This narrative review examines how these and other factors, such as race, serve as barriers to receiving genetic testing and aims to understand health system inequities in receiving targeted therapy. Therefore, the research question was as follows: What disparities in health care contribute to precision medicine testing and targeted therapy in individuals with NSCLC?
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) format (Moher et al., 2009), a literature search was conducted using MEDLINE®, CINAHL®, PsycINFO®, Embase®, and Google ScholarTM databases. Search terms, used singly or in combination, were lung cancer OR lung neoplasms, pharmacogenetic testing OR genetic testing, healthcare disparities OR health status disparities OR minority health, precision medicine, access, and review. Figure 1 illustrates the search and review process. The search included peer-reviewed articles of original studies published in English. Data abstracted included author(s); publication year; title; data source; population studied; variables assessed in the study, including demographic characteristics; statistical analysis; and relevant results. Inclusion criteria were published quantitative studies of genomic testing for lung cancer diagnosis in the past 10 years; study data, including demographic and health equity information, as reflected in SDOH; and studies written in English and conducted in the United States. Studies that were systematic reviews, abstracts, and poster presentations were omitted. 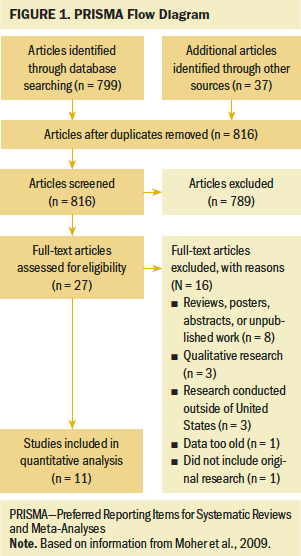
This study encompasses care measures during the emerging process of targeted therapy interventions. Although study publication dates are within the past 10 years (2016–2020), data that the studies represent date back as far as 2007, when precision medicine measures were first emerging for NSCLC. Erlotinib was approved by the U.S. Food and Drug Administration (FDA) in 2013 as a first-line treatment for those with an epidermal growth factor receptor (EGFR) variant, becoming the first targeted therapy to be used as a first-line treatment for advanced NSCLC (Spira et al., 2016). However, in 2004, erlotinib was initially FDA-approved for use in locally advanced or metastatic NSCLC (Palazzo et al., 2019), demonstrating its value as a promising treatment. Therefore, based on development and state of the science for precision medicine initiatives, the current study captures the rise of these initiatives for use in individuals with NSCLC.
Quality Appraisal
The studies were independently appraised for applicability and quality by the research team (M.C. and S.S.D.), who then reached a consensus. Rigor was assessed by considering the criteria to rate the strength of scientific evidence published by the Agency for Healthcare Research and Quality (West et al., 2002). Table 1 summarizes the strength of scientific evidence, including quality assessments of the studies and risk of bias. 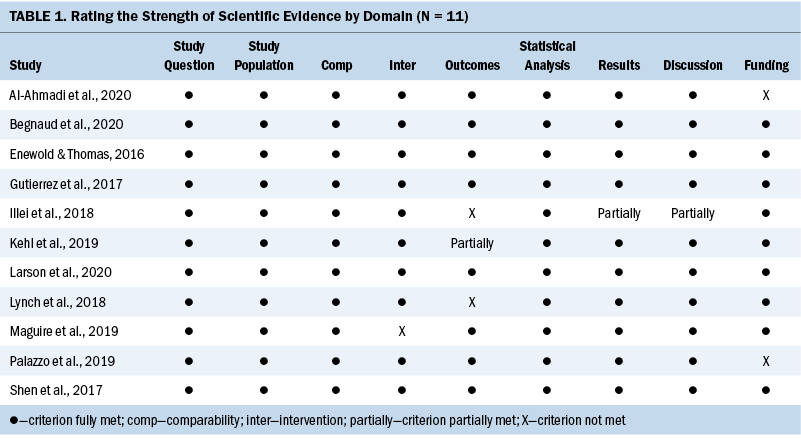
Data Evaluation
Search efforts returned 816 articles after removing duplicates. Initial review of titles and/or abstracts revealed 27 articles that possibly met the inclusion criteria. Following full-text review, 16 articles were excluded because they did not represent original research; were posters, abstracts, or unpublished work; reported qualitative results or data considered too old to be relevant; or represented international data. Eleven articles met the inclusion criteria for this study.
All studies included in this review were retrospective and involved a review of medical records or insurance claims. The databases that were used varied from providing national data, such as from the Surveillance, Epidemiology, and End Results Program database to regional data from medical claims in specific areas. Specific areas included Minnesota, New Jersey, Maryland, Kentucky, California, and Ohio. The potential overlap from use of the same national database is acknowledged and accepted in this study because each article addresses the relationship between precision medicine testing and treatment in those with NSCLC or lung cancer and varying demographic factors. The authors’ efforts to illuminate various disparities differ between studies, making all data valuable. Of note, more than 1.1 million cases were reported from an analysis of Medicare claims from individuals with a diagnosis of lung cancer during a three-year period in one study (Lynch et al., 2018). Although this study provides important information for analysis, the other studies focused on advanced-stage NSCLC and precision medicine efforts and ranged from 200 to 35,000 reviewed records.
