Higher Levels of Stress and Neuropsychological Symptoms Are Associated With a High Nausea Profile in Patients With Cancer Receiving Chemotherapy
Objectives: To evaluate differences in the severity of global, cancer-specific, and cumulative life stress, resilience, and common neuropsychological symptoms among four subgroups of patients with distinct chemotherapy-induced nausea (CIN) profiles.
Sample & Setting: Adult patients with cancer (N = 1,343) receiving chemotherapy.
Methods & Variables: Patients completed stress, resilience, and neuropsychological symptom severity measures. The Memorial Symptom Assessment Scale was used to assess CIN occurrence six times over two cycles of chemotherapy. Parametric and nonparametric statistics were used to evaluate differences among subgroups of patients with distinct CIN profiles.
Results: The high class had significantly higher levels of global, cancer-specific, and cumulative life stress; significantly higher levels of depression, anxiety, sleep disturbance, morning and evening fatigue, and pain; and lower levels of morning and evening energy and cognitive dysfunction.
Implications for Nursing: Clinicians need to evaluate CIN occurrence across each cycle of chemotherapy and assess patients for various types of stress and common neuropsychological symptoms.
Jump to a section
Despite advances in evidence-based antiemetic regimens, 30%–60% of patients with cancer report unrelieved chemotherapy-induced nausea (CIN) (Röhrl et al., 2019). This large range in prevalence rates suggests a significant amount of interindividual variability in this symptom. Given that the known risk factors for CIN do not explain all its interindividual variability, additional risk factors warrant evaluation (Singh et al., 2018). For example, a cancer diagnosis and associated treatments and fear of recurrence are stressful experiences for most patients (Mazor et al., 2019). Equally important, an individual’s level of resilience can affect their response to these events (García-León et al., 2019; Oppegaard, Harris, Shin, Paul, Cooper, Levine, et al., 2021). However, research on associations between CIN and stress and resilience is limited.
Although patients with cancer can experience several types of stress (e.g., global stress, cancer-specific stress, cumulative life stress) (Langford et al., 2020), except for two previous studies (Singh et al., 2018; Singh, Paul, et al., 2020), the evidence to support an association between CIN and stress has been inferred from intervention studies that evaluated the efficacy of a variety of stress reduction techniques. For example, findings from one systematic review suggested that progressive muscle relaxation, a stress-reducing intervention, decreases CIN and vomiting in patients with breast cancer (Kapogiannis et al., 2018). Additional, albeit inconclusive, evidence was reported in two exercise intervention studies (Haller et al., 2021; Johnsson et al., 2019). In the first study, which evaluated the effects of a single session of endurance or resistance training in women with breast cancer within the first week of chemotherapy (Johnsson et al., 2019), only general stress levels decreased in the endurance group, whereas general stress and CIN decreased in the resistance group over time. In the second pilot study (Haller et al., 2021), the effects of an integrative mind–body–medicine group program on stress and CIN in women with breast cancer were evaluated. Although global stress scores, evaluated using the Perceived Stress Scale (PSS), decreased over time, CIN scores increased.
In the current authors’ first study, which evaluated risk factors for the occurrence of CIN in patients prior to their second or third cycle of chemotherapy, patients with CIN reported higher levels of global stress (assessed using the PSS) and cancer-specific stress (assessed using the Impact of Event Scale–Revised [IES-R]) in the univariable analyses (Singh et al., 2018). Of note, in the multivariable analysis, each one-point increase in PSS scores was associated with a 3% increase in the odds of belonging to the CIN group. In the authors’ second study, which used hierarchical linear modeling to evaluate which demographic, clinical, stress, and symptom characteristics were associated with initial levels and the trajectories of CIN severity (Singh, Paul, et al., 2020), higher levels of intrusive thoughts (assessed using the IES-R) were associated with higher CIN severity scores at enrollment. Across various studies (Haller et al., 2021; Johnsson et al., 2019; Kapogiannis et al., 2018; Singh et al., 2018; Singh, Paul, et al., 2020), direct and indirect evidence supports the hypothesis that positive associations exist between CIN occurrence and/or severity and stress. However, none of these studies evaluated for associations among CIN occurrence and all three types of stress (global, cancer-specific, and cumulative life stress) in the same sample of patients.
Resilience is an individual’s ability to handle adversity (Campbell-Sills & Stein, 2007). In the only cross-sectional study identified, which used the Connor-Davidson Resilience Scale to assess resilience in patients with breast cancer (Ristevska-Dimitrovska et al., 2015), lower levels of resilience were associated with higher CIN and vomiting severity scores. However, the conclusions that can be drawn from this study are limited because CIN and vomiting were assessed together as a single item on the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30.
Regarding associations between CIN and neuropsychological symptoms, findings from a limited number of studies suggest that higher levels of sleep disturbance (Crane et al., 2020; Hockenberry et al., 2017; Singh et al., 2018; Singh, Paul, et al., 2020), depression (Crane et al., 2020; Hockenberry et al., 2017; Singh et al., 2018; Singh, Paul, et al., 2020), fatigue (Crane et al., 2020; Hockenberry et al., 2017; Singh et al., 2018; Singh, Paul, et al., 2020), and anxiety (Singh et al., 2018; Whisenant et al., 2019) are associated with higher occurrence rates for and/or severity of CIN. In addition, pain (Fink et al., 2020; Kwekkeboom et al., 2018) and cognitive dysfunction (Bajic et al., 2018; Whisenant et al., 2019) may co-occur with CIN. Although these studies provide preliminary evidence of positive associations among CIN and a number of neuropsychological symptoms, none of these studies evaluated for differences in the severity of these symptoms in patients with distinct CIN profiles.
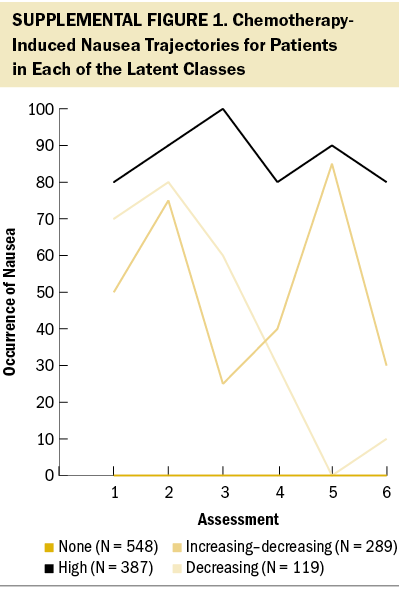
Using latent class analysis (LCA), Singh et al. (2023) identified subgroups of patients (N = 1,343) with four distinct CIN occurrence profiles (none [N = 548, 41%], increasing–decreasing [N = 289, 22%], decreasing [N = 119, 9%], and high [N = 387, 29%]) (see Supplemental Figure 1). In addition, differences in demographic and clinical characteristics and co-occurring common gastrointestinal symptoms among these profiles were described. Given the need to identify additional risk factors for unrelieved CIN, this article builds on the findings of Singh et al. (2023) and evaluates differences in the severity of global, cancer-specific, and cumulative life stress, as well as resilience and common neuropsychological symptoms, among four subgroups of patients with distinct CIN occurrence profiles.
