Symptom Clusters in Patients With Colorectal Cancer and Diabetes Over Time
Objectives: To examine symptoms and symptom clusters in patients with colorectal cancer (CRC) with or without diabetes at three key periods (0–6 months, 12–18 months, and 24–30 months) post–initial chemotherapy.
Sample & Setting: Patients with CRC from a cancer center in the midwestern United States between January 2007 and December 2017.
Methods & Variables: Eight of the most common symptoms (fatigue, gastrointestinal issues, depression, anxiety, peripheral neuropathy, physical function, cognition, and sleep disturbance) reported by patients with CRC and patients with diabetes were extracted from electronic health records. Exploratory factor analysis was used to identify symptom clusters, which were assessed for patterns and clinical relevance.
Results: Gastrointestinal issues and fatigue were the most prevalent symptoms in patients with CRC at each period. Across the three periods, patients with CRC and diabetes had more symptom clusters (n = 7) compared to patients with CRC without diabetes (n = 4). No stable symptom clusters were identified for either group.
Implications for Nursing: Oncology clinicians must recognize that patients with CRC and diabetes may present with exacerbated symptoms or symptom clusters. Ongoing assessment and monitoring of patients with CRC and diabetes for symptoms and symptom clusters is important because they may be at an increased risk for higher symptom burden.
Jump to a section
Estimates suggest that there are 1.5 million patients with colorectal cancer (CRC) in the United States (American Cancer Society, 2023). Advances in prevention, screening, and treatment for CRC have improved overall survivor rates (Benitez Majano et al., 2019; Van Cutsem et al., 2014). With increased cancer survival rates, the risk of living with other comorbidities is also increased (Pule et al., 2019; Rowley et al., 2017). About 33% of patients with CRC have at least one comorbid condition (Michalopoulou et al., 2021). Type 2 diabetes is a common comorbidity in patients with CRC and is prevalent in as much as 20% of the CRC population (Storey et al., 2021) compared to 11% in the noncancer population (Centers for Disease Control and Prevention, 2022). Given the increasing cancer survival rates, diabetes as a comorbidity among patients with cancer is an issue of growing importance for patients and healthcare providers.
Researchers describe similar symptoms, such as fatigue, gastrointestinal (GI) issues, depression, anxiety, peripheral neuropathy, decrements in physical function and cognition, and sleep disturbance, in patients with CRC and diabetes (Brady et al., 2022; Hershey et al., 2012; Hershey & Pierce, 2015; Vissers et al., 2016). CRC and diabetes initiate inflammatory processes (Giovannucci et al., 2010; Hammer et al., 2019), which trigger physiologic interactions that may contribute to or intensify symptoms. In addition, symptoms may co-occur, forming symptom clusters which are defined as two or more co-occurring symptoms (Kim et al., 2005; Miaskowski et al., 2004, 2007). Symptom clusters have not been well studied across multiple chronic conditions like diabetes and CRC.
In the authors’ previous work with patients with breast cancer, patients with diabetes as a comorbidity reported greater symptoms (e.g., poorer physical and attention function, more sleep disturbance, greater fatigue) than patients without this comorbidity (Storey et al., 2019). However, little is known regarding how diabetes affects the symptoms of patients with CRC. Among patients with CRC, only one study examined neuropathic symptoms in patients with CRC and diabetes (n = 218) compared to patients with CRC without diabetes (n = 957) (Vissers et al., 2015). The researchers noted that patients with CRC and diabetes experienced more neuropathic symptoms than patients with CRC without diabetes (Vissers et al., 2015). However, the study focused on only that one symptom. Even less is known about the symptom clusters of patients with CRC and diabetes. Although symptom cluster research has been conducted in patients with CRC (Agasi-Idenburg et al., 2017; Hao et al., 2021; Li et al., 2019; Potosky et al., 2022) and in patients with diabetes (Brady et al., 2022; García et al., 2019; Hammer et al., 2003), there is a paucity of research examining symptom clusters in patients with CRC and diabetes throughout the cancer trajectory. In the presence of comorbid diabetes, it is plausible that the summative consequences of symptoms and symptom clusters experienced by patients with CRC may be exacerbated. As the prevalence of CRC and diabetes is predicted to increase, it is imperative that clinicians understand the effect of diabetes on the symptom clusters in patients with CRC across time (Rowley et al., 2017).
To the authors’ knowledge, no longitudinal studies have examined symptom clusters in patients with CRC and diabetes from initiation of chemotherapy through cessation of treatment. Therefore, the purpose of this study was to use data from electronic health records (EHRs) to examine individual symptoms and symptom clusters in patients with CRC and diabetes compared to patients with CRC without diabetes at three key periods across the cancer trajectory (after initial chemotherapy and through cessation of treatment). Specific aims included the following: (a) Determine the prevalence of individual symptoms in patients with CRC with and without diabetes; (b) identify the individual symptoms that constitute symptom clusters in patients with CRC with or without diabetes; and (c) compare symptom clusters between patients with CRC with or without diabetes at the following three key periods: time 1 (T1) (0–6 months), time 2 (T2) (12–18 months), and time 3 (T3) (24–30 months). Because patients with cancer are living longer with their disease, a comprehensive understanding and characterization of symptom clusters specific to patients with CRC and diabetes are critical to guide clinical practice. Findings from this study will provide pertinent information to clinicians who treat patients with CRC and diabetes.
