A Mobile Application for Symptom Management in Patients With Breast Cancer
Objectives: To evaluate the effect of a symptom management mobile application on quality of life and symptom severity in women with breast cancer undergoing chemotherapy.
Sample & Setting: This parallel randomized pilot study consisted of women with breast cancer admitted to oncology outpatient clinics between November 2019 and January 2021 in Turkey.
Methods & Variables: Participants (N = 40) were randomly assigned to the intervention (n = 20) or control group (n = 20). The intervention group used the mobile application in conjunction with usual care. The control group received usual care. Participants were assessed during the first, third, and last chemotherapy cycles. Data were collected using the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 and the Edmonton Symptom Assessment System.
Results: During the study, the decrease in general health and physical functioning and the increase in the severity of depression/sadness in the intervention group were statistically lower than in the control group.
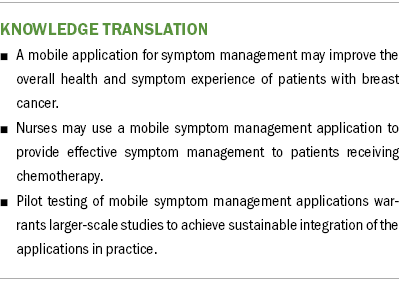
Implications for Nursing: The use of a mobile application for symptom management may promote general well-being and physical function and may alleviate symptoms of depression/sadness in women with breast cancer undergoing chemotherapy. Further studies are needed to evaluate the application in clinical settings with larger groups.
Jump to a section
Worldwide, breast cancer is the most common type of cancer in women and the second most common overall (Sung et al., 2021). Breast cancer and its treatment may result in various symptoms that affect quality of life (QOL) and level of function in patients and range from mild to life-threatening (Albusoul et al., 2017; Moradian et al., 2018). Evidence-based strategies have been developed for self-management of common symptoms, and guidelines have been created for patient care (Kwekkeboom et al., 2020; National Comprehensive Cancer Network, 2022; Oncology Nursing Society [ONS], n.d.; So et al., 2020).
Although patient education about symptom self-management is integrated into some clinical settings, many patients are unable to adequately manage the side effects of chemotherapy or develop effective self-management strategies (Albusoul et al., 2017; Kwekkeboom et al., 2020; Sullivan et al., 2018). The severity of symptoms experienced by patients may vary according to cancer type, stage, treatments, and comorbidities (Henson et al., 2020). This highlights the need for effective and innovative delivery models to provide patients with evidence-based information on the management of side effects resulting from cancer and its treatment.
Mobile health (mHealth) applications are a promising yet underutilized strategy for delivering personalized symptom self-management support to patients with cancer (Azizoddin et al., 2021). Use of mHealth can provide a dynamic platform to continually monitor and track symptoms, provide resources for patients and their caregivers, and educate patients on the self-management of symptoms (Kapoor et al., 2020). A recent systematic review identified 12 mobile applications developed specifically for patients with breast cancer. However, none of these care management applications holistically targeted all possible side effects and symptoms that may arise during chemotherapy (Jongerius et al., 2019).
Another systematic review reported that mobile technology–based interventions might be beneficial to symptom management, communication, and patient empowerment. However, mixed findings regarding the effects of mobile technology–based interventions on patients’ QOL and anxiety were reported (Richards et al., 2018). Using mHealth technology to provide cancer type– and treatment-specific patient education and support for symptom self-management remains a challenge. The development of mHealth requires collaboration between software programmers, graphic designers, and healthcare providers. Researchers must also find ways to incorporate patients’ perspectives to develop content that addresses the unique needs of targeted populations (Azizoddin et al., 2021).
Patients diagnosed with cancer usually do not have just one single symptom related to cancer or its treatment; they often have several symptoms that occur in clusters. Symptom clusters are defined as the cooccurrence of two or more symptoms that are related to each other and make up stable groups of symptoms (So et al., 2021; Sullivan et al., 2017). Some symptoms may cause or may result from other symptoms (Kwekkeboom et al., 2020; Sullivan et al., 2018). Fatigue or lack of energy, general aches and pain, and restless sleep or sleep disturbances are the most common symptoms reported by patients with breast cancer undergoing chemotherapy (Sullivan et al., 2018). To understand and relieve individual symptoms and symptom clusters during and after chemotherapy, it is essential to target symptoms together.
In this study, the authors aimed to address the gap in providing evidence-based mHealth interventions for the most common symptoms in patients with breast cancer receiving chemotherapy. The overall aim of this study was to develop and test the effect of a symptom management mobile application on the QOL of women with breast cancer undergoing chemotherapy. This pilot study had the following aims: (a) to provide patients with evidence-based strategies for symptom management, (b) to evaluate the effect of mobile applications for symptom management on the QOL of patients with breast cancer, and (c) to evaluate participants’ general satisfaction with symptom management during chemotherapy.
Methods
This study was conducted as a parallel randomized controlled pilot study. Participants were allocated equally to the intervention and control groups at a 1:1 ratio. This study was conducted in two phases. In the first phase, the content and mobile symptom management application were developed. In the second phase, the effects of the mobile application on QOL and symptom frequency and severity were pilot tested.
Phase 1: Mobile Phone Application Development
For this study, the researchers developed a mobile application called Mobile Symptom Management (in Turkish, Mobil Semptom Yönetimi [MSY]). The application was designed and revised in collaboration with medical oncologists, nurse scientists, computer scientists, and nursing and computer engineering students. Before the pilot testing, the application was tested with five healthy individuals and was revised to improve user experience and ease of navigation.