Methods
Conceptual Framework
The theory of symptom management served as the theoretical framework for the entire study, which was funded by the National Cancer Institute (Weiss et al., in press). Specifically, the symptom experience domain and person characteristics were the foci for this analysis.
Patients and Settings
As previously described (Singh et al., 2023), eligible patients were aged 18 years or older; had a diagnosis of breast, gastrointestinal, gynecologic, or lung cancer; had received chemotherapy within the past four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read, write, and understand English; and provided written informed consent. Patients were recruited from two comprehensive cancer centers, one Veterans Affairs hospital, and four community-based oncology programs.
Study Procedures
The study was approved by the institutional review board at the University of California and each of the study sites. Of 2,234 patients approached, 1,343 consented to participate and provided evaluable data on the occurrence of CIN for this analysis. Patients’ refusal to participate was primarily because of being overwhelmed with their cancer treatment. Eligible patients were approached on the infusion unit during their first or second cycle of chemotherapy to discuss participation in the study. Patients completed the CIN occurrence item on the Memorial Symptom Assessment Scale (MSAS) (Portenoy et al., 1994) six times in their homes during their next two cycles of chemotherapy. Assessments 1 and 4 were completed prior to chemotherapy administration, assessments 2 and 5 were completed about one week after chemotherapy administration, and assessments 3 and 6 were completed about two weeks after chemotherapy administration. All other questionnaires were completed at enrollment prior to the patient’s second or third cycle of chemotherapy.
Instruments
Demographic and clinical characteristics: Patients completed a demographic questionnaire, the Karnofsky Performance Status (KPS) scale (Karnofsky, 1977), the Self-Administered Comorbidity Questionnaire (Sangha et al., 2003), and a smoking history questionnaire. Disease and treatment information was obtained from patients’ medical records.
Assessment of CIN occurrence: The occurrence of CIN was measured using the nausea item from the MSAS at each of the six assessments. The MSAS is a valid and reliable symptom assessment instrument for patients with cancer that evaluates the occurrence, severity, frequency, and distress of 32 common symptoms (Portenoy et al., 1994).
Stress and resilience measures: The 14-item PSS was used as a measure of global perceived stress according to the degree that life circumstances are appraised as stressful during the course of the previous week (Cohen et al., 1983). Total PSS scores range from 0 to 56. In this study, the Cronbach’s alpha was 0.85.
The 22-item IES-R was used to measure cancer-related distress (Horowitz et al., 1979). Patients rated each item based on how distressing each potential difficulty was for them during the past week “with respect to their cancer and its treatment.” Levels of intrusion, avoidance, and hyperarousal as perceived by the patient are evaluated using three subscales. Sum scores of 24 or greater indicate clinically meaningful post-traumatic symptomatology, and scores of 33 or greater indicate probable post-traumatic stress disorder (PTSD) (Creamer et al., 2003). In this study, the Cronbach’s alpha for total IES-R score was 0.92.
The 30-item Life Stressor Checklist–Revised is an index of lifetime trauma exposure (e.g., death of a loved one, a sexual assault) (Wolfe & Kimerling, 1997). Total scores are obtained by summing the total number of events endorsed. If patients endorsed an event, they were asked to indicate how much that stressor affected their life during the past year. These responses were averaged to yield a mean “affected” score. In addition, a PTSD sum score was created based on the number of positively endorsed items (of 21) that reflect the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) PTSD Criteria A for having experienced a traumatic event.
The 10-item Connor-Davidson Resilience Scale evaluates a patient’s personal ability to handle adversity (e.g., “I am able to adapt when changes occur”) (Campbell-Sills & Stein, 2007). Total scores range from 0 to 40, with higher scores indicating higher self-perceived resilience. The normative adult mean score in the United States is 31.8 (SD = 5.4) (Campbell-Sills et al., 2009). In this study, the Cronbach’s alpha was 0.9.
Assessment of neuropsychological symptoms: An evaluation of other common symptoms was performed using valid and reliable instruments. These symptoms and their respective measures were as follows: depressive symptoms using the Center for Epidemiological Studies–Depression scale (Radloff, 1977), trait and state anxiety using the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1983), cognitive function using the Attentional Function Index (Cimprich et al., 2005), sleep disturbance using the General Sleep Disturbance Scale (Lee, 1992), morning and evening fatigue and energy using the Lee Fatigue Scale (Lee et al., 1991), and pain using the Brief Pain Inventory (Daut et al., 1983).
Data Analyses
Descriptive statistics and frequency distributions were generated for sample characteristics at enrollment using IBM SPSS Statistics, version 27.0. As previously described (Singh et al., 2023), unconditional LCA was used to identify distinct profiles of CIN occurrence that characterized unobserved subgroups of patients (i.e., latent classes) during the six assessments. Prior to performing the LCA, patients who responded “no” to the nausea item on the MSAS for five or six assessments (i.e., these patients did not experience nausea across the two cycles of chemotherapy) were identified and labeled as the “none” class (N = 548). Then, LCA was performed using data from the remaining 795 patients.
Estimation was carried out with full information maximum likelihood with standard errors and a chi-square test that was robust to non-normality and nonindependence of observations (“estimator = MLR”) using a logit link because the items are binary. Model fit was evaluated to identify the solution that best characterized the observed latent class structure with the Bayesian Information Criterion, Vuong–Lo–Mendell–Rubin likelihood ratio test, entropy, and latent class percentages that were large enough to be reliable (i.e., likely to replicate in new samples) (Muthén & Muthén, 1998–2017). Missing data were accommodated with the use of the Expectation-Maximization algorithm (Muthén & Shedden, 1999). Mixture models, like LCA, are known to produce solutions at local maxima. Therefore, the models for this study were fit with from 800 to 2,400 random starts. This approach ensured that the estimated model was replicated many times and was not because of a local maximum. Estimation was done using Mplus, version 8.2.
Parametric and nonparametric tests were used to evaluate differences among the latent classes in stress, resilience, and neuropsychological symptom scores at enrollment. Statistical significance was set at p < 0.05. Post hoc contrasts were done using a Bonferroni-corrected p value of < 0.008 (0.05/6 possible pairwise contrasts).