Conceptual Framework
The conceptual framework for this study was adapted from the conceptual model of hyperglycemia in patients with cancer (Hammer et al., 2019). This original model describes the physiologic links between hyperglycemia (a characteristic of diabetes) and cancer and identifies the downstream effects of hyperglycemia on four primary symptoms (pain, fatigue, depression, and sleep disturbance) frequently experienced by patients with cancer. However, the model does not specifically examine symptom clusters. For this study, the current authors expanded the model in the following ways: (a) included three additional symptoms (GI issues, anxiety, and peripheral neuropathy) that are common among patients with CRC and patients with diabetes; (b) identified the prevalence of symptoms and the grouping of symptoms into clusters; and (c) examined the symptoms and symptom clusters across three key periods during the cancer trajectory.
Methods
Study Design, Setting, and Sample
This retrospective cohort study used EHRs from a large statewide health repository from the Regenstrief Institute that included clinical health data from five health systems in central Indiana (McDonald et al., 1999). Eligibility criteria included the following: (a) having a nonmetastatic diagnosis of CRC (stage II–III) per statewide cancer registry data and International Classification of Diseases (ICD) codes; (b) receiving chemotherapy alone or in combination with other adjuvant therapy (e.g., surgery, radiation therapy); (c) having CRC with no diagnosis of diabetes or had CRC and a diagnosis of type 2 diabetes prior to the CRC diagnosis per ICD codes and medication lists for antidiabetes medications; and (d) being aged 21 years or older. Exclusion criteria included the following factors: (a) having another diagnosis of cancer, except basal or squamous skin cancer; (b) having metastatic CRC; and (c) receiving subsequent chemotherapy after the initial chemotherapy for their cancer diagnosis. Data from January 2007 through December 2017 were identified through a statewide cancer registry and ICD codes. There were 5,629 patients with CRC identified during the study time period. Indiana University’s institutional review board approved this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Procedures
A natural language processing pathway was developed to identify and extract symptom data from clinical notes in the EHR (Luo et al., 2021). The most common symptoms reported by patients with cancer and patients with diabetes (fatigue, GI issues, depression, anxiety, peripheral neuropathy, physical function, cognition, and sleep disturbance) were selected a priori to facilitate data extraction of symptoms from the clinical notes. Relevant seed terms (i.e., synonyms and phrases) that corresponded with the symptoms of interest were used to capture the varied descriptions of symptoms. For example, synonyms used to identify depression included depression, symptoms of depression, failure to thrive, sad, despair, misery, unhappy, melancholy, hopeless, and others. The natural language processing pathway first used the Unified Medical Language System MetaMap (Aronson & Lang, 2010) to extract the symptom expressions. The word sense disambiguation and negation detection were configured to ignore negative cases. Then, based on the given seed terms, a semantic analysis was used to include more expressions of the symptoms of interest that were not in the list of seed terms but were mentioned in the clinical notes (Luo et al., 2021). For example, the semantic analysis added new symptom expressions, such as feeling down, to the synonyms for depression, and added terms like situational stress, nervousness, shaking, trembling, and feeling jittery to the synonyms for anxiety.
Because of the large number of individual descriptive terms for GI symptoms in the clinical notes of the EHR, symptoms were combined into the GI symptom category based on previous literature (García et al., 2019; Han et al., 2020; Hao et al., 2021; Potosky et al., 2022).
Data Collection
Demographic (e.g., age, body mass index, gender, race) and medical (e.g., Charlson Comorbidity Index, cancer stage) characteristics were extracted from the EHRs. A modified Charlson Comorbidity Index (excluding diabetes and CRC) was used to account for comorbidities.
Symptom data were collected at three key periods in the cancer treatment trajectory post–initial chemotherapy: 0–6 months (T1), 12–18 months (T2), and 24–30 months (T3). These periods were chosen because symptom clusters are typically related to treatment and/or disease and may vary over time. Studying only the period around the initiation of chemotherapy may not be an accurate reflection of symptoms and symptom clusters, given the acute side effects associated with the days following initial treatment (Foltran et al., 2014; Giesinger et al., 2014; Pettersson et al., 2014; Röhrl et al., 2019). A more accurate picture of the symptom profile may be found at later periods in the cancer trajectory because these symptoms and symptom clusters may fluctuate across time (Röhrl et al., 2019).
Therefore, the rationale for the three key periods was as follows: (a) 0–6 months is typically when chemotherapy is initiated and acute symptoms are first noticed; (b) 12–18 months is when treatment regimens are continuing or beginning to cease, and patients may demonstrate the cumulative effect of treatment on symptom clusters; and (c) 24–30 months represents the cessation of therapy, when patients with CRC may experience lingering symptoms post-treatment.
Data Analysis
Group comparisons of demographic and medical characteristics were evaluated using the chi-square test for categorical variables and TIBCO Analysis of Variance for continuous variables. Statistical significance was declared at alpha = 0.05. Exploratory factor analysis was used to identify clusters among the eight symptoms (fatigue, GI issues, depression, anxiety, peripheral neuropathy, physical function, cognition, and sleep disturbance) during each of the three measurement periods for patients with CRC with or without diabetes. Exploratory factor analysis with the IBM Promax rotation was performed. Promax rotation, an oblique rotation, is used in factor analysis when it is anticipated that two or more variables are correlated (Field, 2018). A Cronbach’s alpha greater than 0.6 and item-total correlations greater than 0.3 were considered acceptable (Oh et al., 2016). The results of the exploratory factor analysis for two and three factors were examined for each period. The three-factor solution was selected because it led to the best clinical interpretation. Symptom clusters were categorized by the symptoms with the highest factor loading. All statistical analyses were conducted with R software, version 4.1.2.