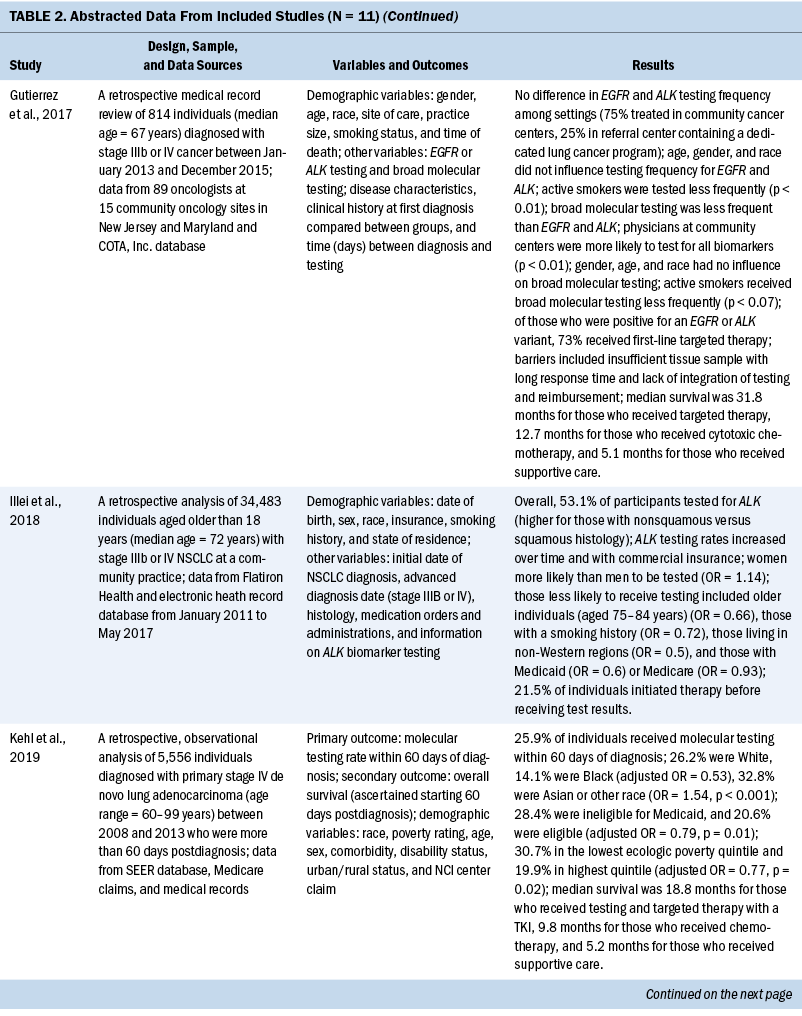
The years that the data analyzed for this review span from 2007 to 2017. For studies that reported age, the mean age of participants ranged from 65 to 72 years. When comparing the prevalence of molecular testing and targeted therapy among participants, eight studies addressed disparities based on age, six studies based on SES, six studies based on medical insurance status, eight studies based on race, six studies based on geographic location or spatial significance, and seven studies based on gender. For the abstracted data from each article, see Table 2. 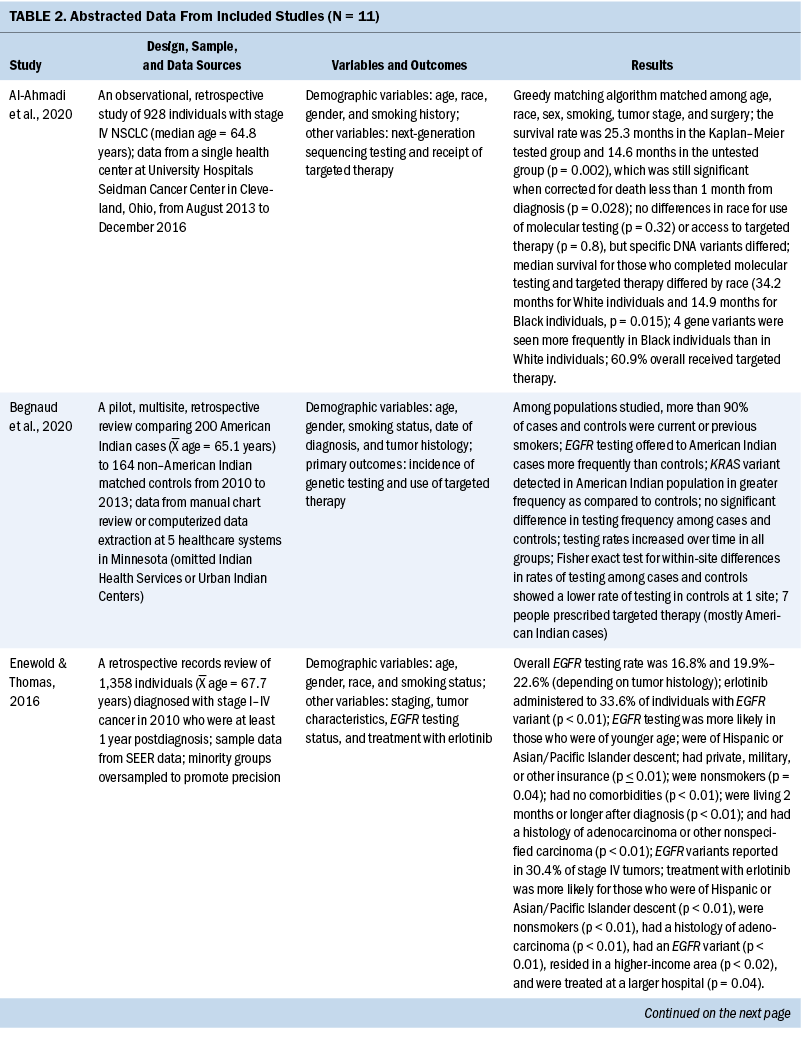

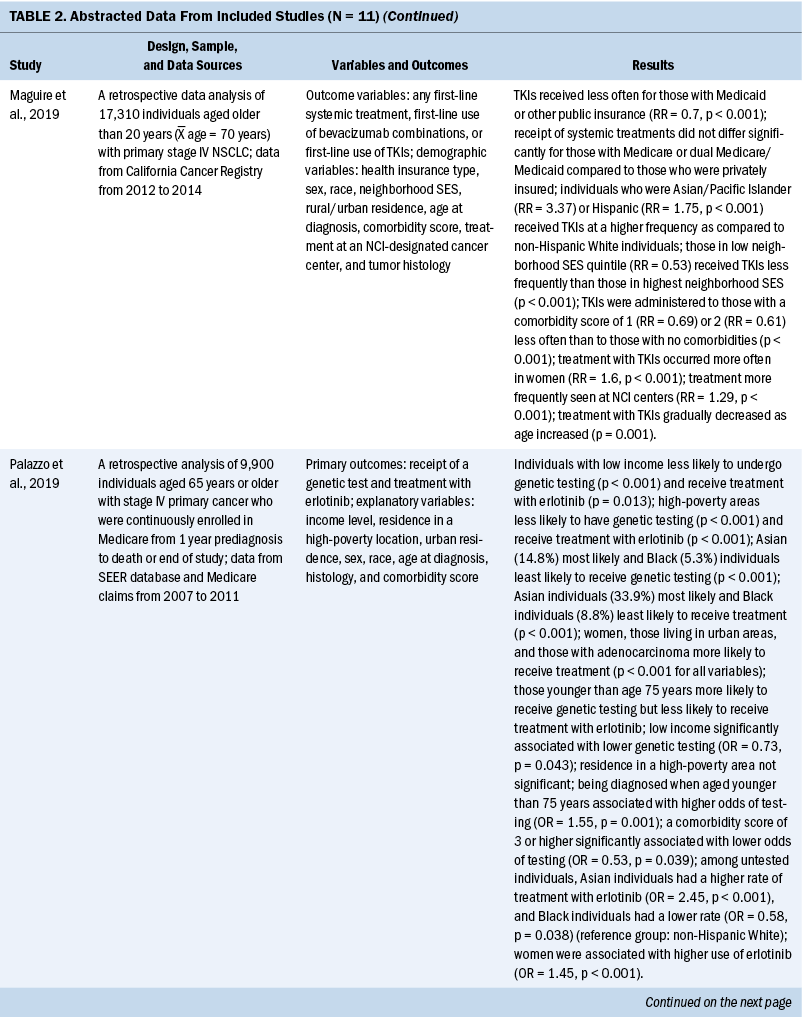
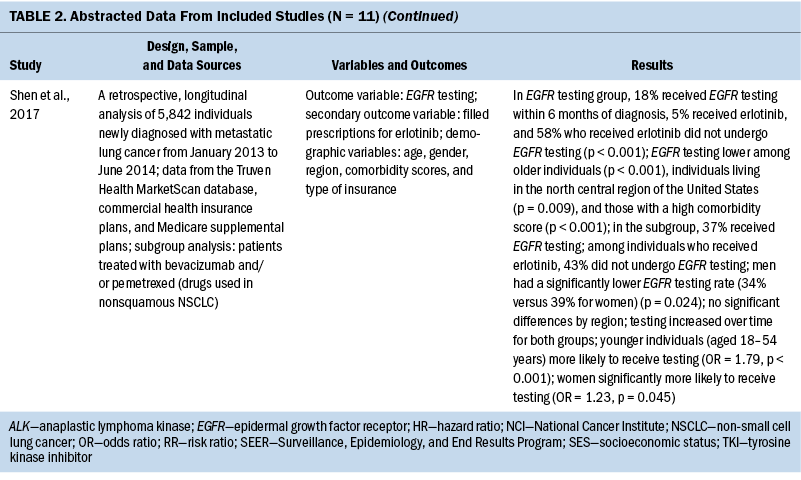
Results
Retrospective Data Analysis of Included Studies
Eleven quantitative studies met inclusion criteria. Key variables in most studies were genetic testing (also termed molecular testing) on tumor tissue and targeted therapeutic treatment using precision medicine techniques. Explanatory variables included SES, medical insurance type, race, geographic region, and type of treatment facility. In addition, age and gender are examined, as well as comorbidity score and smoking status.
Socioeconomic status and health insurance: SES and type of insurance were collectively discussed in seven studies. Six of these studies identified that precision medicine testing was more common if the individual had private insurance and a higher income. Enewold and Thomas (2016) reported significant associations (p < 0.01) between undergoing EGFR testing and having private or military insurance. These results were echoed by Kehl et al. (2019), who reported testing rates to be significantly lower (p = 0.01) in those who qualified for Medicaid, which was an indication of lower income. This same study evaluated the molecular testing rate based on poverty quintile and found that testing was completed 10% less often in the lowest poverty quintile as compared to the highest (p = 0.02). Palazzo et al. (2019) also reported that those with low income, as indicated by eligibility or receipt of prescription drug subsidies (Part D Medicare), are significantly less likely to receive genetic testing (p = 0.0001).
Some heterogeneity was noted in how researchers considered Medicaid and Medicare coverage. Medicaid coverage is based on need, with eligibility primarily dependent on income, whereas Medicare eligibility is based on age or disability (Centers for Medicare and Medicaid Services, 2018). Medicare recipients who meet Medicaid eligibility because of chronic illness, long-term care needs, or social risk factors can qualify for a dual Medicare/Medicare insurance plan (Centers for Medicare and Medicaid Services, 2020). Among the reviewed studies, some inconsistency was seen in the reporting of Medicare and Medicaid insurance status, primarily in how dual Medicare/Medicaid was considered (either alone, grouped