Results
LCA
As previously reported (Singh et al., 2023), 548 patients (41%) who had one or fewer occurrences of CIN over the six assessments were labeled as the none class. A three-class solution was selected for the remaining 795 patients whose data were entered into the LCA. For the increasing–decreasing class (N = 289, 22%), the CIN occurrence rate increased from the first to the second assessment, decreased at the third assessment, and increased again at the fourth and fifth assessments before decreasing at the sixth assessment. For the decreasing class (N = 119, 9%), the occurrence rate for CIN increased slightly from the first to the second assessment, then gradually decreased over the remaining four assessments. For the high class (N = 387, 29%), the occurrence rates for CIN remained consistently high over the six assessments.
Demographic and Clinical Characteristics
As previously described (Singh et al., 2023), compared to the none class, the high class was significantly younger, more likely to have a lower annual household income, and more likely to have childcare responsibilities (see Supplemental Table 1). In addition, these patients had lower KPS scores and higher Self-Administered Comorbidity Questionnaire scores; were more likely to self-report diagnoses of ulcer/stomach disease, anemia or blood disease, or depression; and were more likely to have received only chemotherapy, chemotherapy on a 14-day cycle, and a highly emetogenic chemotherapy regimen. Compared to the increasing–decreasing class, patients in the high class had lower KPS scores, were less likely to exercise on a regular basis, and were more likely to have gastrointestinal cancer and less likely to have gynecologic cancer.
Compared to the none class, the increasing–decreasing class was younger and more likely to be female. In addition, they had higher MAX2 scores and lower KPS scores, were more likely to self-report a diagnosis of depression, and were less likely to receive a minimal/low emetogenic chemotherapy regimen. Compared to the none class, patients in the decreasing class had lower KPS scores.
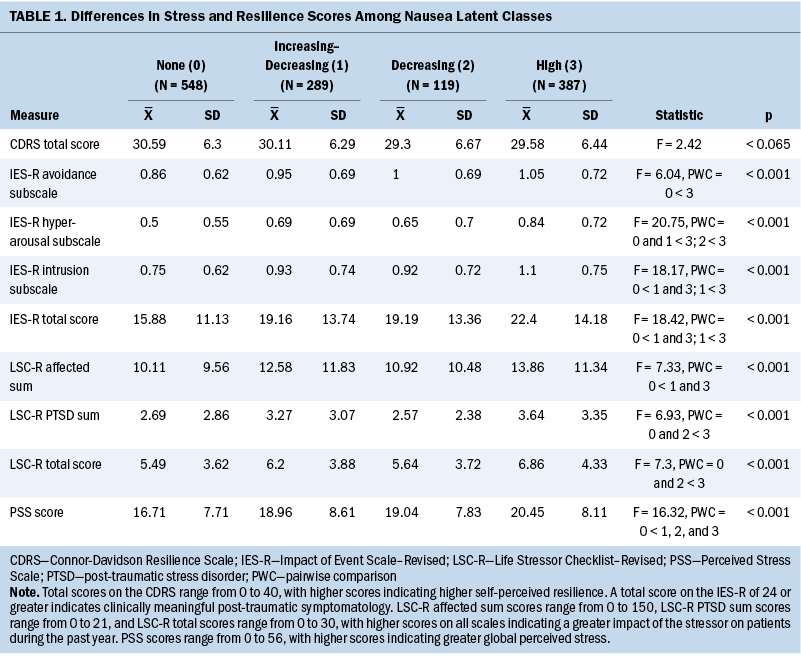
Stress and Resilience Scores
Compared to the none class, the other three classes had higher PSS scores (see Table 1). The increasing–decreasing and high classes had higher IES-R intrusion subscale and total scores compared to the none class. The high class had higher IES-R avoidance and hyperarousal subscale scores, as well as higher Life Stressor Checklist–Revised affected sum, PTSD, and total scores compared to the none class. No differences were found among the classes in Connor-Davidson Resilience Scale scores.
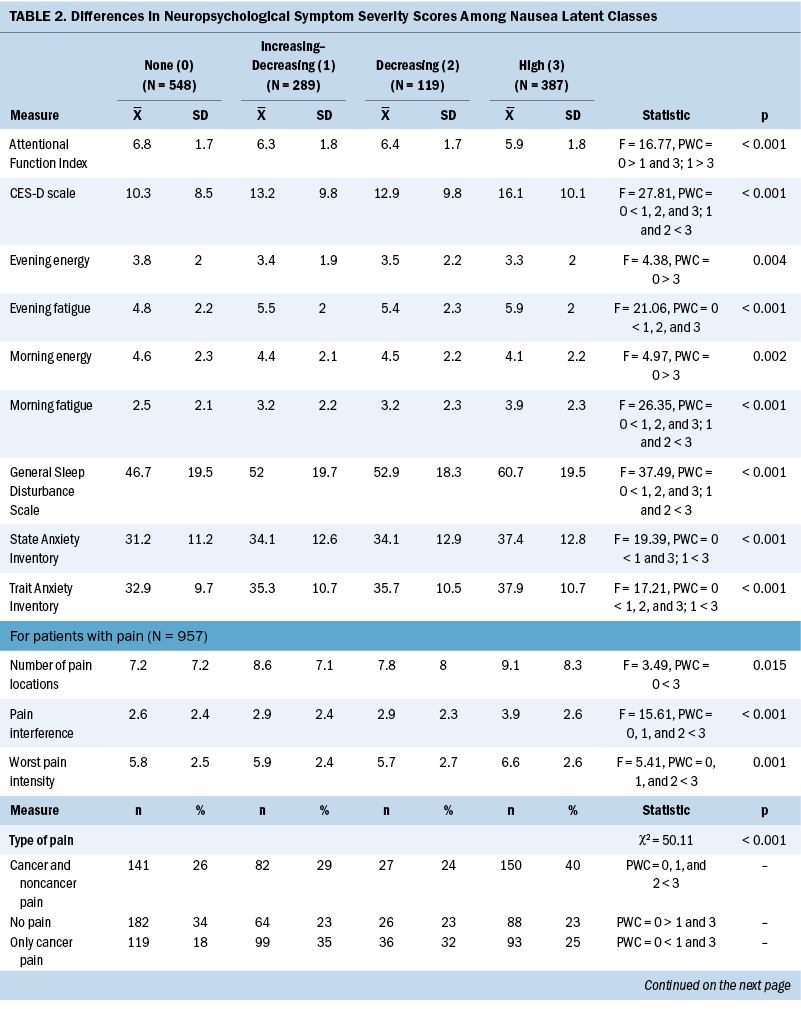
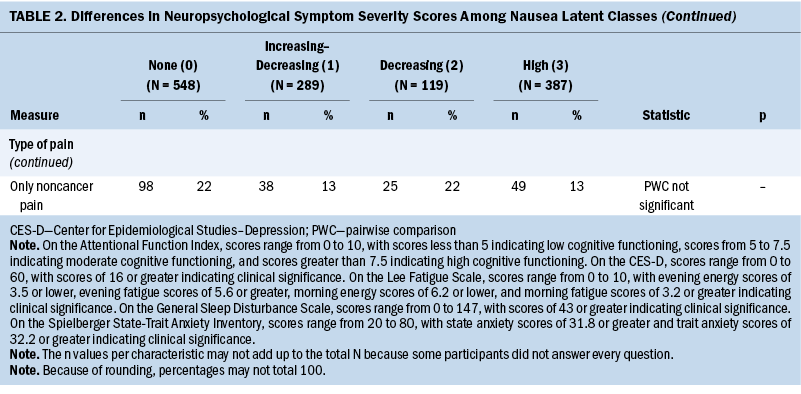
Neuropsychological Symptom Scores
Compared to the none class, the other three classes reported significantly higher levels of depression, trait anxiety, sleep disturbance, and morning and evening fatigue (see Table 2). The increasing–decreasing and high classes reported higher levels of state anxiety and lower levels of attentional function compared to the none class. Compared to the none class, the high class reported lower levels of morning and evening energy and a higher number of pain locations. Compared to the increasing–decreasing and decreasing classes, the high class reported higher levels of depression, sleep disturbance, and morning fatigue. Compared to the other three classes, a higher percentage of patients in the high class reported the occurrence of cancer and noncancer pain, as well as greater worst pain intensity and pain interference scores.