The content of MSY includes evidence-based interventions and strategies from the literature and clinical guidelines, including the following: (a) ONS (n.d.) GuidelinesTM, (b) the National Comprehensive Cancer Network (2022) Clinical Practice Guidelines in Oncology, and (c) the European Society for Medical Oncology (n.d.) Clinical Practice Guidelines. The authors also searched the literature for up-to-date evidence on the management of specific symptoms. The content of the mobile application was pilot tested with three patients with cancer and minor revisions were made to improve clarity.
MSY has the following three sections: (a) general information on chemotherapy, (b) symptom assessment and management recommendations for symptoms, and (c) social support group.
General information on chemotherapy: This section includes information about chemotherapy regimens, delivery method, side effects, and healthy lifestyle habits for patients undergoing chemotherapy. Information about a healthy lifestyle includes details on basic hygiene practices, healthy diet, and physical activity recommendations.
Symptom assessment and recommendations for symptoms: This section includes symptom assessment and recommendations based on the evidence for nonpharmacologic symptom management. Twelve symptoms are assessed based on the Turkish version of the Edmonton Symptom Assessment System (ESAS). Assessed symptoms are pain, fatigue/tiredness, nausea, depression/sadness, worry, insomnia, loss of appetite, state of well-being, shortness of breath, changes in the skin and nails, dry mouth, and numbness or tingling in the hands or feet. MSY allows patients to record symptoms and symptom severity quickly using reminder notifications. Patients use customized slider controls to visually indicate symptom severity ranging from 0 to 10.
The authors included evidence-based recommendations for symptom management interventions and strategies for the most common symptoms under 11 sections in the mobile application. These sections were pain, fatigue/tiredness, nausea and vomiting (including loss of appetite), anxiety and depression (including worry), insomnia, shortness of breath or dyspnea, changes in the skin and nails, dry mouth and mouth sores, peripheral neuropathy (numbness or tingling in the hands or feet), constipation/diarrhea, and sexual problems. Recommended interventions and strategies for each symptom were listed under the application’s headings “Recommended for Practice” and “Likely to Be Effective.” For each symptom, interventions and strategies were briefly described, and information was provided on how to apply the interventions.
Social support group: This section was planned to allow study participants to communicate and share their experiences. However, this feature was not used because of the small number of participants.
Phase 2: Pilot Testing of the MSY Application
The sample size was calculated with an estimated 20 points difference between the QOL of women in the intervention and control group. This estimation considered the standard deviation of the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 (EORTC QLQ-C30) in its Turkish validity and reliability study with 80% power, 95% confidence interval, and type 1 error of 0.05%. The sample size was calculated to be 20 participants for each group (Cankurtaran et al., 2008).
Inclusion criteria for participants were as follows: (a) diagnosed with breast cancer and scheduled to receive the first cycle of adjuvant or neoadjuvant chemotherapy, (b) adult women aged 18 years or older, (c) in possession of a smartphone with either Android or iPhone operating system, (d) capable of using the mobile application, and (e) able to read and provide informed consent in Turkish. Participants were excluded who had had chemotherapy before for breast cancer or any other type of cancer. All participants had planned to have four chemotherapy cycles, with each cycle occurring every 14 or every 21 days.
Instruments
Participant information form (PIF): The PIF was used to collect data on sociodemographic characteristics and medical history of participants. The first part included sociodemographic characteristics such as age, educational status, occupation, and health insurance. The second part included obstetric history (e.g., number of births, menopausal status), cancer stage, date of diagnosis, and treatments.
ESAS: The ESAS is commonly used to screen and monitor symptoms and symptom severity among patients in oncology settings. The ESAS scores for nine core symptoms: pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, feeling of well-being, and shortness of breath, with constipation as an optional tenth symptom (Hui & Bruera, 2017). ESAS has 11-point numeric rating scales ranging from 0 (no symptom) to 10 (worst possible). In practice, ESAS scores of 0, 1–3, 4–6, and 7–10 are considered none, mild, moderate, and severe, respectively. The Turkish version of the ESAS assesses three additional symptoms: changes in the skin and nails, dry mouth, and numbness or tingling in the hands or feet (Yeşilbalkan et al., 2008).
EORTC QLQ-C30: The EORTC QLQ-C30 is the most used patient-reported outcome measure evaluating all dimensions of QOL. It has three subscales that evaluate global health status, functional status (physical, role, emotional, cognitive, social functioning), and symptom experience (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties). The total and subscale scores range from 0 to 100. For the functional and global health subscales, a higher score represents a better level of functional and global health status, but for the symptoms subscale, a higher score indicates more numerous or more severe symptoms (Nolte et al., 2019). In the Turkish version of the EORTC QLQ-C30, Cronbach’s alpha was measured as 0.56–0.85 among patients with cancer (Cankurtaran et al., 2008).
Participant satisfaction: Participant satisfaction with symptom management care was measured on a visual scale ranging from 0 (not satisfied at all) to 10 (very satisfied). The participants were asked, “How do you rate your satisfaction with the care to manage your symptoms during the chemotherapy?”
Recruitment and Data Collection
The study was conducted at the medical oncology outpatient clinic at Koç University Hospital in Istanbul, Turkey. Before the data collection, approval was obtained from Koç University Clinical Research Ethics Board. All eligible patients were approached by a researcher at the outpatient clinic. The researcher explained the purpose and methods of the study and obtained written informed consent from participants. Recruitment was conducted between November 2019 and March 2020 and between September 2020 and January 2021 because of COVID-19 pandemic restrictions affecting clinical research in Turkey.