with Medicare, or grouped with Medicaid). Despite reporting differences, some trends were observed. Illei et al. (2018) found that individuals with Medicaid or Medicare alone and dual Medicare/Medicaid were less likely to be tested than those with commercial insurance. Another study supported this finding, in addition to significantly lower survival (p = 0.0053) for those in public (Medicaid and Medicare) insurance groups (Larson et al., 2020). Lynch et al. (2018) reported that individuals with dual Medicare/Medicaid received molecular testing significantly less often than individuals with Medicare only (p = 0.000).
Enewold and Thomas (2016) reported that treatment with erlotinib for stage IV NSCLC (adenocarcinoma subtype) was significantly more likely for individuals who reside in a higher-income area (p < 0.02). Similarly, Larson et al. (2020) reported that those enrolled in public health insurance (Medicaid or Medicare) in Kentucky were significantly less likely to receive targeted therapy (p = 0.007) when compared to those with private insurance, as well as individuals living in moderate to high poverty (p = 0.008). Maguire et al. (2019) reported that individuals with private insurance were significantly more likely to receive treatment than those with Medicaid (p < 0.001) and that decreasing neighborhood SES was associated with decreased treatment (p < 0.001). However, this same study stated that individuals with Medicare or dual Medicare/Medicaid did not differ significantly from those with private insurance in the receipt of targeted therapy.
Race: Enewold and Thomas (2016) noted that individuals of Hispanic and Asian/Pacific Islander descent were associated with increased rates of EGFR testing compared to other races (p < 0.01). Lynch et al. (2018) stated that individuals of Asian/Pacific Islander descent underwent molecular testing with the highest percentage of those studied when compared to other racial groups, and Kehl et al. (2019) reported a testing rate of 32.8% among Asian individuals and 26.2% among White individuals. Of note, the 14.1% rate of testing among Black individuals was almost half the rate for White individuals and less than half the rate of testing for Asian individuals. Palazzo et al. (2019) also made this distinction, reporting that Asian individuals were the most likely to receive molecular testing and targeted therapy, whereas Black individuals received the lowest rate of testing and therapy (p < 0.0001).
Several other studies also supported the observation that individuals of Asian descent received targeted therapy more often than individuals of other racial backgrounds (Enewold & Thomas, 2016; Maguire et al., 2019; Palazzo et al., 2019). Maguire et al. (2019) reported that individuals of Asian/Pacific Islander and Hispanic descent received targeted therapy at a significantly higher rate when compared to non-Hispanic White individuals (p < 0.001).