Discussion
The current study, which builds on the authors’ previous findings (Singh et al., 2023), is the first to identify associations among stress, resilience, and neuropsychological symptoms in patients with distinct CIN occurrence profiles. Because the researchers’ previous study provided in-depth explanations for the associations between the distinct CIN profiles and various demographic and clinical characteristics, this discussion focuses on the common and distinct risk factors across the CIN occurrence profiles related to stress, resilience, and neuropsychological symptoms (see Table 3).
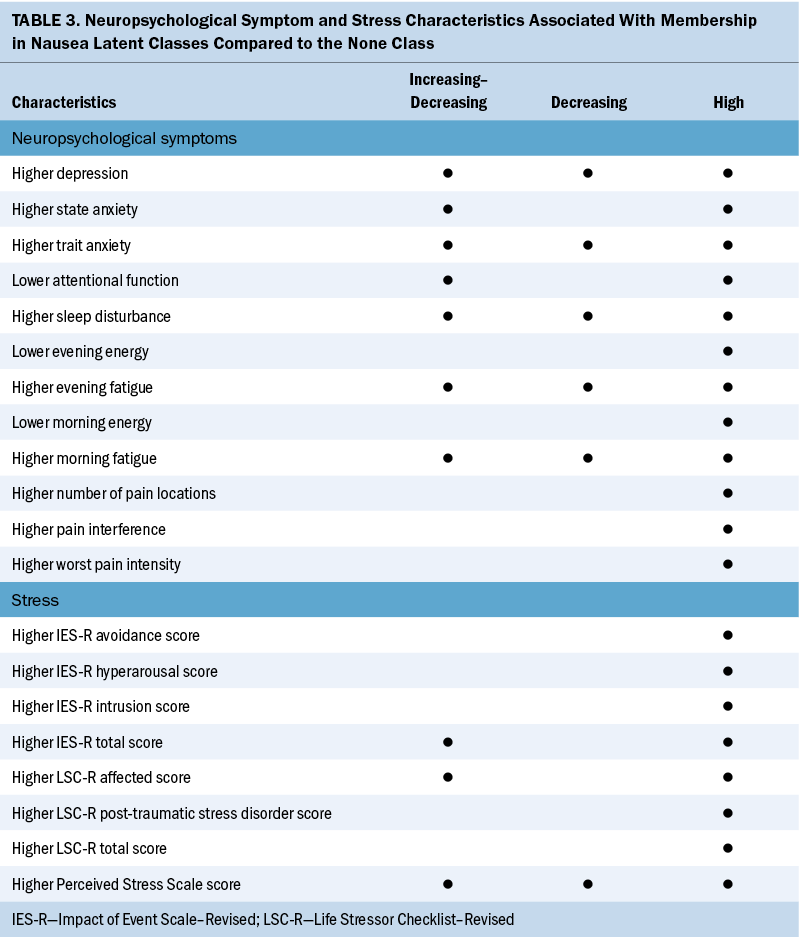
Stress Characteristics and Worse CIN Profiles
This study is the first to evaluate associations between CIN occurrence and three distinct types of stress. In terms of global stress, it is notable that compared to the none class, the other three classes had significantly higher but comparable PSS scores. Although a clinically meaningful cutoff score is not available for the PSS, the scores for the three highest CIN classes are comparable to scores reported by men and women one month after a myocardial infarction (Xu et al., 2015). Because global stress activates the hypothalamic–pituitary–adrenal axis (Chu et al., 2023) and the three highest CIN classes had similar levels of global stress, additional research is warranted on the threshold of stress that contributes to the occurrence of CIN.
Regarding cancer-specific stress, although all IES-R subscale and total scores were higher in the high class, only the intrusion and IES-R total scores were higher in the increasing–decreasing class compared to the none class. Although the IES-R total score for the high class approached the cutoff for post-traumatic symptomatology, 40% of these patients exceeded this cutoff and 20% met the criteria for PTSD. This rate of PTSD is higher than the 6.1%–9.2% reported for the general population of the United States (Sareen, 2022). In addition, the high class’s IES-R total score is similar to that of a sample of postpartum women with severe nausea (Kjeldgaard et al., 2019). Clinicians need to assess for cancer-related stress and PTSD in patients receiving chemotherapy. Cognitive behavioral therapies, mindfulness-based approaches, and telehealth interventions may reduce stress and PTSD symptoms, as well as CIN occurrence and severity (Beerse et al., 2020; Boyd et al., 2018).
In terms of cumulative life stress, compared to the none class, the high class reported higher levels of cumulative exposure to and effects of stressful life events. In addition, compared to the none class, Life Stressor Checklist–Revised affected scores were higher in the increasing–decreasing class. This association between cumulative life stress and CIN may be partially explained by an increase in allostatic load. Allostatic load is defined as the cumulative burden of chronic stress and life events that can result in overwhelming physiologic changes (Guidi et al., 2021). Stress can initiate changes in the autonomic nervous system and/or hypothalamic–pituitary–adrenal axis that result in increases in inflammatory responses (Shields & Slavich, 2017). This hypothesis is supported by reported associations between increases in allostatic load and more severe nausea in patients with migraines (Blumenfeld et al., 2021).