All women who consented to participate in the study filled out the surveys (EORTC QLQ-C30, ESAS, PIF) at the first chemotherapy cycle (time 1) in the outpatient clinic. Then, participants were assigned to the control group or intervention group using the simple randomization method. Each participant was given a number from 1 to 50 (considering dropout during the follow-up) according to the order of admission to the study. The numbers were assigned to columns I (intervention) and II (control) by randomization on the website random.org.
Intervention
Women in the intervention group were instructed on how to download MSY under the guidance of the researcher. The researcher educated participants on how to use MSY and introduced the content of the application to help patients understand how they could benefit from the application. Participants were asked to fill out the symptom screening assessment at least once within the first three days after the chemotherapy and to read the individualized symptom-specific recommendations based on their responses to the symptom screening.
At the outpatient clinic, the usual care included patient education at the initiation of treatment about chemotherapy, its side effects, and management strategies. This patient education was provided by an oncology nurse. At each chemotherapy cycle, the nurse also screened patients for side effects using a symptom checklist to assess the patient’s general health status. In the outpatient clinic where the study was conducted, patients with cancer were provided with no other specific care for symptom management. All women in the intervention and control groups received usual care before the randomization.
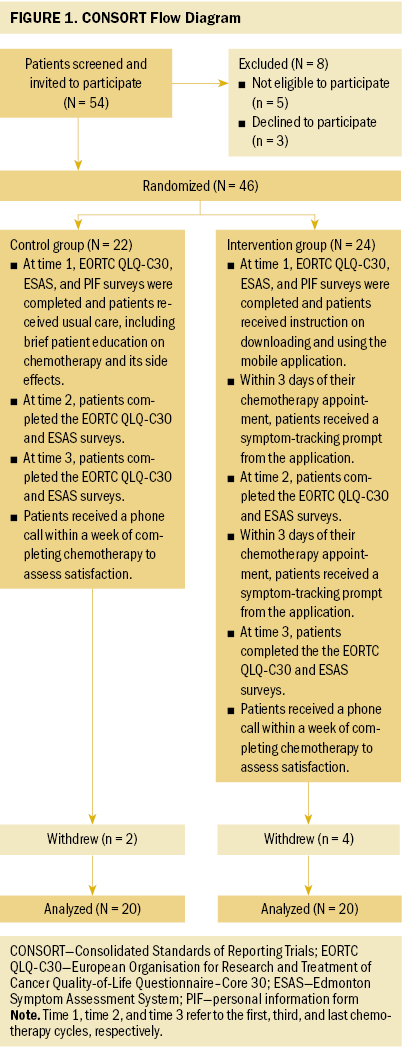
After baseline data collection at the first chemotherapy cycle (time 1), follow-up data were collected from all participants in the intervention and control groups at the third chemotherapy cycle (time 2) and at the last chemotherapy cycle (time 3). Data were collected at the outpatient clinic by the researcher using the EORTC QLQ-C30 and the ESAS. All participants were contacted by phone one week after completion of chemotherapy to evaluate their satisfaction with their symptom management during the chemotherapy on a scale ranging from 0 to 10. The participants and the researchers who collected data were not blinded. The CONSORT flow diagram is given in Figure 1.
Data Analysis
IBM SPSS Statistics, version 27.0, was used to analyze data. Descriptive statistics including numbers and percentages for categorical variables and mean and standard deviation for continuous variables were used. The normality of data was examined using the Kolmogorov–Smirnov normality test.
For comparisons between two groups, independent groups t tests, Mann–Whitney U tests, and chi-square tests were used for sociodemographic and disease-related characteristics of women in the intervention and control group. A two-way repeated measures analysis of variance was used to compare patients’ QOL and symptoms across all the repeated measures. The Tukey post hoc test was used to determine which groups in the sample differ. For the repeated measures analysis of variance, the normality of the data was evaluated with the skewness and kurtosis statistics test, and data with a value of 2.0 or less were considered to fit the normal distribution. Mauchly’s test of sphericity was used to assess whether the assumption of sphericity was violated. When Mauchly’s test of sphericity is not significant (p > 0.05), the assumption of sphericity is not violated. If the sphericity assumption was violated based on Mauchly’s test of sphericity, the F value for Greenhouse-Geisser was reported. The results were evaluated at the 95% confidence interval and reported in the significance level of p < 0.05.
Results
In the intervention group, 24 participants were recruited; however, four women did not complete the study because of (a) withdrawal from the study, (b) hospital admission with severe nausea and vomiting, and (c) transfer to another hospital for chemotherapy. In the control group, 22 participants were recruited, and 2 women withdrew from the study after their first or second cycle of chemotherapy. The attrition rate was 16.66% in the intervention group and 9.09% in the control group. A total of 40 participants (20 in the intervention and 20 in the control group) participated in this study. The mean age of the women was 52.1 (SD = 10.3) in the intervention group and 55.7 (SD = 13.7) in the control group. There were no statistically significant differences in sociodemographic characteristics (age, education status, marital status, employment status, health insurance) of the women between the intervention and control groups (p > 0.05) (see Table 1). There were also no statistically significant differences in medical histories (cancer stage, chemotherapy frequency, family history of cancer) between the intervention and control groups (p > 0.05).