Two studies in this review reported conflicting information regarding race in relation to molecular testing and targeted therapy. Gutierrez et al. (2017) and Al-Ahmadi et al. (2020) found that race had no significant influence on molecular testing frequency. However, both studies represented regional data, with the Gutierrez et al. (2017) study representing 15 regional clinics in New Jersey and Maryland and the Al-Ahmadi et al. (2020) study representing one large institution in Cleveland, Ohio. Although race was not a factor for testing in these two studies, one of the studies reported that survival for individuals with NSCLC undergoing molecular testing and targeted therapy differed, with a median survival of 34.2 months for White individuals and only 14.9 months for Black individuals (p = 0.015) (Al-Ahmadi et al., 2020).
In a study by Begnaud et al. (2020), genetic testing and targeted therapy among American Indian individuals with lung cancer was compared with non–American Indian individuals with lung cancer in a regional healthcare system in Minnesota. Of note, only one-third of individuals from each group received genetic testing. In addition, the variant most frequently identified in American Indian individuals was the KRAS gene, a finding most often associated with a history of smoking and deemed difficult to treat (Rajurkar et al., 2020). This finding emphasizes the importance of a diverse population in precision medicine efforts.
Access: Several studies agreed that molecular testing of tumors in individuals with NSCLC was more likely if the individual lived in an urban or metropolitan area (Larson et al., 2020; Lynch et al., 2018; Palazzo et al., 2019). Palazzo et al. (2019) made the additional claim that targeted treatments were more commonly given to individuals residing in urban areas (p < 0.0001). Larson et al. (2020), who studied healthcare trends involving precision medicine in Kentucky, made the distinction that those who lived in rural areas—Appalachian areas or non-Appalachian areas—received precision medicine testing significantly less often than those in metropolitan areas (p = 0.001). In their study, Lynch et al. (2018) reported that Los Angeles, California, and Boston, Massachusetts, had the highest rates of testing (p = 0.000).
Regional observations were also reported. Illei et al. (2018) found that those who lived in non-Western states were less likely to get tested. Shen et al. (2017) reported that the north central region of the United States had lower rates of precision medicine testing for eligible individuals when compared to other regions (p = 0.009). Two studies reported that residing close to a National Cancer Institute testing center was associated with higher rates of testing (Lynch et al., 2018) and higher rates of targeted therapy use (p < 0.001) (Maguire et al., 2019).
Age and gender: Most studies reported that molecular testing, either specific tests such as EGFR or panel testing for multiple markers, was associated with increased testing at younger ages (Enewold & Thomas, 2016; Illei et al., 2018; Larson et al., 2020; Palazzo et al., 2019; Shen et al., 2017). The only researchers who disagreed with this association were Lynch et al. (2018), who reported less molecular testing at younger ages (aged less than 55 years). However, the population for this study was individuals with Medicare, with age eligibility of 65 years or older. Similarly, several studies described a relationship of greater incidence of targeted therapy at younger ages (Larson et al., 2020; Maguire et al., 2019), and one study noted no significant difference (Gutierrez et al., 2017). Palazzo et al. (2019) reported that although younger age (less than 75 years) was associated with more testing, it was also associated with a decreased incidence of receiving targeted therapy.
Most studies were consistent regarding gender and molecular testing and targeted therapy. Women were significantly more likely to undergo testing according to five studies (Illei et al., 2018; Larson et al., 2020; Lynch et al., 2018; Palazzo et al., 2019; Shen et al., 2017), and three studies reported that women were also more likely to receive targeted therapies (Larson et al., 2020; Maguire et al., 2019; Palazzo et al., 2019). Only one study reported no difference between gender and genetic testing (Gutierrez et al., 2017).
Smoking and comorbidity status: Four studies reported on the smoking status of individuals and the likelihood of receiving genetic testing and treatment. All reported lower odds for testing in active smokers or increased incidence of testing for nonsmokers (Enewold & Thomas, 2016; Gutierrez et al., 2017; Illei et al., 2018; Larson et al., 2020), whereas one study reported that nonsmokers with no smoking history had a higher likelihood of receiving treatment with targeted therapy (p < 0.01) (Enewold & Thomas, 2016).
Four studies measured individual comorbidity index. All results showed a significant association between higher comorbidity scores and lower molecular testing rates (Enewold & Thomas, 2016; Lynch et al., 2018; Palazzo et al., 2019; Shen et al., 2017). Maguire et al. (2019) reported that lower average comorbidity scores were also associated with an increased rate of receiving targeted therapy (p < 0.001).
Quality Review
All studies met the criteria for the study question, study population, and comparability of participants adequately. Although most studies had a homogeneous population regarding diagnosis of NSCLC, there was variability among participants in specific demographic variables, such as race, SES, medical insurance status, geographic location, age, and gender. All but four studies addressed the intervention of molecular testing and the outcome of targeted therapy. The study by Maguire et al. (2019) explored outcomes only, including tyrosine kinase inhibitor targeted therapy (Rajurkar et al., 2020). All studies conducted appropriate statistical analyses, with most reporting results clearly and appropriately, and presenting thoughtful discussions, including limitations and future directions. All studies were retrospective in nature and primarily involved review of medical billing records. Only two studies did not report a funding source.