Neuropsychological Symptoms and Worse CIN Profiles
Compared to the none class, the other three classes reported higher severity scores for 5 of 10 neuropsychological symptoms (depression, trait anxiety, sleep disturbance, morning fatigue, and evening fatigue). This finding is not surprising given that previous studies reported on the co-occurrence of these symptoms with CIN in patients with ovarian (Donovan et al., 2016), breast (Crane et al., 2020; Jung et al., 2016; Kwekkeboom et al., 2018; Peoples et al., 2017; Whisenant et al., 2019), gastrointestinal (Hong et al., 2020; Kwekkeboom et al., 2018), and lung (Kwekkeboom et al., 2018) cancers. Of note, for the three highest CIN classes, scores for trait anxiety, sleep disturbance, and fatigue (morning and evening) exceeded these symptoms’ clinically meaningful cutoff scores. In addition, for the high class, Center for Epidemiological Studies–Depression scale scores exceeded the clinically meaningful cutoff. Similar to stress, the co-occurrence of common neuropsychological symptoms in the three highest CIN classes suggests common biologic mechanisms (Kim et al., 2012).
For example, evidence suggests that common neuropsychological symptoms associated with the administration of chemotherapy may be related to increases in levels of proinflammatory cytokines (Bajic et al., 2018; Bower, 2019; Kim et al., 2012; Miller et al., 2013; Vichaya et al., 2015) and/or alterations in the microbiome–gut–brain axis (Bajic et al., 2018; Jordan et al., 2018; Singh, Dhruva, et al., 2020). Postchemotherapy increases in levels of proinflammatory cytokines may exert direct effects (e.g., cross the blood–brain barrier) and/or indirect effects (e.g., vagal stimulation) within the brain that result in the perception of CIN, as well as various neuropsychological symptoms (Bajic et al., 2018; Bower, 2019; Kim et al., 2012). Additional evidence to support this hypothesis comes from studies that identified associations between the occurrence of CIN and perturbations in a number of inflammatory (Singh et al., 2021) and gut–brain axis (Singh, Dhruva, et al., 2020) pathways.
Changes in gut microbiome ecology after chemotherapy may alter bidirectional signaling in the microbiome–gut–brain axis (Jordan et al., 2018). In addition to CIN (Singh, Dhruva, et al., 2020), emerging evidence suggests that changes in the gut microbiome are associated with a number of neuropsychological symptoms (Jordan et al., 2018). For example, in studies of patients with major depressive disorder (Jiang et al., 2015) and fatigue (Nagy-Szakal et al., 2017), decreases in the abundance of a short-chain fatty acid synthesizing microbiome, Faecalibacterium spp., was associated with depression and fatigue. In addition, sleep disturbance was associated with a decrease in abundance of Streptococcus spp. (Jackson et al., 2015). Future studies can investigate for associations among CIN and psychoneurological symptoms and changes in gut microbiome diversity, composition, and metabolites. Given that findings from preclinical studies suggest that prebiotic- and probiotic-induced changes in the gut may alleviate depression and anxiety (Liu et al., 2015), these interventions warrant investigation in patients with cancer.
Compared to the none class, the increasing–decreasing and high classes reported lower Attentional Function Index scores, which suggests a moderate level of cognitive impairment. Although cognitive impairment was shown to be part of a neuropsychological symptom cluster (Hormozi et al., 2019), the current study is the first to identify an association with CIN. Increases in the levels of proinflammatory cytokines may explain this association (Kim et al., 2012). For example, in a preclinical study (Briones & Woods, 2014), administration of a highly emetogenic chemotherapy regimen that increased levels of interleukin-1 beta and tumor necrosis factor-alpha in the corpus callosum of rats was associated with decreases in cognitive function. In addition, in a study with the same sample (Oppegaard, Harris, Shin, Paul, Cooper, Chan, et al., 2021), two of the perturbed pathways associated with cognitive dysfunction were associated with CIN occurrence (e.g., cytokine–cytokine receptor interaction pathway and mitogen-activated protein kinase signaling pathway) (Singh et al., 2021).
Finally, compared to the none class, the high class was more likely to report decrements in morning and evening energy, as well as higher rates of cancer and noncancer pain, an average of nine pain locations, pain intensity scores in the moderate to severe range, and moderate levels of pain interference. In addition, patients in the high class reported the highest levels of comorbidity. The cumulative effects of these factors undoubtedly contribute to decrements in morning and evening energy levels.
Limitations
Despite several novel findings, certain limitations warrant consideration. First, stress and resilience were evaluated only at enrollment. To demonstrate causal relationships, future studies need to evaluate for changes in CIN, stress, and resilience over time. Second, several risk factors for CIN, such as occurrence of CIN in the first cycle (Molassiotis et al., 2014), motion sickness (Naito et al., 2020), and morning sickness (Naito et al., 2020), were not evaluated. Future studies need to evaluate these risk factors as well as the dose and duration of antiemetics taken at home. Third, because the majority of patients were female and White, future studies need to include a more diverse sample to increase generalizability of the results. Lastly, future studies need to evaluate dose and duration of self-care measures (e.g., ginger, cannabis) that patients take to alleviate symptoms at home.
Implications for Nursing and Conclusion
This study identified a number of stress characteristics and neuropsychological symptoms associated with worse CIN profiles. With 1,343 (59.2%) patients reporting CIN, this symptom continues to be a significant clinical problem. To manage this distressing symptom, clinicians need to continuously assess patients for CIN, stress, and co-occurring symptoms. For patients in the high class, an evaluation of their level of adherence to their antiemetic regimen and the need for changes in their prescription(s) warrant careful consideration. In addition, based on the risk factors identified in this study, appropriate interventions may include the following: mental health referral/counseling services for depression and anxiety; dietary interventions (Najafi et al., 2019); guided imagery and progressive muscle relaxation for attentional function, fatigue, and energy (Charalambous et al., 2016); and mindfulness- and exercise-based interventions to reduce stress and improve sleep (Beerse et al., 2020; Boyd et al., 2018). Equally important, clinicians can determine whether patients require changes in their pharmacologic interventions to decrease specific symptoms (e.g., changes in analgesic prescriptions). Finally, clinicians can monitor patients’ level of adherence to and the efficacy of various pharmacologic and nonpharmacologic symptom management interventions and recommend alternative strategies if warranted.