Results
Characteristics of Cohort
A total of 5,629 patients with CRC were identified for the study, of which 2,293 met the inclusion criteria for analysis. Table 1 provides the demographic and medical characteristics of patients with CRC in the study cohort (N = 2,293). Overall, most of the sample was male and White, and had stage III CRC. Overall, 712 (31%) patients with CRC also had a diagnosis of diabetes. Statistically significant differences were noted between patients with CRC and diabetes and patients with CRC without diabetes. In particular, patients with CRC and diabetes were older, with stage III CRC, had higher body mass index, and had modified Charlson Comorbidity Index scores. Cognitive and sleep disturbance symptoms were excluded from the analysis because of low occurrences at each key period (less than 0.1%), leaving six symptoms (fatigue, GI issues, depression, anxiety, peripheral neuropathy, and physical function) for symptom and symptom cluster analysis.
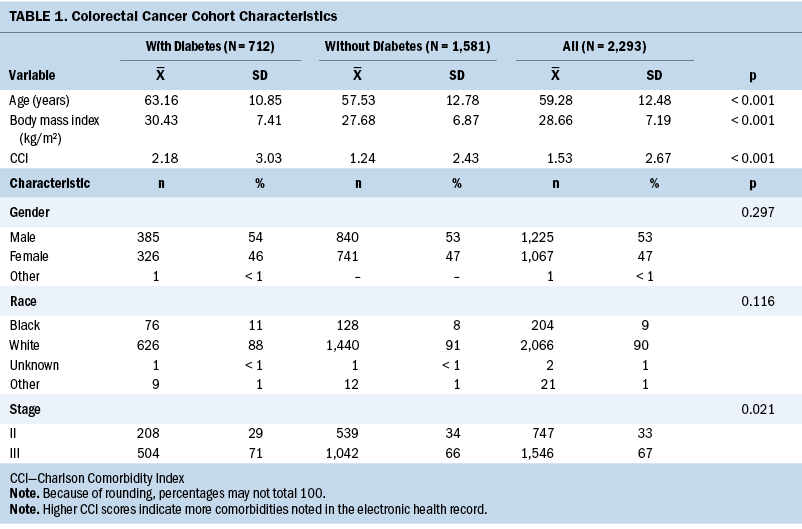
Symptom Prevalence
Table 2 shows the prevalence of the individual symptoms across the three periods by diabetes status. Across the three periods, the most prevalent symptoms in patients with CRC, with or without diabetes, were GI issues and fatigue.
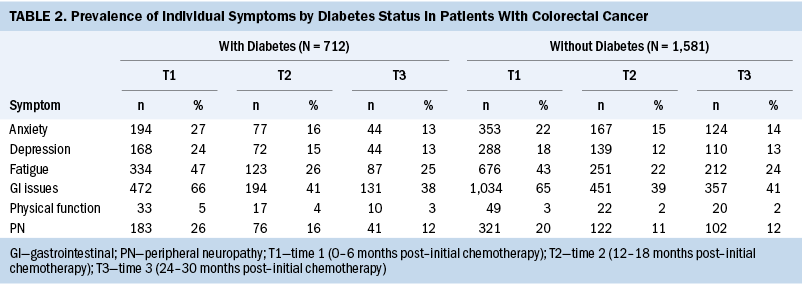
Symptom Clusters
T1: Table 3 shows the symptom clusters based on their highest loading on a factor within each period post–initial chemotherapy. At T1, the authors found three symptom clusters made up of two individual symptoms in each cluster in patients with CRC and diabetes, and only one symptom cluster made up of three individual symptoms in patients with CRC without diabetes.
Among patients with CRC and diabetes, the symptom clusters identified included fatigue/GI issues (cluster 1), anxiety/depression (cluster 2), and peripheral neuropathy/physical function (cluster 3). For patients with CRC without diabetes, the symptom cluster included GI issues/fatigue/anxiety (cluster 1). Overall, for T1, patients with CRC and diabetes had more symptom clusters (three versus one) that consisted of more individual symptoms (six versus three) than patients with CRC without diabetes.
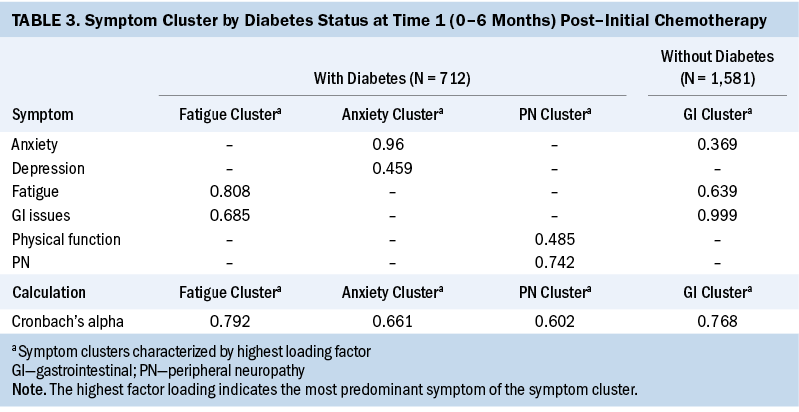
T2: At T2, two symptom clusters were identified for patients with CRC with or without diabetes. The two symptom clusters identified in patients with CRC and diabetes were GI issues/fatigue/depression (cluster 1) and anxiety/peripheral neuropathy/depression (cluster 2). The two symptom clusters in patients with CRC without diabetes were depression/anxiety/GI issues (cluster 1) and peripheral neuropathy/fatigue (cluster 2). Although the number of symptom clusters was the same for each group, like T1, the symptom clusters in patients with CRC and diabetes contained more individual symptoms (six versus five) than the clusters in patients with CRC without diabetes at T2 (see Table 4).