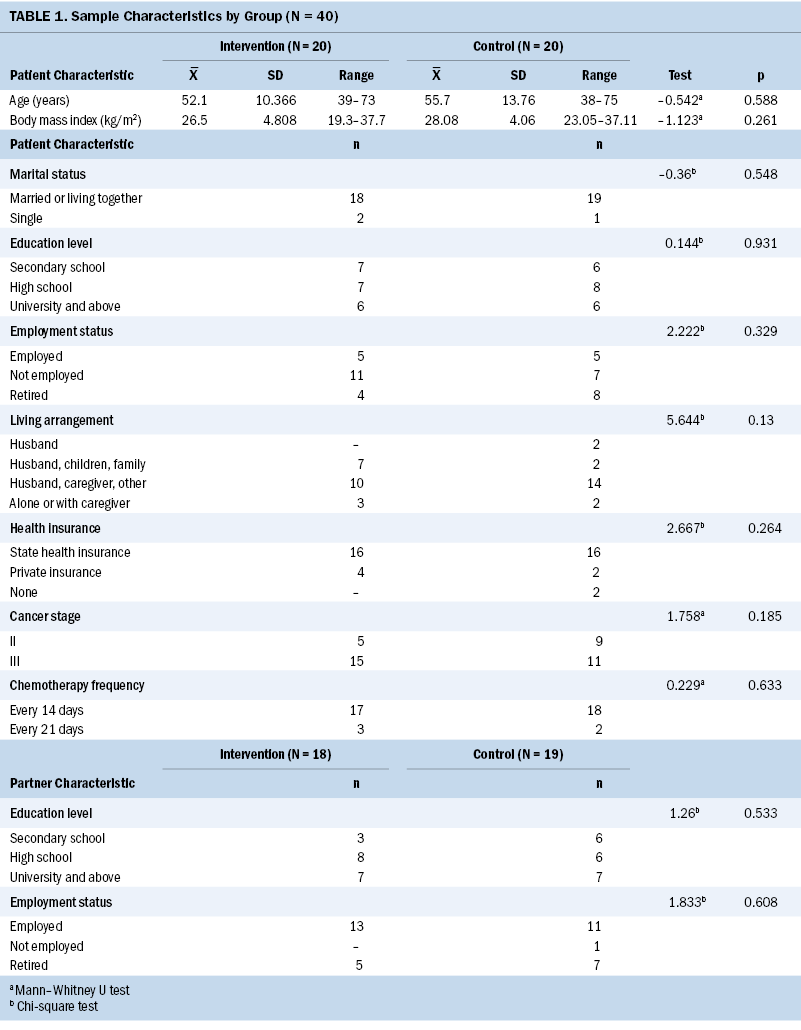
Regardless of group, the changes in functional subscale total scores and subscores for the physical, role, and social functioning of women over time 1, time 2, and time 3 were statistically significant (p < 0.05). The interaction between groups and the physical functioning scores at the three time points was statistically significant (p < 0.05) (see Table 2). This suggests that the physical functioning score decreased less in the intervention group than in the control group.
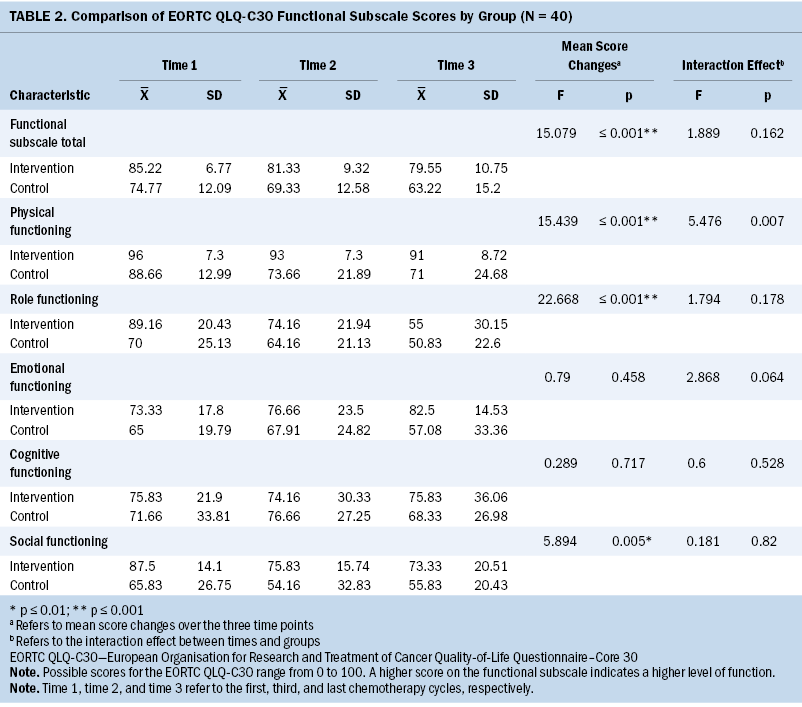
Regardless of group, the changes in symptom subscale total scores and symptom-specific scores for fatigue, nausea and vomiting, pain, insomnia, appetite loss, constipation, diarrhea, and financial difficulties over time 1, time 2, and time 3 were statistically significant (p < 0.05). The interaction between groups and any symptom-specific scores at the three time points was not statistically significant (p > 0.05) (see Table 3).