Discussion
This review confirms that health disparities must be acknowledged when considering precision medicine initiatives in the realm of molecular testing and targeted therapeutic treatment for individuals with NSCLC in the United States. The main disparities were seen in SES, race, and access (regional area). SDOH domains are closely aligned with the disparities that were observed in this review. The SDOH domains of economic stability and healthcare system become important when considering disparities observed in SES and health insurance status. Race plays a role in discriminatory practices assigned to the social and community context domain. In addition, the social context of smoking is relevant in this study population as demonstrated in the studies reviewed. Lastly, access is a function of the domain of neighborhood and built environment in many ways. Table 3 highlights the SDOH domains and their relationship to studies examined. 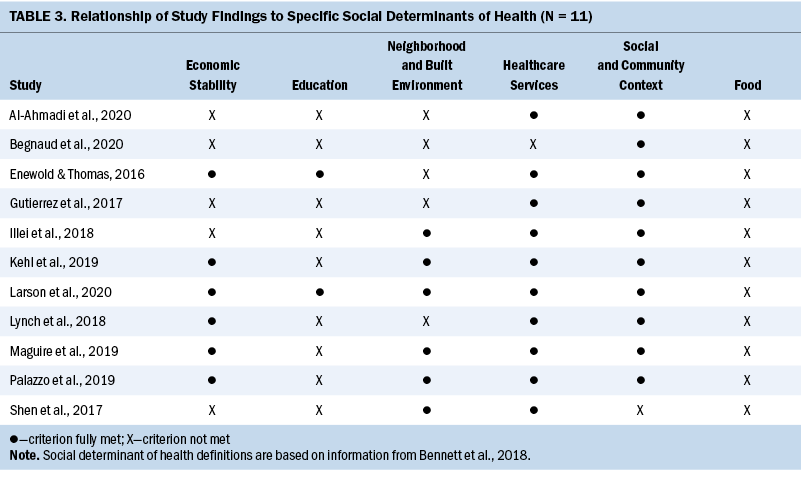
Economic Stability and Healthcare System
It was consistently reported that those of lower SES are less represented in individuals who receive molecular testing and/or targeted therapy when compared to the NSCLC population as a whole (Enewold & Thomas, 2016; Illei et al., 2018; Kehl et al., 2019; Larson et al., 2020; Lynch et al., 2018; Maguire et al., 2019; Palazzo et al., 2019). Health insurance type and status are often a measure of one’s economic situation. According to a U.S. Census report (Berchick et al., 2019), about 8% of the U.S. population was uninsured in 2018. Of those who were insured, about two-thirds had private insurance and about one-third had public insurance. Public insurance was split between Medicaid and Medicare. The percentage of individuals with private insurance increased as income level increased, and the percentage of individuals with public insurance or no insurance was highest at low-income levels (Berchick et al., 2019). Because Medicare eligibility is primarily based on age rather than need, it is interesting to see how it compares to other insurance types. Individuals who had Medicaid alone or paired with Medicare received less molecular testing and targeted therapy than those with private insurance. Only one study (Maguire et al., 2019) demonstrated that dual Medicare/Medicaid insurance recipients received the same likelihood for targeted therapy as those with commercial insurance. The authors acknowledge that this finding contrasts with that of other researchers and hypothesize that perhaps the dual coverage defrayed some costs related to care and treatment that would be incurred with Medicare alone. Dual eligibility for Medicare/Medicaid offers an interesting comparison to Medicare or Medicaid alone or other insurance. In the U.S. healthcare system, different levels of insurance coverage translate to different levels of financial coverage for particular care measures (Goodell, 2020; Ridic et al., 2012). Individuals are often in a position where they must assume the burden of the cost for precision medicine initiatives (Krzyszczyk et al., 2018), with the cost for testing and one round of treatment approximated to be $10,000 (Burris et al., 2018). Therefore, it is unsurprising to observe inequities in this realm.
Social and Community Context
Black Americans are shown to be consistently underrepresented in testing and treatment populations when compared to all other racial backgrounds (Kehl et al., 2019; Lynch et al., 2018; Palazzo et al., 2019). The disparity that is observed in Black individuals with NSCLC is concerning because this population far exceeds those from other racial backgrounds in mortality even in early-stage cancer (Al-Ahmadi et al., 2020; Soneji et al., 2017). The importance of testing individuals from diverse racial and ethnic backgrounds has become apparent in precision medicine because specific genetic variations can differ in frequency among different races. For example, among non-Hispanic White individuals, the rate of EGFR variants in the tumor tissue of individuals with NSCLC is about 10%–20%, whereas for individuals with the same clinical findings who are of South American or Asian descent, the incidence is as high as 50% (Tan et al., 2016). An early understanding of the incidence of the EGFR variant in individuals with NSCLC in Asian individuals was achieved through the IPASS studies (Fukuoka et al., 2011). This, in part, may contribute to the increased incidence and treatment among Asian individuals with NSCLC. The importance of testing individuals from diverse backgrounds is even more crucial so that treatment can be optimized for all individuals.
Studies that examined smoking status all reported that molecular testing and targeted therapy were less likely in individuals who smoked (Enewold & Thomas, 2016; Gutierrez et al., 2017; Illei et al., 2018; Larson et al., 2020). The retrospective nature of most of the studies prevents further examination of this association, but one explanation may be that those who continue to smoke may be unable to modify this behavior. It has been reported that those who smoke and develop advanced lung cancer express feelings of responsibility and inevitability for their diagnosis (Dickerson et al., 2012). In one study that reported a low genetic testing rate of about one-third of individuals with advanced lung cancer, more than 90% of participants reported that they had a current or former smoking habit (Begnaud et al., 2020). Care must be taken in this realm because smoking status is known as an unreliable data point, particularly in electronic health records (Patel et al., 2020).
Neighborhood and Built Environment
Regional area analysis indicated that individuals residing in urban areas were more likely to undergo molecular testing and/or receive targeted therapy when compared to those residing in rural areas (Larson et al., 2020; Lynch et al., 2018; Palazzo et al., 2019). Further trends suggest that populations in Western states have higher testing and treatment rates (Illei et al., 2018), and populations in the middle Northern states have lower rates (Shen et al., 2017). This could be suggestive of the concentration of urban areas in coastal regions. In addition, practice types in urban areas are varied, and there are more opportunities to be treated at hospital-, research-, or specialty-based settings, all of which report higher testing and treatment rates than general and community-based healthcare settings. Urban areas are also where National Cancer Institute–designated centers are likely to be found (Lynch et al., 2018; Maguire et al., 2019). The National Institutes of Health (2017) noted that 85% of cancer care in the United States is provided in community settings, which is the most prevalent format available in rural areas. Center practice distinctions also were apparent in a survey study of oncologists conducted by Gray et al. (2017), who reported that molecular testing for individuals with NSCLC occurs significantly less often in nonprofit integrated health systems when compared to hospital-based specialty groups or solo practices. Therefore, the concept of spatial significance is a strong contributor to health disparities when examining the use of precision medicine initiatives among those who live in rural and urban areas. For individuals with NSCLC to benefit from specific treatments, it is necessary to gain access to facilities and healthcare providers who are cognizant of the standards of care. Of note, many healthcare providers have expressed concern with maintaining the experience and knowledge necessary to provide up-to-date care. This is particularly true in primary care, where providers are hesitant to implement care because of lack of knowledge of or resources on genetics (Carroll et al., 2016).