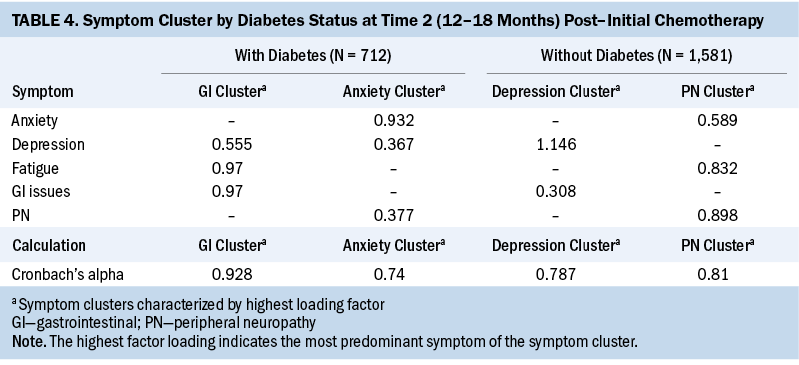
T3: At T3, two symptom clusters were identified in patients with CRC and diabetes. Cluster 1 consisted of anxiety/depression/fatigue, and cluster 2 consisted of peripheral neuropathy/GI issues. In patients with CRC without diabetes, only one symptom cluster was identified: peripheral neuropathy/fatigue/depression/anxiety (cluster 1). Similar to T1, the number of symptom clusters was higher (two versus one), and, as in T1 and T2, the number of individual symptoms was higher (five versus four) in patients with CRC and diabetes at T3 (see Table 5).
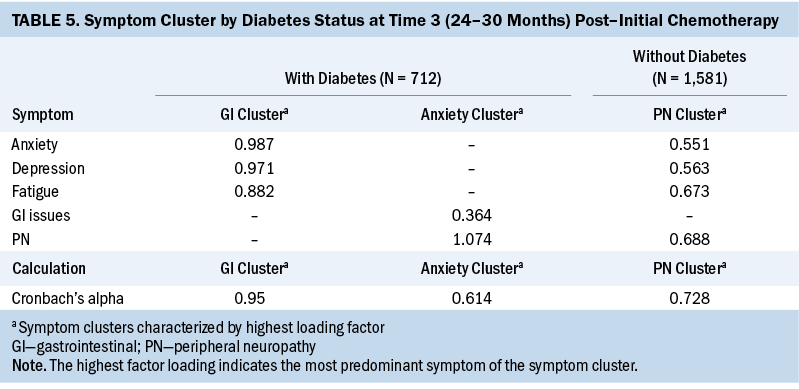
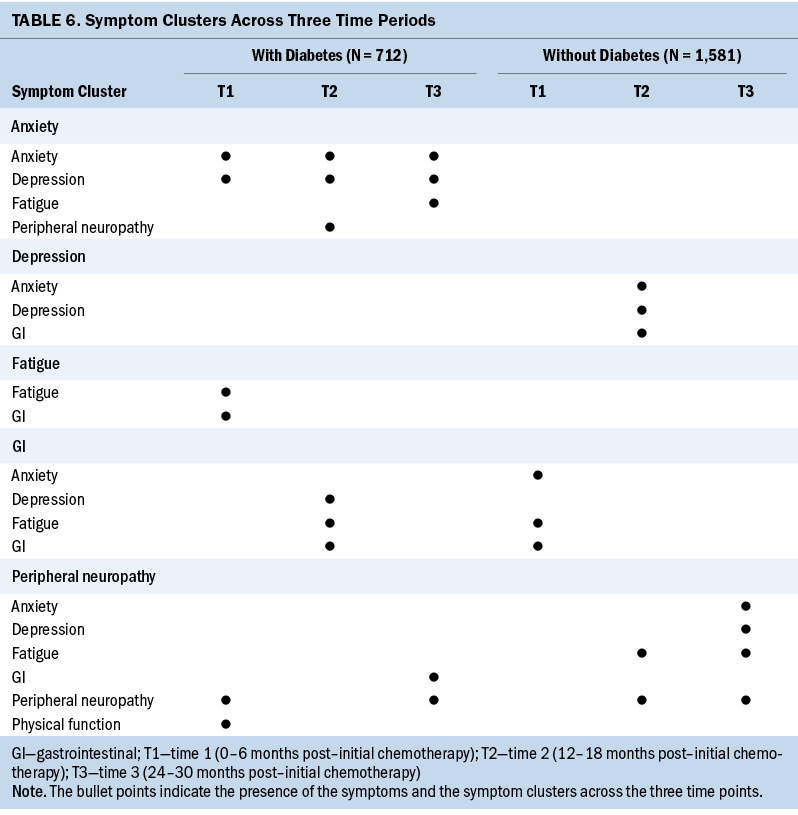
The number of individual symptoms that made up the symptom clusters across the three key periods ranged from a minimum of two to a maximum of four for each group. Table 6 shows the symptom clusters across the three time periods. Patients with CRC and diabetes had more symptom clusters (n = 7) than patients with CRC without diabetes (n = 4). Although the symptom clusters in patients with CRC and diabetes and in patients with CRC without diabetes consisted of various configurations of individual symptoms, the authors did not find any symptom clusters to be stable (i.e., repeated) across the three periods in either group.