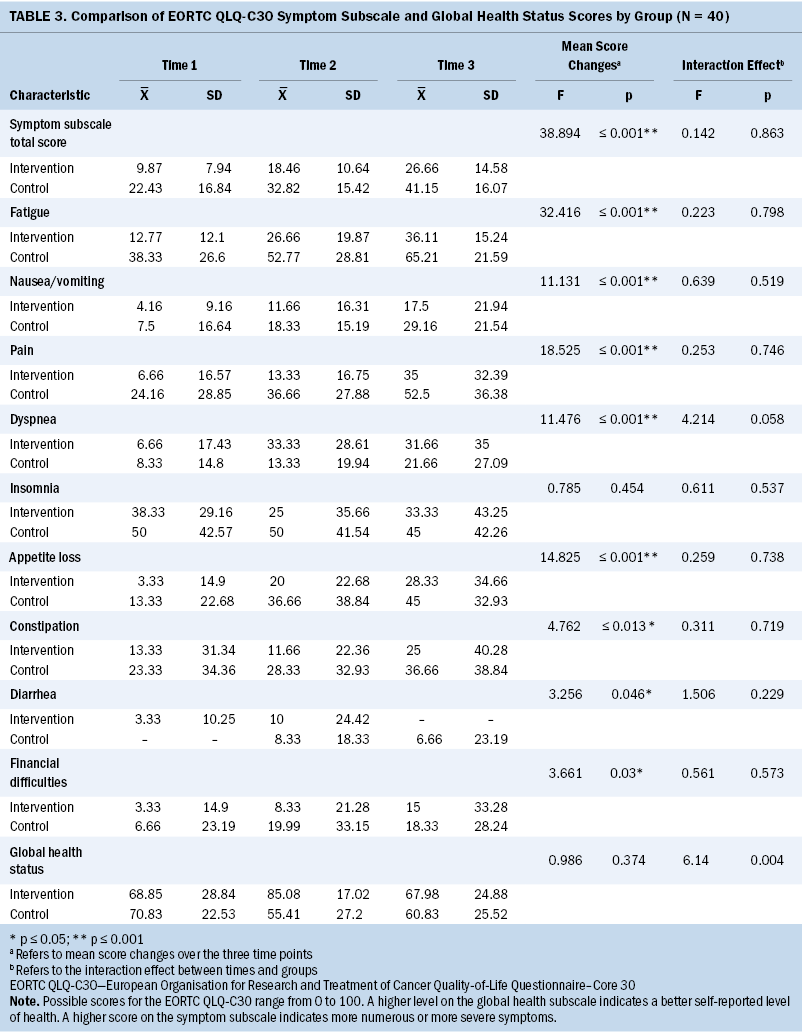
In the intervention group, the EORTC QLQ-C30 global health status subscale mean scores were 68.85 (SD = 28.84) at time 1, 85.08 (SD = 17.02) at time 2, and 67.98 (SD = 24.88) at time 3. In the control group, the EORTC QLQ-C30 global health status subscale mean scores were 70.83 (SD = 22.53) at time 1, 55.41 (SD = 27.2) at time 2, and 60.83 (SD = 25.52) at time 3. Changes in the global health status subscale scores over time were not statistically significant (p > 0.05).However, the interaction between groups and the subscale scores at the three time points was statistically significant (p < 0.05). The global health status scores of all participants did not significantly change over the course of chemotherapy; however, the global health status score in the intervention group decreased less than in the control group.
In addition to the EORTC QLQ-C30 symptom subscale, which assesses overall symptom frequency, the authors used ESAS to evaluate symptom severity. Although not shown in a table, the changes in the means of the ESAS total scores between the three time points (i.e., between time 1 and 2, between time 1 and 3, and between time 2 and 3) were statistically significant (p < 0.05), indicating an increase in symptom severity over the time points. The interaction between groups and the ESAS depression/sadness item score was statistically significant at three different time points (p < 0.05). Comparing the scores measured in the first and the last chemotherapy cycles, there was an increase in depression/sadness score (indicating increased severity) in the control group; however, in the intervention group, there was a decrease in depression/sadness score (p < 0.05).
The mean satisfaction score for symptom management care was 9.06 (SD = 1.11) in the intervention group and 7.64 (SD = 2.02) in the control group, out of a possible range of 0–10. At time 3, the differences in satisfaction scores of the intervention and the control groups were statistically significant (p < 0.05).
Discussion
Systematic reviews suggest that there is a need to test the use of evidence-based mobile applications in randomized clinical trials (Cruz et al., 2019; Jongerius et al., 2019). It is important to develop new evidence-based mHealth interventions to proactively manage symptoms and symptom clusters during chemotherapy. This study aimed to develop and test the effect of the first such Turkish mobile application, MSY, on QOL and symptom experience of women with breast cancer undergoing chemotherapy.
Among all participants in this study, functional status (physical, role, social functioning) decreased in time 2 and time 3 compared to time 1. Symptom severity increased overall, and severity of symptoms such as fatigue, nausea/vomiting, dyspnea, appetite loss, constipation, diarrhea, and financial difficulties increased in both intervention and control groups during the chemotherapy. Chemotherapy-related symptoms and symptom burden may increase as the number of chemotherapy cycles increases, especially in the first week of chemotherapy (Albusoul et al., 2017; Hsu et al., 2017; Li et al., 2019). Sullivan et al. (2018) reported that different symptom clusters based on symptom severity, such as a nutritional cluster including nausea, lack of appetite, dysgeusia, weight loss, and diarrhea might occur after the first chemotherapy cycle. These findings show the importance of managing the cumulative effects of chemotherapy and warrant careful and ongoing assessment and management of symptoms in patients with cancer.