Individual-Related Risk Factors
Studies that examined comorbidity scores all reported that molecular testing and targeted therapy were less likely if the individual had a higher comorbidity score (Enewold & Thomas, 2016; Lynch et al., 2018; Maguire et al., 2019; Palazzo et al., 2019; Shen et al., 2017). One explanation may be that comorbidities can contribute to a more urgent health crisis or may make a lung tissue biopsy procedure too dangerous. Another possibility is that the individual died before testing and/or treatment could be completed. One study reported an association between increased molecular testing rates and living at least two months after diagnosis (Enewold & Thomas, 2016). Gutierrez et al. (2017) reported that 4% of their study population died within one month of diagnosis, but this association was not consistently reported in all studies. Most cases of lung cancer are diagnosed at advanced stages (Crinò et al., 2010), making molecular testing an intervention that requires much valuable time. Although molecular testing techniques vary, results can take two weeks to be completed (Krzyszczyk et al., 2018) and are sometimes not received in the clinic for as many as 23 days (Gutierrez et al., 2017). Often, providers will start targeted therapy prior to receiving molecular testing results or without completing molecular testing (Illei et al., 2018) to allow the individual the potential for benefit. There is the possibility of reliably detecting circulating tumor cells or circulating tumor DNA in the bloodstream (Heitzer et al., 2017) in the future, which would allow testing in a safe and minimally invasive manner, as well as potentially provide earlier detection.
Consideration can also be given to possible feelings of mistrust and misunderstanding that surround new protocols in health care. Many individuals view clinical trial participation as burdensome and inconsequential (Miller et al., 2013) or leading to a financial burden in exchange for modest clinical benefit (Carrera & Ormond, 2015). Others report mistrust of the healthcare system and unfavorable attitudes toward research (Rogers et al., 2018), including how their personal genetic data are used and whether they will gain knowledge from the testing (Edwards et al., 2016). Qualitative exploration of these factors may offer insight.
This review illustrates the continuing disparities that exist in the healthcare system regarding SDOH. Although new advances are ongoing and promising, it is difficult to appreciate the benefit when segments of the population are not receiving the same care. This is unjust not only on a social level, but also on a scientific level, as shown by the importance of studying the varying genetic changes among those of different racial backgrounds. It is beneficial to explore ways to mitigate disparities on all levels and in all realms of health care. One consistency among most individuals in the United States is that they seek medical care when they are sick. The focus of mitigation efforts should be on education and outreach for frontline providers. This is possible with a knowledgeable provider in primary care, oncology, inpatient, or emergency services, paired with outreach capabilities that equip healthcare centers beyond research facilities with the tools needed for the latest advances in care. Although awareness is an important first step, it is not enough. Efforts in this realm, starting with policy changes and provision of resources, can be coordinated on a national level. Awareness of inequities continues with dissemination of existing practices, such as in the current review. Finally, educational efforts in the formal educational settings, as well as continuing education formats, are crucial.
Limitations
Not all studies reviewed were comprehensive about SDOH, which poses a limitation. Future studies could focus on precision medicine efforts in NSCLC and SDOH in particular. Of note are the vast and rapid discoveries in precision medicine efforts, which make it challenging for providers of clinical care to stay up to date on research advances. As new and promising initiatives become available, researchers’ continued attention on disparities and ways to mitigate them will ensure that equity is not forgotten. Another limitation is that this review focused on retrospective studies that analyzed medical and billing records, so the views of the affected individuals were not considered. Future studies exploring this perspective could provide a deeper understanding of factors that contribute to disparities. By examining the reasons why individuals did not pursue molecular testing, researchers can gain insight into individuals’ desires to get tested, their mistrust or skepticism of testing, or lack of knowledge. Complementing this narrative with qualitative efforts can enhance understanding. A third limitation is the lack of detail that was discussed regarding medical insurance. The complexities of insurance coverage are vast, and the general nature of what was provided in this review only allowed for broad associations. Lastly, the authors acknowledge that some articles that may have contributed to the findings of this review may have been missed.
Implications for Nursing
Precision medicine is becoming integrated into almost all aspects of health care. As understanding of the human genome experiences exponential growth, so does the ability to treat genetic changes that are responsible for illness. Nurses are also involved in most aspects of health care, so the need for comprehension in this realm is great.
Nursing literature often adopts the concept of precision health to offer broader meaning. Valuing the scientific advances that are leading to strides in health care with a holistic view, nurses have stressed the importance of considering social, societal, and environmental determinants of health (Dorsey et al., 2019). Expanding on the National Institute of Nursing Research Symptom Science Model, Hickey et al. (2019) developed the Nursing Science Precision Health Model, in which phenotypic characterizations included lifestyle and environmental factors, and clinical applications included self-management of interventions. Together with a clinical presentation and the details of genetic and biochemical test results, there is a need for nurses to help with the translation of complex information to individuals to whom they provide care.
A further implication for nurses is the need for advocacy and policy changes to ensure that health care is delivered in a fair and equitable manner. Precision medicine initiatives can be powerful tools in the treatment of devastating illnesses, and their benefits should apply to all with an appropriate diagnosis. The disparities illuminated in this review illustrate the need for work in this realm. Nurses, making up the largest segment of healthcare workers in the United States (Fayer & Watson, 2015), are poised to have an important voice in this change. Several researchers have called for the creation of an overall policy to direct precision medicine efforts (Bertier et al., 2016). This would serve to offer guidelines for all stakeholders to use in developing and providing care equitably. Through these approaches, nurses are positioned to lead the way in mitigating many of the SDOH that exist not only in the realm of precision medicine, but in health care itself. 
Conclusion
Rapid advances in molecular testing and the development of targeted therapeutic treatments have demonstrated promising results for individuals with NSCLC. This narrative explored the current literature to comprehensively identify disparities in precision medicine initiatives for individuals with NSCLC. The reviewed studies contribute to greater understanding of the provision of care. Gaining awareness of the inequities in the provision of precision medicine initiatives is the first step toward mitigation.
The authors gratefully acknowledge Grace Dean, PhD, RN, for her wisdom and expertise that she shared during the final revisions of the manuscript. Her suggestions and endorsement were very meaningful and allowed the authors to publish this work with greater confidence.