About the Authors
Komal P. Singh, PhD, RN, is a nurse scientist at the Mayo Clinic Cancer Center and an assistant professor in the Edson College of Nursing and Health Innovation at Arizona State University, both in Phoenix; Bruce A. Cooper, PhD, is a research data analysist III in the Department of Physiological Nursing at the University of California, San Francisco; Cindy S. Tofthagen, PhD, APRN, AOCNP®, FAANP, FAAN, is a nurse scientist and an associate professor of nursing in the Department of Nursing at the Mayo Clinic in Jacksonville, FL; John D. Fryer, PhD, is a professor in the Department of Neuroscience at the Mayo Clinic in Phoenix, AZ; Parminder Singh, MD, is assistant professor of medicine at the Mayo Clinic Cancer Center in Phoenix, AZ; Keenan Pituch, PhD, is a research professor in the Edson College of Nursing and Health Innovation at Arizona State University in Phoenix; Qiyun Zhu, PhD, is an assistant professor in the Biodesign Center for Fundamental and Applied Microbiomics at Arizona State University in Tempe; Haiwei Gu, PhD, is an associate professor of Environmental Health Sciences at Florida International University in Port St. Lucie; Marilyn J. Hammer, PhD, RN, DC, FAAN, is the director of the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at Dana Farber Cancer Institute in Boston, MA; Yvette P. Conley, PhD, FAAN, is a professor in the School of Nursing at the University of Pittsburgh in Pennsylvania; and Jon D. Levine, MD, PhD, is a professor in the School of Medicine and Christine Miaskowski, RN, PhD, is a professor in the School of Nursing, both at the University of California, San Francisco. This research was supported, in part, by a grant from the National Cancer Institute (CA134900). Miaskowski is an American Cancer Society Clinical Research Professor. K. Singh is supported by a grant from the Louis V. Gerstner Jr. Fund at Vanguard Charitable. K. Singh and Miakowski contributed to the conceptualization and design. K. Singh, Hammer, and Miakowski completed the data collection. K. Singh, Cooper, Pituch, Zhu, and Miakowski provided statistical support. K. Singh, Cooper, Fryer, Zhu, Gu, Conley, and Miakowski provided the analysis. K. Singh, Tofthagen, Fryer, P. Singh, Pituch, Zhu, Gu, Hammer, Conley, Levine, and Miakowski contributed to the manuscript preparation. Miaskowski can be reached at chris.miaskowski@ucsf.edu, with copy to ONFEditor@ons.org. (Submitted July 2022. Accepted January 19, 2023.)
References
Bajic, J.E., Johnston, I.N., Howarth, G.S., & Hutchinson, M.R. (2018). From the bottom-up: Chemotherapy and gut–brain axis dysregulation. Frontiers in Behavioral Neuroscience, 12, 104. https://doi.org/10.3389/fnbeh.2018.00104
Beerse, M.E., Van Lith, T., Pickett, S.M., & Stanwood, G.D. (2020). Biobehavioral utility of mindfulness-based art therapy: Neurobiological underpinnings and mental health impacts. Experimental Biology and Medicine, 245(2), 122–130. https://doi.org/10.1177/1535370219883634
Blumenfeld, A., Durham, P.L., Feoktistov, A., Hay, D.L., Russo, A.F., & Turner, I. (2021). Hypervigilance, allostatic load, and migraine prevention: Antibodies to CGRP or receptor. Neurology and Therapy, 10(2), 469–497. https://doi.org/10.1007/s40120-021-00250-7
Bower, J.E. (2019). The role of neuro-immune interactions in cancer-related fatigue: Biobehavioral risk factors and mechanisms. Cancer, 125(3), 353–364. https://doi.org/10.1002/cncr.31790
Boyd, J.E., Lanius, R.A., & McKinnon, M.C. (2018). Mindfulness-based treatments for posttraumatic stress disorder: A review of the treatment literature and neurobiological evidence. Journal of Psychiatry and Neuroscience, 43(1), 7–25. https://doi.org/10.1503/jpn.170021
Briones, T.L., & Woods, J. (2014). Dysregulation in myelination mediated by persistent neuroinflammation: Possible mechanisms in chemotherapy-related cognitive impairment. Brain, Behavior, and Immunity, 35, 23–32. https://doi.org/10.1016/j.bbi.2013.07.175
Campbell-Sills, L., Forde, D.R., & Stein, M.B. (2009). Demographic and childhood environmental predictors of resilience in a community sample. Journal of Psychiatric Research, 43(12), 1007–1012. https://doi.org/10.1016/j.jpsychires.2009.01.013
Campbell-Sills, L., & Stein, M.B. (2007). Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–1028. https://doi.org/10.1002/jts.20271
Charalambous, A., Giannakopoulou, M., Bozas, E., Marcou, Y., Kitsios, P., & Paikousis, L. (2016). Guided imagery and progressive muscle relaxation as a cluster of symptoms management intervention in patients receiving chemotherapy: A randomized control trial. PLOS ONE, 11(6), e0156911. https://doi.org/10.1371/journal.pone.0156911
Chu, B., Marwaha, K., Sanvictores, T., & Ayers, D. (2023). Physiology, stress reaction. StatPearls. https://pubmed.ncbi.nlm.nih.gov/31082164
Cimprich, B., So, H., Ronis, D.L., & Trask, C. (2005). Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psycho-Oncology, 14(1), 70–78. https://doi.org/10.1002/pon.821
Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://www.ncbi.nlm.nih.gov/pubmed/6668417
Crane, T.E., Badger, T.A., Sikorskii, A., Segrin, C., Hsu, C.-H., & Rosenfeld, A.G. (2020). Symptom profiles of Latina breast cancer survivors: A latent class analysis. Nursing Research, 69(4), 264–271. https://doi.org/10.1097/NNR.0000000000000434
Creamer, M., Bell, R., & Failla, S. (2003). Psychometric properties of the Impact of Event Scale–Revised. Behaviour Research and Therapy, 41(12), 1489–1496. https://doi.org/10.1016/j.brat.2003.07.010
Daut, R.L., Cleeland, C.S., & Flanery, R.C. (1983). Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain, 17(2), 197–210. https://doi.org/10.1016/0304-3959(83)90143-4
Donovan, H.S., Hagan, T.L., Campbell, G.B., Boisen, M.M., Rosenblum, L.M., Edwards, R.P., . . . Horn, C.C. (2016). Nausea as a sentinel symptom for cytotoxic chemotherapy effects on the gut–brain axis among women receiving treatment for recurrent ovarian cancer: An exploratory analysis. Supportive Care in Cancer, 24(6), 2635–2642. https://doi.org/10.1007/s00520-015-3071-4