In this study, global health status and physical functioning decreased less, and the severity of depression/sadness increased less in the intervention group than in the control group. For participants in both groups, an oncology nurse provided usual care, including patient education on chemotherapy at the initiation of treatment. In each chemotherapy cycle, information on symptom management was provided based on symptom assessment. Although there was no improvement in QOL during chemotherapy, global health status and physical functioning decreased less, and depression/sadness increased less, in the intervention group. This suggests that participants benefited from the evidence-based interventions and strategies provided through the MSY application. Systematic reviews have found that mHealth interventions increase QOL, symptom management, and patient empowerment (Richards et al., 2018; Rincon et al., 2017). For example, mPRO Mamma, a mobile application that allows daily tracking of symptoms and symptom severity and sends reports to the oncologist, resulted in a better QOL and coping with symptoms in patients with breast cancer receiving systemic treatment (Grašič Kuhar et al., 2020). Another mobile application, Cancer Symptom Management System: SMILE, was developed based on ONS Putting Evidence Into Practice for patients diagnosed with cancer starting adjuvant or palliative chemotherapy (Rha et al., 2020). Rha et al. (2020) found that this application helped participants effectively manage fatigue and sleep disturbance.
The authors found only one study conducted in Turkey in which a mobile phone application was developed for women with breast cancer taking adjuvant endocrine hormone therapy. Women with breast cancer who used this application for 12 weeks had improved QOL and lower symptom distress levels (Çınar et al., 2021). Although use of the MSY application did not improve QOL or relieve symptom severity, it was associated with a less severe decline in global health status, physical functioning, and depression/sadness symptoms over the chemotherapy cycles. The MSY application is the first mobile application developed in Turkish specifically for patients with breast cancer to manage multiple symptoms during chemotherapy; therefore, it is important to test the effects and feasibility in a larger group.
The Untire mHealth application, developed in the Netherlands, consists of information on exercises, physical activity, and suggestions for fatigue management. In one study, the Untire mHealth application significantly improved the full recovery from fatigue in the intervention versus the control group (Spahrkäs et al., 2020). Similarly, Hou et al. (2020) reported that a Taiwanese symptom management support application for patients with breast cancer improved the general QOL of patients. Although applications have been developed to facilitate the entire post-surgery cancer treatment process to improve compliance with medical treatment or empower patients in decision-making for a treatment plan, current applications mostly target specific symptoms or focus on specific periods of the cancer trajectory such as chemotherapy, surgery, or survivorship (Hou et al., 2020; Petrocchi et al. 2021; Rha et al., 2020; Siebenhüner et al., 2021; Yu et al. 2021). However, in a systematic review, Richards et al. (2018) highlighted the need for mHealth interventions to meet patients’ full range of cancer-related information needs, from psychological support to management of finances during and beyond treatment completion. Further studies are needed to expand the content and extent of the MSY application for survivors by addressing the long-term effects of chemotherapy.
In this study, dropout was higher in the intervention group among women who used the MSY app for 8–12 weeks. However, during the satisfaction assessment, those in the intervention group reported a greater level of satisfaction with symptom management received during chemotherapy than those in the control group. Similarly, a systematic review on the use of mobile applications for symptom management reported that interventions were generally perceived as useful, and adherence was consistent and high for five days to six months (Richards et al., 2018). Mobile applications may offer various advantages over providing verbal or written patient education on symptom management (Putranto & Rochmawati, 2020).
Nurses are well-positioned to integrate the use of mobile applications into practice, ultimately improving overall patient satisfaction and well-being. However, integration of any mobile application into a clinical setting requires more evidence on outcomes such as reach, effectiveness, adoption, implementation, and maintenance (RE-AIM). Siebenhüner et al. (2021) used the RE-AIM framework to evaluate the implementation of a mobile application. They reported that decreased levels of distress might reduce patients’ motivation to continue with a self-care intervention. Although this pilot study did not evaluate the implementation of the application, future studies are needed to evaluate MSY using an implementation science framework such as RE-AIM to facilitate its sustainable adoption and effective integration into practice.
Limitations
Although this study provides pilot results on the effect of the first Turkish mobile symptom management application, it has some limitations. This was a nonblinded study, and participants were recruited from a single outpatient clinic. The high attrition rate in the intervention group might have resulted in a better QOL and symptom experience at the baseline because of the dropout of patients with severe symptoms who transferred to other hospitals or inpatient clinics. Satisfaction was measured with a scale that might have been improved by the addition of an in-depth interview to understand areas to improve the use of MSY. Another limitation was the need to recruit participants before and after the COVID-19 pandemic restriction was lifted for research in hospital settings in Turkey.
Implications for Practice and Research
Oncology nurses are in a unique position to improve the QOL of people affected by cancer through more effective symptom management during and after cancer treatment. Technology such as mHealth may provide opportunities to address challenges in symptom self-management when patients are at home with limited access to reliable sources for health information or to their healthcare providers. Although use of mobile applications has been emerging in clinical practice and evidence of the efficacy of mHealth-based symptom management strategies is increasing, nurses should proactively seek ways to integrate the technology into their practice.
Based on the findings of this pilot study, future research should focus on the feasibility and acceptability of the MSY application in larger studies to achieve sustainable integration of mHealth into practice. Future studies are also needed for expanding and updating the content and the extent of the MSY app by addressing the long-term, ongoing side effects of chemotherapy. Adding interactive features to connect patients affected by cancer with their peers and healthcare providers would improve social support and support timely symptom management interventions.