About the Author(s)
Martha Curtin, MS, RN, is a PhD student, Darryl Somayaji, PhD, MSN, CNS, CCRC, is an assistant professor, and Suzanne S. Dickerson, DNS, RN, is a professor, all in the School of Nursing at the State University of New York at Buffalo. No financial relationships to disclose. Curtin and Dickerson contributed to the conceptualization and design. Curtin completed the data collection and provided statistical support. All authors provided the analysis and contributed to the manuscript preparation. Curtin can be reached at marthacu@buffalo.edu, with copy to ONFEditor@ons.org. (Submitted July 2021. Accepted September 29, 2021.)
References
Al-Ahmadi, A., Ardeshir-Larijani, F., Fu, P., Cao, S., Lipka, M.B., Dowlati, A., & Bruno, D.S. (2020). Next generation sequencing of advanced non-small cell lung cancer—Utilization based on race and impact on survival. Clinical Lung Cancer, 22(1), 16–22. https://doi.org/10.1016/j.cllc.2020.08.004
Begnaud, A., Yang, P., Robichaux, C., Rubin, N., Kratzke, R., Melzer, A., . . . Jacobson, P. (2020). Evidence that established lung cancer mortality disparities in American Indians are not due to lung cancer genetic testing and targeted therapy disparities. Clinical Lung Cancer, 21(3), e164–e168. https://doi.org/10.1016/j.cllc.2019.10.012
Bennett, N.M., Brown, M.T., Green, T., Hall, L.L., & Winkler, A.M. (2018). Addressing social determinants of health (SDOH): Beyond the clinic walls. AMA: Stepsforward. https://edhub.ama-assn.org/steps-forward/video-player/18608659?resultCl…
Berchick, E.R., Barnett, J.C., & Upton, R.D. (2019). Health insurance coverage in the United States: 2018. U.S. Government Printing Office.
Bertier, G., Carrot-Zhang, J., Ragoussis, V., & Joly, Y. (2016). Integrating precision cancer medicine into healthcare—Policy, practice, and research challenges. Genome Medicine, 8(1), 108. https://doi.org/10.1186/s13073-016-0362-4
Burris, H.A., Saltz, L.B., & Yu, P.P. (2018). Assessing the value of next-generation sequencing tests in a dynamic environment. American Society of Clinical Oncology Educational Book, 38, 139–146. https://doi.org/10.1200/EDBK_200825
Carrera, P.M., & Ormond, M. (2015). Current practice in and considerations for personalized medicine in lung cancer: From the patient’s molecular biology to patient values and preferences. Maturitas, 82(1), 94–99. https://doi.org/10.1016/j.maturitas.2015.04.008
Carroll, J.C., Makuwaza, T., Manca, D.P., Sopcak, N., Permaul, J.A., O’Brien, M.A., . . . Grunfeld, E. (2016). Primary care providers’ experiences with and perceptions of personalized genomic medicine. Canadian Family Physician, 62(10), e626–e635.
Centers for Medicare and Medicaid Services. (2018). Medicare and Medicaid basics. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-ML…
Centers for Medicare and Medicaid Services. (2020). People dually eligible for Medicare and Medicaid. https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicai…
Connors, L., & Schorn, M. (2018). Genetics and genomics content in nursing education: A national imperative. Journal of Professional Nursing, 34(4), 235–237. https://doi.org/10.1016/j.profnurs.2018.06.003
Crinò, L., Weder, W., van Meerbeeck, J., & Felip, E. (2010). Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 21(Suppl. 5), v103–v115. https://doi.org/10.1093.annonc/mdq207
Dickerson, S.S., Sabbah, E.A., Ziegler, P., Chen, H., Steinbrenner, L.M., & Dean, G. (2012). The experience of a diagnosis of advanced lung cancer: Sleep is not a priority when living my life. Oncology Nursing Forum, 39(5), 492–499. https://doi.org/10.1188/12.ONF.492-499
Dorsey, S.G., Griffioen, M.A., Renn, C.L., Cashion, A.K., Colloca, L., Jackson-Cook, C.K., . . . Lyon, D. (2019). Working together to advance symptom science in the precision era. Nursing Research, 68(2), 86–90. https://doi.org/10.1097/NNR.0000000000000339
Edwards, K.L., Korngiebel, D.M., Pfeifer, L., Goodman, D., Renz, A., Wenzel, L., . . . Condit, C.M. (2016). Participant views on consent in cancer genetics research: Preparing for the precision medicine era. Journal of Community Genetics, 7(2), 133–143. https://doi.org/10.1007/s12687-015-0259-8
Enewold, L., & Thomas, A. (2016). Real-world patterns of EGFR testing and treatment with erlotinib for non-small cell lung cancer in the United States. PLOS ONE, 11(6), e0156728. https://doi.org/10.1371/journal.pone.0156728
Fayer, S., & Watson, A. (2015). Employment and wages in healthcare occupations. U.S. Bureau of Labor Statistics. https://www.bls.gov/spotlight/2015/employment-and-wages-in-healthcare-o…
Fukuoka, M., Wu, Y.-L., Thongprasert, S., Sunpaweravong, P., Leong, S.-S., Sriuranpong, V., . . . Mok, T.S.K. (2011). Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small cell lung cancer in Asia (IPASS). Journal of Clinical Oncology, 29(21), 2866–2874. https://doi.org/10.1200/JCO.2010.33.4235
Ginsburg, G.S., & Phillips, K.A. (2018). Precision medicine: From science to value. Health Affairs, 37(5), 694–701. https://doi.org/10.1377/hlthaff.2017.1624
Goebel, C., Louden, C.L., McKenna, J.R., Onugha, O., Wachtel, A., & Long, T. (2019). Diagnosis of non-small cell lung cancer for early stage asymptomatic patients. Cancer Genomics and Proteomics, 16(4), 229–244. https://doi.org/10.21873/cgp.20128
Goodell, S. (2020). Different types of health plans: How they compare. https://www.webmd.com/health-insurance/types-of-health-insurance-plans
Gray, S.W., Kim, B., Sholl, L., Cronin, A., Parikh, A.R., Klabunde, C.N., . . . Keating, N.L. (2017). Medical oncologists’ experiences in using genomic testing for lung and colorectal cancer care. Journal of Oncology Practice, 13(3), e185–e196. https://doi.org/10.1200/jop.2016.016659
Gutierrez, M.E., Choi, K., Lanman, R.B., Licitra, E.J., Skrzypczak, S.M., Pe Benito, R., . . . Goldberg, S.L. (2017). Genomic profiling of advanced non-small cell lung cancer in community settings: Gaps and opportunities. Clinical Lung Cancer, 18(6), 651–659. https://doi.org/10.1016/j.cllc.2017.04.004
Heitzer, E., Perakis, S., Geigl, J.B., & Speicher, M.R. (2017). The potential of liquid biopsies for the early detection of cancer. NPJ Precision Oncology, 1(1), 36. https://doi.org/10.1038/s41698-017-0039-5
Hickey, K.T., Bakken, S., Byrne, M.W., Bailey, D.E., Demiris, G., Docherty, S.L., . . . Grady, P.A. (2019). Precision health: Advancing symptom and self-management science. Nursing Outlook, 67, 462–475. https://doi.org/10.1016/j.outlook.2019.01.003
Illei, P.B., Wong, W., Wu, N., Chu, L., Gupta, R., Schultze, K., & Gubens, M.A. (2018). ALK testing trends and patterns among community practices in the United States. JCO Precision Oncology, 2, 1–11. https://doi.org/10.1200/po.18.00159
Kehl, K.L., Lathan, C.S., Johnson, B.E., & Schrag, D. (2019). Race, poverty, and initial implementation of precision medicine for lung cancer. Journal of the National Cancer Institute, 111(4), 431–434. https://doi.org/10.1093/jnci/djy202
Knutsen, R.M. (2016). The oncology onslaught. Medical Marketing and Media, 51(3), 40–41.