Discussion
To the authors’ knowledge, this is the first study to use clinical data from EHRs to comprehensively examine symptoms and symptom clusters of patients with CRC and diabetes across three key periods throughout the cancer trajectory. Using a large dataset, a cohort of patients with CRC was identified, 31% of whom had diabetes, which exceeds previous studies in which the prevalence of diabetes among patients with CRC was 20% (Storey et al., 2021). The current study expanded the conceptual model of hyperglycemia in patients with cancer (Hammer et al., 2019) to include the examination of symptoms and symptom clusters in patients with CRC and diabetes at three key periods in the cancer treatment trajectory. Findings from this study demonstrate the relationship between diabetes and the number of symptoms and symptom clusters in patients with CRC. These findings support and expand the relationships in the model and elucidate the need for future prospective studies to examine the role of diabetes and/or hyperglycemia on symptoms and symptom clusters.
Symptom Prevalence
In this study, GI symptoms were the most prevalent, followed by fatigue, for patients with CRC with or without diabetes in each period. In a systematic review and meta-analysis of 35 studies by Han et al. (2020), fatigue was found to be the most prevalent symptom among patients with CRC during active treatment and for years beyond treatment. It is plausible that GI symptoms associated with chemotherapy (nausea, vomiting, diarrhea) contribute to fatigue in patients with CRC (Husson et al., 2015). In addition, poor nutritional status (Wei & Li, 2018), malabsorption, and bowel motility (Han et al., 2023) issues may persist beyond treatment and may continue to contribute to fatigue. Future studies should examine the role of diabetes on fatigue and explore the differences in GI symptoms between patients with CRC and diabetes and patients with CRC without diabetes. Understanding whether patients with CRC and diabetes experience unique symptoms can inform the development of tailored interventions to mitigate symptoms.
Symptom Clusters
The results from the current exploratory factor analysis demonstrated that patients with CRC and diabetes had a greater number of symptom clusters at the T1 and T3 periods. These findings from T1 indicate that patients with CRC and diabetes may come into treatment with poorer overall health (Dowling et al., 2013; O’Shea et al., 2015) and have more latent symptoms than patients with CRC without diabetes. In particular, across all time periods, patients with CRC and diabetes had seven symptom clusters, and patients with CRC without diabetes had four symptom clusters. However, none of the symptom clusters in this study were repeated across the three periods, limiting the ability for direct comparison. More research is needed to identify key periods in the cancer trajectory during which patients with CRC and diabetes may experience more symptom clusters, which can inform oncology clinicians of critical time periods that may require more intense assessment and treatment of symptom clusters.
Studies of symptom clusters in patients with CRC are limited, with even fewer studies examining symptom clusters in patients with CRC and diabetes. The authors found only four studies that examined symptom clusters among patients with CRC. Agasi-Idenburg et al. (2017) conducted a secondary analysis comparing symptom clusters associated with fatigue in younger and older patients with CRC. These researchers found three clusters (emotional, pain, and dyspnea) in both age groups. However, they did not report the time frame (active or post-treatment) of the sample at the time of the study, which may affect symptoms and symptom clusters (Agasi-Idenburg et al., 2017). Hao et al. (2021) examined patients with CRC (N = 149) and identified five symptom clusters (psychologic, GI issues, urinary, lack of energy, and pain) during the first year postcolostomy. However, the study focused on patients with colostomies, which may result in different symptom clusters. Potosky et al. (2022) studied co-occurring symptoms and functioning deficits in patients with CRC (N = 909) to identify subgroups with a low, moderate, or high quality of life. These researchers found that about one-third of patients with CRC experienced a physical and psychosocial symptom cluster highly correlated with functional deficits and poorer quality of life. However, the study did not define the specific symptoms within the clusters (Potosky et al., 2022). Li et al. (2022) examined whether biomarkers predicted symptom clusters in patients with radically resected CRC. The authors identified four chemotherapy-related symptom clusters that included fatigue–psychological, GI issues, neurotoxic symptoms, and constipation–abdominal distension (Li et al., 2022). However, it was a cross-sectional study conducted three days after chemotherapy administration, which captured symptoms at their peak but did not reflect symptom clusters across the cancer treatment trajectory (Li et al., 2022). All four of these studies failed to examine symptom clusters by diabetes diagnosis, which may alter symptom clusters. Future studies should examine the role of glycemic biomarkers such as blood glucose (fasting or random) or glycated hemoglobin on the symptom clusters of patients with CRC and diabetes. Glycemia is a modifiable risk factor, which may improve symptom clusters with optimal monitoring and management.
The authors found only one exploratory study that examined the severity and patterns of preselected symptoms (pain, fatigue, change in appetite, nausea, and vomiting) in a heterogeneous population of individuals with cancer and diabetes (N = 43) during chemotherapy administration (Hershey & Pierce, 2015). These researchers found two distinct clusters based on severity. In cluster 1, patients with cancer and diabetes experienced mild symptoms (i.e., no pain, mild fatigue, change in appetite, and nausea) at baseline, which remained mild throughout the study period. In cluster 2, patients with cancer and diabetes experienced mild symptom severity progressing to moderate symptom severity, with marked increases in fatigue, nausea, numbness, and tingling (Hershey & Pierce, 2015). However, only four patients in the study had CRC, limiting generalizability. The study also included patients with types 1 and 2 diabetes, which are distinctly different diseases and may have influenced the findings (Hershey & Pierce, 2015). Finally, the study was conducted over only an eight-week period and did not examine key periods within the treatment trajectory (Hershey & Pierce, 2015). The findings from the current study suggest that patients with CRC and diabetes experience multiple and unique symptom clusters at key periods in the cancer treatment trajectory. Understanding the contribution of diabetes to symptom clusters can inform the development of screening tools to identify at-risk patients with cancer. Prospective research is needed to confirm the findings from this study.