Conclusion
Use of the MSY in conjunction with usual care may support global health status and physical functioning and mitigate depression/sadness in women with breast cancer undergoing chemotherapy. In this study, women who used the mobile application were more satisfied with their symptom management than those who received usual care only. Because current practice mainly focuses on individual symptom management through patient education in outpatient clinics, mobile technology may provide a more comprehensive, less burdensome, and more effective patient education for various symptoms and symptom clusters.
About the Authors
Memnun Seven, RN, PhD, is an assistant professor in the Elaine Marieb College of Nursing at the University of Massachusetts, Amherst, and Koc University School of Nursing (at the time of the study); Şeyma İnciser Paşalak, RN, MSc, PhD, is a research assistant, and Gulcan Bagcivan, PhD, is an assistant professor, both in the School of Nursing, Oznur Ozkasap, PhD, is a professor in the Department of Computer Engineering, and Fatih Selçukbiricik, MD, is an associate professor in the School of Medicine, all at Koç University in Istanbul, Turkey. This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) Starting R&D Projects Support Program (award number 118S442). All authors contributed to the conceptualization and design. Seven, Paşalak, and Selçukbiricik completed the data collection. Seven and Bagcivan provided statistical support. Seven and Ozkasap provided the analysis. Bagcivan and Ozkasap contributed to the manuscript preparation. Seven can be reached at memnunseven@gmail.com, with copy to ONFEditor@ons.org. (Submitted November 2021. Accepted January 4, 2022.)
References
Albusoul, R.M., Berger, A.M., Gay, C.L., Janson, S.L., & Lee, K.A. (2017). Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. Journal of Pain and Symptom Management, 53(5), 880–886. https://doi.org/10.1016/j.jpainsymman.2016.12.332
Azizoddin, D.R., Adam, R., Kessler, D., Wright, A.A., Kematick, B., Sullivan, C., . . . Enzinger, A.C. (2021). Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Supportive Care in Cancer, 29(10), 5741–5751. https://doi.org/10.1007/s00520-021-06146-4
Cankurtaran, E.S., Ozalp, E., Soygur, H., Ozer, S., Akbiyik, D.I., & Bottomley, A. (2008). Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. European Journal of Cancer Care, 17(1), 98–104. https://doi.org/10.1111/j.1365-2354.2007.00827.x
Çınar, D., Karadakovan, A., & Erdoğan, A.P. (2021). Effect of mobile phone app-based training on the quality of life for women with breast cancer. European Journal of Oncology Nursing, 52, 101960. https://doi.org/10.1016/j.ejon.2021.101960
Cruz, F.O.A.M., Vilela, R.A., Ferreira, E.B., Melo, N.S., & Reis, P.E.D. (2019). Evidence on the use of mobile apps during the treatment of breast cancer: Systematic review. JMIR mHealth and uHealth, 7(8), e13245. https://doi.org/10.2196/13245
European Society for Medical Oncology. (n.d.). ESMO Clinical Practice Guidelines: Supportive and palliative care. https://esmo.org/guidelines/supportive-and-palliative-care?page=2
Grašič Kuhar, C., Gortnar Cepeda, T., Kovač, T., Kukar, M., & Ružić Gorenjec, N. (2020). Mobile app for symptom management and associated quality of life during systemic treatment in early stage breast cancer: Nonrandomized controlled prospective cohort study. JMIR mHealth and uHealth, 8(8), e17408. https://doi.org/10.2196/17408
Henson, L.A., Maddocks, M., Evans, C., Davidson, M., Hicks, S., & Higginson, I.J. (2020). Palliative care and the management of common distressing symptoms in advanced cancer: Pain, breathlessness, nausea and vomiting, and fatigue. Journal of Clinical Oncology, 38(9), 905–914. https://doi.org/10.1200/JCO.19.00470
Hou, I.C., Lin, H.Y., Shen, S.H., Chang, K.J., Tai, H.C., Tsai, A.J., & Dykes, P.C. (2020). Quality of life of women after a first diagnosis of breast cancer using a self-management support (mHealth) app in Taiwan: Randomized controlled trial. JMIR mHealth and uHealth, 8(3), e17084. https://doi.org/10.2196/17084
Hsu, H.T., Lin, K.C., Wu, L.M., Juan, C.H., Hou, M.F., Hwang, S.L., . . . Dodd, M.J. (2017). Symptom cluster trajectories during chemotherapy in breast cancer outpatients. Journal of Pain and Symptom Management, 53(6), 1017–1025. https://doi.org/10.1016/j.jpainsymman.2016.12.354
Hui, D., & Bruera, E. (2017). The Edmonton Symptom Assessment System 25 years later: Past, present, and future developments. Journal of Pain and Symptom Management, 53(3), 630–643. https://doi.org/10.1016/j.jpainsymman.2016.10.370
Jongerius, C., Russo, S., Mazzocco, K., & Pravettoni, G. (2019). Research-tested mobile apps for breast cancer care: Systematic review. JMIR mHealth and uHealth, 7(2), e10930. https://doi.org/10.2196/10930
Kapoor, A., Nambisan, P., & Baker, E. (2020). Mobile applications for breast cancer survivorship and self-management: A systematic review. Health Informatics Journal, 26(4), 2892–2905. https://doi.org/10.1177/1460458220950853