Krist, A.H., Davidson, K.W., Mangione, C.M., Barry, M.J., Cabana, M., Caughey, A.B., . . . Wong, J.B. (2021). Screening for lung cancer: US Preventive Services Task Force recomendation statement. JAMA, 325(10), 962–970. https://doi.org/10.1001/jama.2021.1117
Krzyszczyk, P., Acevedo, A., Davidoff, E.J., Timmins, L.M., Marrero-Berrios, I., Patel, M., . . . Yarmush, M.L. (2018). The growing role of precision and personalized medicine for cancer treatment. Technology, 6(3–4), 79–100. https://doi.org/10.1142/S2339547818300020
Larson, K.L., Huang, B., Chen, Q., Tucker, T., Schuh, M., Arnold, S.M., & Kolesar, J.M. (2020). EGFR testing and erlotinib use in non-small cell lung cancer patients in Kentucky. PLOS ONE, 15(8), e0237790. https://doi.org/10.1371/journal.pone.0237790
Lynch, J.A., Berse, B., Rabb, M., Mosquin, P., Chew, R., West, S.L., . . . Kautter, J. (2018). Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010–2013. BMC Cancer, 18(1), 306. https://doi.org/10.1186/s12885-018-4190-3
Maguire, F.B., Morris, C.R., Parikh-Patel, A., Cress, R.D., Keegan, T.H.M., Li, C.S., . . . Kizer, K.W. (2019). Disparities in systemic treatment use in advanced-stage non-small cell lung cancer by source of health insurance. Cancer, Epidemiology, Biomarkers and Prevention, 28(6), 1059–1066. https://doi.org/10.1158/1055-9965.EPI-18-0823
Miller, F.A., Hayeems, R.Z., Bytautas, J.P., Bedard, P.L., Ernst, S., Hirte, H., . . . Siu, L.L. (2013). Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. European Journal of Human Genetics, 22(3), 391–395. https://doi.org/10.1038/ejhg.2013.158
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
National Institutes of Health. (2017). Bridging resources to the community to reduce cancer disparities. https://www.cancer.gov/research/areas/disparities/chanita-hughes-halber…
Palazzo, L.L., Sheehan, D.F., Tramontano, A.C., & Kong, C.Y. (2019). Disparities and trends in genetic testing and erlotinib treatment among metastatic non-small cell lung cancer patients. Cancer Epidemiology, Biomarkers and Prevention, 28(5), 926–934. https://doi.org/10.1158/1055-9965.Epi-18-0917
Patel, N., Miller, D.P., Snavely, A.C., Bellinger, C., Foley, K.L., Case, D., . . . Miller, D.P.J. (2020). A comparison of smoking history in the electronic health record with self-report. American Journal of Preventive Medicine, 58(4), 591–595. https://doi.org/10.1016.j.amepre.2019.10.020
Rajurkar, S., Mambetsariev, I., Pharaon, R., Leach, B., Tan, T., Kulkarni, P., & Salgia, R. (2020). Non-small cell lung cancer from genomics to therapeutics: A framework for community practice integration to arrive at personalized therapy strategies. Journal of Clinical Medicine, 9(6), 1870. https://doi.org/10.3390/jcm9061870
Ridic, G., Gleason, S., & Ridic, O. (2012). Comparisons of health care systems in the United States, Germany, and Canada. Materia Socio-Medica, 24(2), 112–120. https://doi.org/10.5455.msm.2012.24.112-120
Rogers, C.R., Rovito, M.J., Hussein, M., Obidike, O.J., Pratt, R., Alexander, M., . . . Warlick, C. (2018). Attitudes toward genomic testing and prostate cancer research among Black men. American Journal of Preventive Medicine, 55(5, Suppl. 1), S103–S111. https://doi.org/10.1016/j.amepre.2018.05.028
Shen, C., Kehl, K.L., Zhao, B., Simon, G.R., Zhou, S., & Giordano, S.H. (2017). Utilization patterns and trends in epidermal growth factor receptor (EGFR) mutation testing among patients with newly diagnosed metastatic lung cancer. Clinical Lung Cancer, 18(4), e233–e241. https://doi.org/10.1016/j.cllc.2016.11.002
Soneji, S., Tanner, N.T., Silvestri, G.A., Lathan, C.S., & Black, W. (2017). Racial and ethnic disparities in early-stage lung cancer survival. Chest, 152(3), 587–597. https://doi.org/10.1016/j.chest.2017.03.059
Spira, A., Halmos, B., & Powell, C.A. (2016). Update in lung cancer 2015. American Journal of Respiratory and Critical Care Medicine, 194(6), 661–671. https://doi.org/10.1164/rccm.201604-0898UP
Tan, D.S., Mok, T.S., & Rebbeck, T.R. (2016). Cancer genomics: Diversity and disparity across ethnicity and geography. Journal of Clinical Oncology, 34(1), 91–101. https://doi.org/10.1200/jco.2015.62.0096
West, S.L., King, V., Caret, T.S., Lohr, K.N., McKoy, N., Sutton, S.F., & Lux, L. (2002). Systems to rate the strength of scientific evidence. Agency for Healthcare Research and Quality.