Fink, J., Burns, J., Perez Moreno, A.C., Kram, J.J.F., Armstrong, M., Chopp, S., . . . Conway, N. (2020). A quality brief of an oncological multisite massage and acupuncture therapy program to improve cancer-related outcomes. Journal of Alternative and Complementary Medicine, 26(9), 820–824. https://doi.org/10.1089/acm.2019.0371
García-León, M.Á., Pérez-Mármol, J.M., Gonzalez-Pérez, R., Del Carmen García-Ríos, M., & Peralta-Ramírez, M.I. (2019). Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiology and Behavior, 202, 87–93. https://doi.org/10.1016/j.physbeh.2019.02.001
Guidi, J., Lucente, M., Sonino, N., & Fava, G.A. (2021). Allostatic load and its impact on health: A systematic review. Psychotherapy and Psychosomatics, 90(1), 11–27. https://doi.org/10.1159/000510696
Haller, H., Choi, K.-E., Lange, S., Kümmel, S., Paul, A., Cramer, H., . . . Voiss, P. (2021). Effects of an integrative mind–body–medicine group program on breast cancer patients during chemotherapy: An observational study. Current Pharmaceutical Design, 27(8), 1112–1120. https://doi.org/10.2174/1381612826666201211111122
Hockenberry, M.J., Hooke, M.C., Rodgers, C., Taylor, O., Koerner, K.M., Mitby, P., . . . Pan, W. (2017). Symptom trajectories in children receiving treatment for leukemia: A latent class growth analysis with multitrajectory modeling. Journal of Pain and Symptom Management, 54(1), 1–8. https://doi.org/10.1016/j.jpainsymman.2017.03.002
Hong, Y., Wu, C., & Wu, B. (2020). Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy. Integrative Cancer Therapies, 19, 1534735420954912. https://doi.org/10.1177/1534735420954912
Hormozi, M., Hashemi, S.-M., & Shahraki, S. (2019). Investigating relationship between pre- and post-chemotherapy cognitive performance with levels of depression and anxiety in breast cancer patients: A cross-sectional study. Asian Pacific Journal of Cancer Prevention, 20(12), 3831–3837. https://doi.org/10.31557/APJCP.2019.20.12.3831
Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of Event Scale: A measure of subjective stress. Psychosomatic Medicine, 41(3), 209–218. https://doi.org/10.1097/00006842-197905000-00004
Jackson, M.L., Butt, H., Ball, M., Lewis, D.P., & Bruck, D. (2015). Sleep quality and the treatment of intestinal microbiota imbalance in chronic fatigue syndrome: A pilot study. Sleep Science, 8(3), 124–133. https://doi.org/10.1016/j.slsci.2015.10.001
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., . . . Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. https://doi.org/10.1016/j.bbi.2015.03.016
Johnsson, A., Demmelmaier, I., Sjövall, K., Wagner, P., Olsson, H., & Tornberg, A.B. (2019). A single exercise session improves side-effects of chemotherapy in women with breast cancer: An observational study. BMC Cancer, 19(1), 1073. https://doi.org/10.1186/s12885-019-6310-0
Jordan, K.R., Loman, B.R., Bailey, M.T., & Pyter, L.M. (2018). Gut microbiota–immune–brain interactions in chemotherapy-associated behavioral comorbidities. Cancer, 124(20), 3990–3999. https://doi.org/10.1002/cncr.31584
Jung, D., Lee, K.-M., Kim, W.-H., Lee, J.-Y., Kim, T.-Y., Im, S.-A., . . . Hahm, B.-J. (2016). Longitudinal association of poor sleep quality with chemotherapy-induced nausea and vomiting in patients with breast cancer. Psychosomatic Medicine, 78(8), 959–965. https://doi.org/10.1097/PSY.0000000000000372
Kapogiannis, A., Tsoli, S., & Chrousos, G. (2018). Investigating the effects of the progressive muscle relaxation–guided imagery combination on patients with cancer receiving chemotherapy treatment: A systematic review of randomized controlled trials. Explore, 14(2), 137–143. https://doi.org/10.1016/j.explore.2017.10.008
Karnofsky, D. (1977). Performance scale. Plenum Press.
Kim, H.-J., Barsevick, A.M., Fang, C.Y., & Miaskowski, C. (2012). Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nursing, 35(6), E1–E20. https://doi.org/10.1097/NCC.0b013e318233a811
Kjeldgaard, H.K., Vikanes, A., Benth, J.Š., Junge, C., Garthus-Niegel, S., & Eberhard-Gran, M. (2019). The association between the degree of nausea in pregnancy and subsequent posttraumatic stress. Archives of Women’s Mental Health, 22(4), 493–501. https://doi.org/10.1007/s00737-018-0909-z
Kwekkeboom, K., Zhang, Y., Campbell, T., Coe, C.L., Costanzo, E., Serlin, R.C., & Ward, S. (2018). Randomized controlled trial of a brief cognitive-behavioral strategies intervention for the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Psycho-Oncology, 27(12), 2761–2769. https://doi.org/10.1002/pon.4883
Langford, D.J., Cooper, B., Paul, S., Humphreys, J., Hammer, M.J., Levine, J., . . . Miaskowski, C. (2020). Distinct stress profiles among oncology patients undergoing chemotherapy. Journal of Pain and Symptom Management, 59(3), 646–657. https://doi.org/10.1016/j.jpainsymman.2019.10.025
Lee, K.A. (1992). Self-reported sleep disturbances in employed women. Sleep, 15(6), 493–498. https://doi.org/10.1093/sleep/15.6.493
Lee, K.A., Hicks, G., & Nino-Murcia, G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36(3), 291–298. https://doi.org/10.1016/0165-1781(91)90027-m
Liu, X., Cao, S., & Zhang, X. (2015). Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. Journal of Agricultural and Food Chemistry, 63(36), 7885–7895. https://doi.org/10.1021/acs.jafc.5b02404
Mazor, M., Paul, S.M., Chesney, M.A., Chen, L.-M., Smoot, B., Topp, K., . . . Miaskowski, C. (2019). Perceived stress is associated with a higher symptom burden in cancer survivors. Cancer, 125(24), 4509–4515. https://doi.org/10.1002/cncr.32477
Miller, A.H., Haroon, E., Raison, C.L., & Felger, J.C. (2013). Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depression and Anxiety, 30(4), 297–306. https://doi.org/10.1002/da.22084
Molassiotis, A., Aapro, M., Dicato, M., Gascon, P., Novoa, S.A., Isambert, N., . . . Roila, F. (2014). Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: Results from a European prospective observational study. Journal of Pain and Symptom Management, 47(5), 839–848.e4. https://doi.org/10.1016/j.jpainsymman.2013.06.012
Muthén, B., & Shedden, K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55(2), 463–469. https://doi.org/10.1111/j.0006-341x.1999.00463.x