Strengths
This study had several strengths. One strength was that the data were extracted from EHRs. Using EHR data allowed examination of symptoms and symptom clusters in a large cohort of patients with CRC, which would have been costly and difficult to obtain in a longitudinal study. In addition, the descriptions of symptoms reported directly from patients with CRC (with or without diabetes) to healthcare clinicians provide valuable insight into their experiences. Finally, this study provided a foundation for developing prospective studies that can measure symptoms and symptom clusters in patients with CRC and diabetes in real time at key periods during the cancer trajectory.
Limitations
This study had several limitations. First, the descriptive retrospective study design precluded the ability to establish causality. However, this study provided preliminary data useful to guide the development of future prospective studies. One goal of this study was to compare symptoms and symptom clusters of patients with CRC with or without diabetes at three periods in the cancer trajectory. Therefore, other comorbidities that may have also affected symptoms and symptom clusters were not examined. More prospective research is needed to understand the influence of diabetes and multiple comorbid conditions on symptom clusters in patients with cancer. As in most symptom cluster studies, the authors preselected symptoms to include eight of the most common symptoms reported by patients with CRC and patients with diabetes and also collapsed the variety of GI symptoms into a preset GI symptom cluster based on previous literature (García et al., 2019; Hao et al., 2021; Potosky et al., 2022). Future studies may benefit from including additional symptoms for a more in-depth assessment and analysis of GI symptoms. Finally, clinicians may have incorrectly assigned or omitted some health data and ICD codes in the EHR. However, to moderate this risk, the diagnosis of diabetes was verified by reviewing medication lists. Despite these limitations, the findings from this study have implications for clinical practice and future research.
Implications for Nursing
Oncology clinicians will likely be challenged with treating patients with CRC and comorbid diabetes, given that patients with CRC live longer and the prevalence of diabetes is estimated to increase by 51% by 2045 to include more than 548 million people worldwide (Saeedi et al., 2019). Within the context of concurrent comorbidities, symptoms and symptom clusters may be underreported. Therefore, oncology clinicians must recognize that patients with CRC and diabetes may present with exacerbated symptoms or symptom clusters. Ongoing assessment and monitoring of patients with CRC and diabetes for symptoms and symptom clusters is important because they may be at an increased risk for higher symptom burden.
Conclusion
The findings of this study suggest that having a concurrent diagnosis of CRC and diabetes may exacerbate symptoms and symptom clusters, compared to patients with CRC without diabetes. Prospective research is needed to determine whether patients with CRC and diabetes experience specific and/or a greater number of symptoms and symptom clusters throughout the treatment trajectory. It is plausible that challenges in self-management of diabetes during treatment for CRC may further intensify the symptoms or symptom clusters. Future research should include studies with interventions that facilitate self-management of diabetes, which may improve symptoms and/or symptom clusters in patients with CRC and diabetes. In addition, survivorship models that include multispecialty practitioners are warranted to assess and assist in the medical management of patients with CRC and diabetes.
About the Authors
Susan Storey, PhD, RN, AOCNS®, FCNS, is an assistant professor in the School of Nursing at Indiana University; Xiao Luo, PhD, is an associate professor in the School of Engineering and Technology at Indiana University–Purdue University Indianapolis; Jie Ren, PhD, is an assistant professor in the Department of Biostatistics and Health Data Science in the School of Medicine at Indiana University; and Kun Huang, PhD, FAIMBE, is a professor and chair of the Department of Biostatistics and Health Data Science, and chair for genomic data science and director of data science and informatics for the Precision Health initiative, all in the School of Medicine, and an associate director of data science in the Simon Comprehensive Cancer Center, all at Indiana University, and an investigator at the Regenstrief Institute, all in Indianapolis; and Diane Von Ah, PhD, RN, FAAN, is the director of cancer research at the Center for Healthy Aging, Self-Management, and Complex Care and a distinguished professor of cancer research in the College of Nursing, both at the Ohio State University in Columbus. This research was funded, in part, by an RE01 from the Oncology Nursing Foundation (principal investigator: Storey) and the Indiana University Simon Cancer Center Cancer Prevention and Control Pilot Grant. Storey, Luo, Huang, and Von Ah contributed to the conceptualization and design. Ren provided statistical support. All authors provided the analysis and contributed to the manuscript preparation. Storey can be reached at sustorey@iu.edu, with copy to ONFEditor@ons.org. (Submitted August 2022. Accepted January 30, 2023.)
References
Agasi-Idenburg, S.C., Thong, M.S.Y., Punt, C.J.A., Stuiver, M.M., & Aaronson, N.K. (2017). Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Supportive Care in Cancer, 25(2), 625–632. https://doi.org/10.1007/s00520-016-3451-4
American Cancer Society. (2023). Key statistics for colorectal cancer. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-stati…
Aronson, A.R., & Lang, F.-M. (2010). An overview of MetaMap: Historical perspective and recent advances. Journal of the American Medical Informatics Association, 17(3), 229–236. https://doi.org/10.1136/jamia.2009.002733
Benitez Majano, S., Di Girolamo, C., Rachet, B., Maringe, C., Guren, M.G., Glimelius, B., . . . Walters, S. (2019). Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: A population-based study. Lancet Oncology, 20(1), 74–87. https://doi.org/10.1016/S1470-2045(18)30646-6
Brady, V., Whisenant, M., Wang, X., Ly, V.K., Zhu, G., Aguilar, D., & Wu, H. (2022). Characterization of symptoms and symptom clusters for type 2 diabetes using a large nationwide electronic health record database. Diabetes Spectrum, 35(2), 159–170. https://doi.org/10.2337/ds21-0064
Centers for Disease Control and Prevention. (2022). National diabetes statistics report. U.S. Department of Health and Human Services. https://www.cdc.gov/diabetes/data/statistics-report/index.html
Dowling, E.C., Chawla, N., Forsythe, L.P., de Moor, J., McNeel, T., Rozjabek, H.M., . . . Yabroff, K.R. (2013). Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer, 119(18), 3393–3401. https://doi.org/10.1002/cncr.28214
Field, A. (2018). Discovering statistics using IBM SPSS Statistics: North American edition (5th ed.). Sage.