Kwekkeboom, K.L., Wieben, A., Stevens, J., Tostrud, L., & Montgomery, K. (2020). Guideline-recommended symptom management strategies that cross over two or more cancer symptoms. Oncology Nursing Forum, 47(5), 498–511. https://doi.org/10.1188/20.ONF.498-511
Li, H., Sereika, S.M., Marsland, A.L., Conley, Y.P., & Bender, C.M. (2019). Impact of chemotherapy on symptoms and symptom clusters in postmenopausal women with breast cancer prior to aromatase inhibitor therapy. Journal of Clinical Nursing, 28(23–24), 4560–4571. https://doi.org/10.1111/jocn.15047
Moradian, S., Krzyzanowska, M.K., Maguire, R., Morita, P.P., Kukreti, V., Avery, J., . . . Howell, D. (2018). Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed-methods study. JMIR Cancer, 4(2), e10932. https://doi.org/10.2196/10932
National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Supportive care [v.2.2022]. https://www.nccn.org/guidelines/category_3
Nolte, S., Liegl, G., Petersen, M.A., Aaronson, N.K., Costantini, A., Fayers, P.M., . . . Rose, M. (2019). General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the United States. European Journal of Cancer, 107, 153–163. https://doi.org/10.1016/j.ejca.2018.11.024
Oncology Nursing Society. (n.d.) Symptom interventions and guidelines. https://ons.org/ons-guidelines
Petrocchi, S., Filipponi, C., Montagna, G., Bonollo, M., Pagani, O., & Meani, F. (2021). A breast cancer smartphone app to navigate the breast cancer journey: Mixed methods study. JMIR Formative Research, 5(5), e28668. https://doi.org/10.2196/28668
Putranto, D., & Rochmawati, E. (2020). Mobile applications for managing symptoms of patients with cancer at home: A scoping review. International Journal of Nursing Practice, 26(4), e12842. https://doi.org/10.1111/ijn.12842
Rha, S.Y., Nam, J.M., & Lee, J. (2020). Development and evaluation of the Cancer Symptom Management System: Symptom Management Improves your LifE (SMILE)—A randomized controlled trial. Supportive Care in Cancer, 28(2), 713–723. https://doi.org/10.1007/s00520-019-04865-3
Richards, R., Kinnersley, P., Brain, K., McCutchan, G., Staffurth, J., & Wood, F. (2018). Use of mobile devices to help cancer patients meet their information needs in non-inpatient settings: Systematic review. JMIR mHealth and uHealth, 6(12), e10026. https://doi.org/10.2196/10026
Rincon, E., Monteiro-Guerra, F., Rivera-Romero, O., Dorronzoro-Zubiete, E., Sanchez-Bocanegra, C.L., & Gabarron, E. (2017). Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: Systematic review. JMIR mHealth and uHealth, 5(12), e187. https://bit.ly/3SXCC67
Siebenhüner, A.R., Mikolasek, M., Witt, C.M., & Barth, J. (2021). Improvements in health might contradict adherence to mobile health interventions: Findings from a self-care cancer app study. Journal of Alternative and Complementary Medicine, 27(S1), S115–S123. https://doi.org/10.1089/acm.2020.0111
So, W.K.W., Law, B.M.N., Chan, D.N.S., Xing, W., Chan, C., Sing, W., & McCarthy, A.L. (2020). The effect of nonpharmacological interventions on managing symptom clusters among cancer patients: A systematic review. Cancer Nursing, 43(6), E304–E327. https://doi.org/10.1097/NCC.0000000000000730
So, W.K.W., Law, B.M.N., Ng, M.S.N., He, X., Chan, D.N.S., Chan, C.W.H., & McCarthy, A.L. (2021). Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Medicine, 10(8), 2531–2565. https://doi.org/10.1002/cam4.3794
Spahrkäs, S.S., Looijmans, A., Sanderman, R., & Hagedoorn, M. (2020). Beating cancer-related fatigue with the Untire mobile app: Results from a waiting-list randomized controlled trial. Psycho-Oncology, 29(11), 1823–1834. https://doi.org/10.1002/pon.5492
Sullivan, C.W., Leutwyler, H., Dunn, L.B., Cooper, B.A., Paul, S.M., Conley, Y.P., . . . Miaskowski, C.A. (2017). Differences in symptom clusters identified using symptom occurrence rates versus severity ratings in patients with breast cancer undergoing chemotherapy. European Journal of Oncology Nursing, 28, P122–132. https://doi.org/10.1016/j.ejon.2017.04.001
Sullivan, C.W., Leutwyler, H., Dunn, L.B., Cooper, B.A., Paul, S.M., Levine, J.D., . . . Miaskowski, C.A. (2018). Stability of symptom clusters in patients with breast cancer receiving chemotherapy. Journal of Pain and Symptom Management, 55(1), P39–55. https://doi.org/10.1016/j.jpainsymman.2017.08.008
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Yeşilbalkan, Ö.U., Özkütük, N., Karadakovan, A., Turgut, T., & Kazgan, B. (2008). Validity and reliability of the Edmonton Symptom Assessment Scale in Turkish cancer patients. Turkish Journal of Cancer, 38(2), 62–67.
Yu, J., Wu, J., Huang, O., Chen, X., & Shen, K. (2021). A smartphone-based app to improve adjuvant treatment adherence to multidisciplinary decisions in patients with early-stage breast cancer: Observational study. Journal of Medical Internet Research, 23(9), e27576. https://doi.org/10.2196/27576