Muthén, L.K., & Muthén, B.O. (1998–2017). Mplus user’s guide (8th ed.). https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf
Nagy-Szakal, D., Williams, B.L., Mishra, N., Che, X., Lee, B., Bateman, L., . . . Lipkin, W.I. (2017). Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome, 5(1), 44. https://doi.org/10.1186/s40168-017-0261-y
Naito, Y., Kai, Y., Ishikawa, T., Fujita, T., Uehara, K., Doihara, H., . . . Saeki, T. (2020). Chemotherapy-induced nausea and vomiting in patients with breast cancer: A prospective cohort study. Breast Cancer, 27(1), 122–128. https://doi.org/10.1007/s12282-019-01001-1
Najafi, S., Haghighat, S., Raji Lahiji, M., RazmPoosh, E., Chamari, M., Abdollahi, R., . . . Zarrati, M. (2019). Randomized study of the effect of dietary counseling during adjuvant chemotherapy on chemotherapy induced nausea and vomiting, and quality of life in patients with breast cancer. Nutrition and Cancer, 71(4), 575–584. https://doi.org/10.1080/01635581.2018.1527375
Oppegaard, K., Harris, C.S., Shin, J., Paul, S.M., Cooper, B.A., Chan, A., . . . Kober, K.M. (2021). Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine, 148, 155653. https://doi.org/10.1016/j.cyto.2021.155653
Oppegaard, K., Harris, C.S., Shin, J., Paul, S.M., Cooper, B.A., Levine, J.D., . . . Miaskowski, C. (2021). Anxiety profiles are associated with stress, resilience and symptom severity in outpatients receiving chemotherapy. Supportive Care in Cancer, 29(12), 7825–7836. https://doi.org/10.1007/s00520-021-06372-w
Peoples, A.R., Roscoe, J.A., Block, R.C., Heckler, C.E., Ryan, J.L., Mustian, K.M., . . . Dozier, A.M. (2017). Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: A multicenter NCORP study. Supportive Care in Cancer, 25(4), 1271–1278. https://doi.org/10.1007/s00520-016-3520-8
Portenoy, R.K., Thaler, H.T., Kornblith, A.B., Lepore, J.M., Friedlander-Klar, H., Kiyasu, E., . . . Scher, H. (1994). The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer, 30A(9), 1326–1336. https://doi.org/10.1016/0959-8049(94)90182-1
Radloff, L.S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306
Ristevska-Dimitrovska, G., Filov, I., Rajchanovska, D., Stefanovski, P., & Dejanova, B. (2015). Resilience and quality of life in breast cancer patients. Open Access Macedonia Journal of Medical Sciences, 3(4), 727–731. https://doi.org/10.3889/oamjms.2015.128
Röhrl, K., Guren, M.G., Småstuen, M.C., & Rustøen, T. (2019). Symptoms during chemotherapy in colorectal cancer patients. Supportive Care in Cancer, 27(8), 3007–3017. https://doi.org/10.1007/s00520-018-4598-y
Sangha, O., Stucki, G., Liang, M.H., Fossel, A.H., & Katz, J.N. (2003). The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism, 49(2), 156–163. https://doi.org/10.1002/art.10993
Sareen, J. (2022). Posttraumatic stress disorder in adults: Epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. In M.B. Stein (Ed.), UpToDate. Retrieved on May 22, 2023, from https://bit.ly/3Wy1Km2
Shields, G.S., & Slavich, G.M. (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11(8), e12335. https://doi.org/10.1111/spc3.12335
Singh, K., Cao, H., Miaskowski, C., Conley, Y.P., Hammer, M., Wright, F., . . . Kober, K.M. (2021). Perturbations in endocytotic and apoptotic pathways are associated with chemotherapy-induced nausea. Biological Research for Nursing, 23(2), 238–247. https://doi.org/10.1177/1099800420951271
Singh, K., Paul, S.M., Kober, K.M., Conley, Y.P., Wright, F., Levine, J.D., . . . Miaskowski, C. (2020). Neuropsychological symptoms and intrusive thoughts are associated with worse trajectories of chemotherapy-induced nausea. Journal of Pain and Symptom Management, 59(3), 668–678. https://doi.org/10.1016/j.jpainsymman.2019.10.023
Singh, K., Pituch, K., Zhu, Q., Gu, H., Ernst, B., Tofthagen, C., . . . Miaskowski, C. (2023). Distinct nausea profiles are associated with gastrointestinal symptoms in oncology patients receiving chemotherapy. Cancer Nursing, 46(2), 92-102. https://doi.org/10.1097/ncc.0000000000001076
Singh, K.P., Dhruva, A., Flowers, E., Paul, S.M., Hammer, M.J., Wright, F., . . . Kober, K.M. (2020). Alterations in patterns of gene expression and perturbed pathways in the gut–brain axis are associated with chemotherapy-induced nausea. Journal of Pain and Symptom Management, 59(6), 1248–1259.e5. https://doi.org/10.1016/j.jpainsymman.2019.12.352
Singh, K.P., Kober, K.M., Dhruva, A.A., Flowers, E., Paul, S.M., Hammer, M.J., . . . Miaskowski, C. (2018). Risk factors associated with chemotherapy-induced nausea in the week before the next cycle and impact of nausea on quality of life outcomes. Journal of Pain and Symptom Management, 56(3), 352–362. https://doi.org/10.1016/j.jpainsymman.2018.05.019
Spielberger, C.D., Gorsuch, R.L., Lushene, R., Vagg, P.R., & Jacobs, G.A. (1983). Manual for the State-Trait Anxiety Inventory (form Y1–Y2). Consulting Psychologists Press.
Vichaya, E.G., Chiu, G.S., Krukowski, K., Lacourt, T.E., Kavelaars, A., Dantzer, R., . . . Walker, A.K. (2015). Mechanisms of chemotherapy-induced behavioral toxicities. Frontiers in Neuroscience, 9, 131. https://doi.org/10.3389/fnins.2015.00131
Weiss, S.J., Franck, L.S., Leutwyler, H., Dawson-Rose, C.S., Wallhagen, M.I., Staveski, S.L., . . . Miaskowski, C.A. (in press). Theory of symptom management. In M.J. Smith, P.R. Liehr, and R.D. Carpenter (Eds.), Middle range theory for nursing (5th ed., pp. 125–142). Springer.
Whisenant, M., Wong, B., Mitchell, S.A., Beck, S.L., & Mooney, K. (2019). Symptom trajectories are associated with co-occurring symptoms during chemotherapy for breast cancer. Journal of Pain and Symptom Management, 57(2), 183–189. https://doi.org/10.1016/j.jpainsymman.2018.11.010
Wolfe, J., & Kimerling, R. (1997). Gender issues in the assessment of PTSD. In J.P. Wilson & T.M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 192–238). Guilford Press.
Xu, X., Bao, H., Strait, K., Spertus, J.A., Lichtman, J.H., D’Onofrio, G., . . . Krumholz, H.M. (2015). Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation, 131(7), 614–623. https://doi.org/10.1161/circulationaha.114.012826