Foltran, L., Aprile, G., Pisa, F.E., Ermacora, P., Pella, N., Iaiza, E., . . . Fasola, G. (2014). Risk of unplanned visits for colorectal cancer outpatients receiving chemotherapy: A case crossover study. Supportive Care in Cancer, 22(9), 2527–2533. https://doi.org/10.1007/s00520-014-2234-z
García, A.A., Bose, E., Zuñiga, J.A., & Zhang, W. (2019). Mexican Americans’ diabetes symptom prevalence, burden, and clusters. Applied Nursing Research, 46, 37–42. https://doi.org/10.1016/j.apnr.2019.02.002
Giesinger, J.M., Wintner, L.M., Zabernigg, A., Gamper, E.-M., Oberguggenberger, A.S., Sztankay, M.J., . . . Holzner, B. (2014). Assessing quality of life on the day of chemotherapy administration underestimates patients’ true symptom burden. BMC Cancer, 14, 758. https://doi.org/10.1186/1471-2407-14-758
Giovannucci, E., Harlan, D.M., Archer, M.C., Bergenstal, R.M., Gapstur, S.M., Habel, L.A., . . . Yee, D. (2010). Diabetes and cancer: A consensus report. Diabetes Care, 33(7), 1674–1685. https://doi.org/10.2337/dc10-0666
Hammer, J., Howell, S., Bytzer, P., Horowitz, M., & Talley, N.J. (2003). Symptom clustering in subjects with and without diabetes mellitus: A population-based study of 15,000 Australian adults. American Journal of Gastroenterology, 98(2), 391–398. https://doi.org/10.1111/j.1572-0241.2003.07236.x
Hammer, M.J., Eckardt, P., Cartwright, F., & Miaskowski, C. (2021). Prescribed walking for glycemic control and symptom management in patients without diabetes undergoing chemotherapy. Nursing Research, 70(1), 6–14. https://doi.org/10.1097/NNR.0000000000000468
Hammer, M., Storey, S., Hershey, D.S., Brady, V.J., Davis, E., Mandolfo, N., . . . Olausson, J. (2019). Hyperglycemia and cancer: A state-of-the-science review. Oncology Nursing Forum, 46(4), 459–472. https://doi.org/10.1188/19.ONF.459-472
Han, C.J., Reding, K.W., Kalady, M.F., Yung, R., Greenlee, H., & Paskett, E.D. (2023). Factors associated with long-term gastrointestinal symptoms in colorectal cancer survivors in the women’s health initiatives (WHI study). PLOS ONE, 18(5), e0286058. https://doi.org/0.1371/journal.pone.0286058
Han, C.J., Yang, G.S., & Syrjala, K. (2020). Symptom experiences in colorectal cancer survivors after cancer treatments: A systematic review and meta-analysis. Cancer Nursing, 43(3), E132–E158. https://doi.org/10.1097/NCC.0000000000000785
Hao, J., Gu, L., Liu, P., Zhang, L., Xu, H., Qiu, Q., & Zhang, W. (2021). Symptom clusters in patients with colorectal cancer after colostomy: A longitudinal study in Shanghai. Journal of International Medical Research, 49(12), 3000605211063105. https://doi.org/10.1177/03000605211063105
Hershey, D.S., Given, B., Given, C., Von Eye, A., & You, M. (2012). Diabetes and cancer: Impact on health-related quality of life. Oncology Nursing Forum, 39(5), 449–457. https://doi.org/10.1188/12.ONF.449-457
Hershey, D.S., & Pierce, S.J. (2015). Examining patterns of multivariate, longitudinal symptom experiences among older adults with type 2 diabetes and cancer via cluster analysis. European Journal of Oncology Nursing, 19(6), 716–723. https://doi.org/10.1016/j.ejon.2015.05.006
Husson, O., Mols, F., van de Poll-Franse, L.V., & Thong, M.S.Y. (2015). The course of fatigue and its correlates in colorectal cancer survivors: A prospective cohort study of the PROFILES registry. Supportive Care in Cancer, 23(11), 3361–3371. https://doi.org/10.1007/s00520-015-2802-x
Kim, H-J., McGuire, D.B., Tulman, L. & Barsevick, A.M. (2005). Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing, 28(4), 270–282. https://doi.org/10.1097/00002820-200507000-00005
Li, H., Ji, M., Scott, P., & Dunbar-Jacob, J.M. (2019). The effect of symptom clusters on quality of life among patients with type 2 diabetes. Diabetes Educator, 45(3), 287–294. https://doi.org/10.1177/0145721719837902
Li, N., Lu, J., Xia, D., Jiang, X., Wen, X., Qin, X., . . . Wang, T. (2022). Serum biomarkers predict adjuvant chemotherapy-associated symptom clusters in radical resected colorectal cancer patients. Journal of Gastrointestinal Oncology, 13(1), 197–209. https://doi.org/10.21037/jgo-21-904
Luo, X., Storey, S., Gandhi, P., Zhang, Z., Metzger, M., & Huang, K. (2021). Analyzing the symptoms in colorectal and breast cancer patients with or without type 2 diabetes using EHR data. Health Informatics Journal, 27(1), 14604582211000785. https://doi.org/10.1177/14604582211000785
McDonald, C.J., Overhage, J.M., Tierney, W.M., Dexter, P.R., Martin, D.K., Suico, J.G., . . . Wodniak, C. (1999). The Regenstrief Medical Record System: A quarter century experience. International Journal of Medical Informatics, 54(3), 225–253. https://doi.org/10.1016/s1386-5056(99)00009-x
Miaskowski, C., Aouizerat, B.E., Dodd, M., & Cooper, B. (2007). Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. Journal of the National Cancer Institute. Monographs, 37, 39–46. https://doi.org/10.1093/jncimonographs/lgm003
Miaskowski, C., Dodd, M., & Lee, K. (2004). Symptom clusters: The new frontier in symptom management research. Journal of the National Cancer Institute. Monographs, 32, 17–21. https://doi.org/10.1093/jncimonographs/lgh023
Michalopoulou, E., Matthes, K.L., Karavasiloglou, N., Wanner, M., Limam, M., Korol, D., . . . Rohrmann, S. (2021). Impact of comorbidities at diagnosis on the 10-year colorectal cancer net survival: A population-based study. Cancer Epidemiology, 73, 101962. https://doi.org/10.1016/j.canep.2021.101962
Oh, J.H., Thor, M., Olsson, C., Skokic, V., Jörnsten, R., Alsadius, D., . . . Deasy, J.O. (2016). A factor analysis approach for clustering patient reported outcomes. Methods of Information in Medicine, 55(5), 431–439. https://doi.org/10.3414/ME16-01-0035
O’Shea, M.P., Teeling, M., & Bennett, K. (2015). Comorbidity, health-related quality of life and self-care in type 2 diabetes: A cross-sectional study in an outpatient population. Irish Journal of Medical Science, 184(3), 623–630. https://doi.org/10.1007/s11845-014-1190-4
Pettersson, G., Berterö, C., Unosson, M., & Börjeson, S. (2014). Symptom prevalence, frequency, severity, and distress during chemotherapy for patients with colorectal cancer. Supportive Care in Cancer, 22(5), 1171–1179. https://doi.org/10.1007/s00520-013-2069-z
Potosky, A.L., Graves, K.D., Lin, L., Pan, W., Fall-Dickson, J.M., Ahn, J., . . . Reeve, B.B. (2022). The prevalence and risk of symptom and function clusters in colorectal cancer survivors. Journal of Cancer Survivorship, 16(6), 1449–1460. https://doi.org/10.1007/s11764-021-01123-6
Pule, M.L., Buckley, E., Niyonsenga, T., & Roder, D. (2019). The effects of comorbidity on colorectal cancer mortality in an Australian cancer population. Scientific Reports, 9(1), 8580. https://doi.org/10.1038/s41598-019-44969-8
Röhrl, K., Guren, M.G., Småstuen, M.C., & Rustøen, T. (2019). Symptoms during chemotherapy in colorectal cancer patients. Supportive Care in Cancer, 27(8), 3007–3017. https://doi.org/10.1007/s00520-018-4598-y
Rowley, W.R., Bezold, C., Arikan, Y., Byrne, E., & Krohe, S. (2017). Diabetes 2030: Insights from yesterday, today, and future trends. Population Health Management, 20(1), 6–12. https://doi.org/10.1089/pop.2015.0181
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., . . . Williams, R. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843. https://pubmed.ncbi.nlm.nih.gov/31518657/
Storey, S., Cohee, A., Gathirua-Mwangi, W.G., Vachon, E., Monahan, P., Otte, J., . . . Champion, V. (2019). Impact of diabetes on the symptoms of breast cancer survivors. Oncology Nursing Forum, 46(4), 473–484. https://doi.org/10.1188/19.ONF.473-484
Storey, S., Zhang, Z., Luo, X., Von Ah, D., Metzger, M., Zhang, J., . . . Huang, K. (2021). Association of comorbid diabetes with clinical outcomes and healthcare utilization in colorectal cancer survivors. Oncology Nursing Forum, 48(2), 195–206. https://doi.org/10.1188/21.ONF.195-206
Van Cutsem, E., Cervantes, A., Nordlinger, B., & Arnold, D. (2014). Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 25(Suppl. 3), iii1–iii9. https://doi.org/10.1093/annonc/mdu260
Vissers, P.A.J., Falzon, L., van de Poll-Franse, L.V., Pouwer, F., & Thong, M.S.Y. (2016). The impact of having both cancer and diabetes on patient-reported outcomes: A systematic review and directions for future research. Journal of Cancer Survivorship, 10(2), 406–415. https://doi.org/10.1007/s11764-015-0486-3
Vissers, P.A.J., Mols, F., Thong, M.S.Y., Pouwer, F., Vreugdenhil, G., & van de Poll-Franse, L.V. (2015). The impact of diabetes on neuropathic symptoms and receipt of chemotherapy among colorectal cancer patients: Results from the PROFILES registry. Journal of Cancer Survivorship, 9(3), 523–531. https://doi.org/10.1007/s11764-015-0429-z
Wei, J.N. & Li, S.-X. (2018). The relationship between nutritional risks and cancer-related fatigue in patients with colorectal cancer fast-track surgery. Cancer Nursing, 41(6), E41-E47. https://doi.org/10.1097/NCC.0000000000000541

